Abstract
This study aimed to evaluate the impact of Thailand’s hepatitis B virus (HBV) National Program Immunization (NPI), 32 years post-implementation, on infection rates and immunity in various age groups. A cross-sectional study involved 6,068 participants aged 6 months to 80 years from four regions in Thailand. Blood samples were tested for HBsAg, anti-HBs, and anti-HBc using a chemiluminescent immunoassay. Data were compared across age groups and with previous surveys from 2004 to 2014. Individuals born after the implementation of the NPI had significantly lower HBV infection rates (p < 0.0001). No HBsAg was detected in individuals under 20 years old. The prevalence of HBV carriers increased with age, from 0.3% in the 21–30 group to 4.3% in those over 60, with an overall prevalence of 1.7%. Percentages of seroprotected individuals (anti-HBs ≥ 10 mIU/mL) were high in young children but dropped to 19.4% in ages 11–20 and 12.5% in ages 21–30. Anti-HBc was found at very low rates in children but increased significantly after age 30. Thailand’s HBV NPI significantly reduced HBV infection rates, especially in younger populations. This study highlighted the program’s success and guided future elimination efforts to achieve hepatitis elimination goal by 2030.
Similar content being viewed by others
Introduction
Hepatitis B virus (HBV) is a major global health concern, causing acute and chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma. Currently, there are approximately 254 million people worldwide who are chronic HBV carrier in the year 20221. Before the introduction of vaccination programs, Thailand had an HBV carrier rate of around 8.2%. The prevalence of HBsAg was higher in males than females2. In 1986, the prevalence of HBsAg in pregnant women was 6%3. Nearly half of the HBV transmissions were through vertical transmission from carrier mother to infant4. If a mother is an HBV carrier with HBeAg positive or high viral load, there is approximately a 90% chance the infant will acquire the infection5. Infants who become HBV carriers can further transmit the virus horizontally as they grow up. Males who acquire the infection in childhood or from vertical transmission have a high chance of dying from liver diseases such as cirrhosis, liver failure, or liver cancer. In females, the risk of liver disease and death is lower than in males6, but they may pass the virus to their offspring, perpetuating the cycle of transmission.
Breaking this cycle through vaccination is critical. Studies show that administering the HBV vaccine to newborns within 12 h of birth and 1,2 and 12 months (4 doses) or at birth, 1 and 6 months (3 doses) of age is 94% effective in preventing the infection in infants with carrier mothers3,7,8, and when combined with hepatitis B immunoglobulin (HBIg), the prevention rate increases to 98%7,8. Additionally, administering tenofovir to mothers during the last trimester can prevent vertical transmission entirely9. HBV vaccination in newborns provides long-term protection, lasting over 20 years10,11. Even when antibody levels fall below 10 mIU/ml, a booster can rapidly raise the immune response, ensuring effective protection against infection12.
Thailand introduced a pilot program for universal HBV vaccination in 1988 as part of the National Immunization Program, beginning in Chonburi and Chiang Mai provinces13. By 1990, the program expanded to 12 provinces and, in 1992, was implemented nationwide. The current HBV vaccine coverage rate for the first dose of HBV vaccination was high (93.1%)14, largely because most births in Thailand occur in hospitals. The vaccination schedule includes three doses: at birth, at 2 months, and 6 months, alongside the EPI Diphtheria, tetanus and whole cell pertussis (DTPw) vaccines.
In 1994, the Thai Ministry of Public Health piloted the combined DTPw-HB vaccine in Chiang Rai, where monovalent HB was administered at birth, followed by the combined DTPw-HB vaccine at 2, 4, and 6 months, effectively providing four doses of the HB vaccine. This strategy proved highly effective15 and was expanded nationwide in 2005, with all provinces adopting it by 2008. Further studies found that in infants born to HBV carrier mothers who did not receive HBIg, giving an additional monovalent HB dose at one month of age, making it five total doses, offered better protection than the four-dose schedule16. Consequently, in 2009, the Thai Department of Disease Control updated the vaccination schedule for infants born to HBV carrier mothers to include a second monovalent HB dose at one month, followed by combined DTPw-HB at 2, 4, and 6 months.
In 2019, the DTPw-HB vaccine was replaced with the pentavalent vaccine, which includes protection against Haemophilus influenzae (DTPw-HB-Hib), with the pertussis component being whole-cell. The pentavalent vaccine was used in place of the previous tetravalent vaccine. Field studies on the DTPw-HB-Hib vaccine under the EPI program showed that it stimulated a strong immune response against hepatitis B, comparable to that of the hexavalent DTPa-Hib-HB-IPV (diphtheria, tetanus, acellular pertussis, H influenzae, hepatitis B and inactivated polio vaccine) vaccine, which contains an acellular pertussis component16. The pentavalent DTPw-HB-Hib vaccine continues to be currently used.
National surveys conducted in 2004 and 2014, involving approximately 6,000 participants representing different regions of Thailand, revealed that individuals born after the introduction of the HBV vaccine under EPI had a markedly reduced rate of HBV infection, as measured by anti-HBc and HBsAg18,19.
The World Health Organization (WHO) aims to eliminate HBV as a public health threat by 203020. One key strategy is to reduce new HBV infections by at least 90%, and the administration of a birth dose of the HBV vaccine is a highly effective measure. WHO also aims to achieve zero mother-to-child transmission of HBV, with a target of reducing HBV infection in children under 5 years of age to less than 0.1%21.
This study presents a national survey on HBV infection across different age groups, following a pattern of studies conducted every 10 years. The data gathered will serve as a foundation for the ongoing planning and implementation of HBV elimination strategies, in line with the WHO’s 2030 targets. It will also contribute to efforts to prevent mother-to-child transmission of HBV, highlighting the success of Thailand’s national vaccination program and informing future prevention, control, and treatment efforts for hepatitis B.
Results
Population study
The study involved blood testing of the target population in various specified provinces. The target number and population in different age groups, along with details for each province, are shown in Table 1. The population included 1,526 people from Uttaradit Province, 1,518 people from Buriram Province, 1,522 people from Ayutthaya Province, and 1,503 people from Trang Province, with a total of 6,069 people. The study was conducted from May 1 to July 31, 2024.
Prevalence of HBsAg, anti-HBs, anti-HBc among different age groups
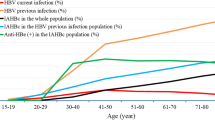
The results of the HBsAg screening, which indicated chronic hepatitis B infection, in different population groups were as follows: In the group of children under 20 years old, no HBsAg was detected, meaning the carrier rate was less than 0.1%. The prevalence of HBsAg increased with age, particularly after 20 years old, and was notably higher in populations born before the national immunization program. The prevalence reached up to 4.3% in the elderly population, or those over 60 years old, as shown in Fig. 1a.
Regarding anti-HBs, the antibody against hepatitis B surface antigen, it was found that young children had high levels of antibodies due to recent vaccination during the first year of life. However, the antibody levels rapidly decreased. When measuring the protective level of anti-HBs (≥ 10 mIU/ml), up to 20% of the population aged 11–20 years tested negative, and 10% of the population aged 21–30 years also tested negative. After that, the measured antibody levels tended to increase, partly due to past infections. However, when considering the overall seropositive rate for anti-HBs, the majority of the population still had detectable antibody levels, albeit at low levels between 1 and 10 mIU/ml, as shown in Fig. 1b.
For the anti-HBc test, which indicates past infection or ongoing infetion, no infection was detected in children as anti-HBc tested negative. Virtually all chronically infected immunocompetent patients have antiHBc. Anti-HBc started to be detected after the age of 10, with a gradual increase in prevalence. By the age of over 60, approximately half of the population had been previously infected with hepatitis B, as indicated by positive anti-HBc results, as shown in Fig. 1c. Isolated antiHBc can be found in 11 cases.
Anti-HB levels among different age groups
The antibody levels are categorized as undetectable or negative, 1–10, 10–100, 100–1000, and greater than 1000 mIU/ml. The detection rates in each age group are shown in Fig. 2. The majority of the population had relatively low antibody levels, ranging between 1 and 10 mIU/ml. The average GMT (Geometric Mean Titer) for each age group is presented in Fig. 2.
The prevalence of anti-HBs and geometric mean titers (GMTs) in the Thai population. The x-axis shows the eight age groups and the sample size in each age group. The scale on the left shows the percentage of the population with positive anti-HBs. The scale on the right shows the GMTs in each age group, with the means indicated with grey triangles. Antibody measurements were negative (white), 1–10 mIU/ml (orange), > 10–100 mIU/ml (pink), > 100–100 mIU/ml (purple), and > 1000 mIU/ml (blue navy). GMTs were calculated from anti-HBs > 1 mIU/ml.
Comparison with past studies from 2004, 2014, and the current study
The studies conducted at 10-year intervals on the prevalence of HBsAg, anti-HBs, and anti-HBc showed a clear decline over each decade following the implementation of hepatitis B vaccination in newborns, completed within the first year of life, along with other preventive measures against hepatitis B infection in Thailand. Over the past 20 years, the prevalence of HBsAg among individuals aged 21–30 has significantly declined from 6.73 to 0.30%. Detailed data are presented in Fig. 3.
Estimated comparison of hepatitis B virus markers detection in different age groups of the Thai population in 2024
Based on the study data, we projected onto the Thai population across different age groups, it was estimated that, on average, 1.68% of the population, or approximately 1.1 million people, were chronic carriers of hepatitis B nationwide. Details for each sample group are shown in Table 2.
Discussion
In 2015, the first World Hepatitis Summit in Glasgow, UK, led to the announcement of the Glasgow Declaration on Hepatitis, which set the goal to eliminate hepatitis by 2030. Subsequently, in 2016, the World Health Organization (WHO) adopted this target, establishing a policy to minimize hepatitis infections globally by 203020. The main objectives are to reduce new hepatitis infections by at least 90%, ensure that 90% of the population is screened for hepatitis B and C, and treat at least 80% of those infected. The ultimate goal is to decrease liver-related deaths caused by hepatitis by at least 65%21,23. Thailand has adopted and implemented this policy, although progress may have been delayed due to the global COVID-19 pandemic.
Efforts to reduce hepatitis B infections in Thailand began with the introduction of an effective hepatitis B vaccine, particularly aimed at preventing mother-to-child transmission by administering the first dose as early as possible (within 12 h after birth). Before the vaccine was introduced, the hepatitis B carrier rate in Thailand was high, with an endemic prevalence of around 6–8%, especially among pregnant women (6%)3. A pilot vaccination program was initiated in 1988, and by 1992, it was extended to all newborns. This led to a significant reduction in hepatitis B infections among infants. Pregnant women are now routinely screened for hepatitis B, and those who test positive receive hepatitis B immune globulin (HBIG) to enhance the efficacy of prevention. Additionally, current guidelines recommend the use of antiviral medication, such as tenofovir, during the last three months of pregnancy in carrier women who had high viral load > 200,000 IU/mL23. This approach aimed to achieve zero mother-to-child transmission of hepatitis B, in alignment with WHO’s objectives. The findings from this study showed that the prevalence of hepatitis B infection in children under the age of five was less than 0.1%, meeting WHO’s target24.
Thailand has also implemented effective blood screening procedures since 1986, 1994, and 1996 by RPHA, ELISA, and Chemiluminescent immunoassay, respectively, and since 2006, highly sensitive nucleic acid testing (NAT) has been used, resulting in a low detection rate of new hepatitis B cases among blood donors25. Furthermore, intravenous drug use in Thailand has significantly decreased, partly due to a shift from injectable drugs, which are expensive and difficult to obtain, to oral drugs like methamphetamine. Public health campaigns that followed the HIV epidemic raised awareness about the risks of sharing needles and other sharp instruments, as well as the importance of safe sex practices, leading to a significant reduction in hepatitis B transmission.
This study demonstrated a significant decline in overall hepatitis B infections, as reflected by anti-HBc markers and the reduced hepatitis B carrier rate compared to previous decades. Surveys conducted 20 and 10 years ago, as well as recent data, showed that the national hepatitis B carrier rate has decreased from 4.0, 3.48 to 1.68%18,20, representing approximately one million people, most of whom were over 30 years old and were born before the hepatitis B vaccine became part of the national immunization schedule. Similar outcomes were observed in Taiwan after introducing hepatitis B vaccination for newborns26. Since 2023, the Thai National Health Security Office (NHSO) has advised that people over 30 undergo hepatitis B and C screening at least once, with those testing positive being referred for treatment.
In this study, the level of anti-HBs antibodies among children vaccinated in their first year of life declined rapidly. By age 10, only 20% had protective antibody levels (≥ 10 mIU/ml), and by age 20, this number dropped to 10%. However, the majority of the population remained seropositive (≥ 1 mIU/ml). The number of cases with detectable HBsAg and anti-HBs was found to be 14 cases in total. Generally, individuals with anti-HBs immunity do not test positive for HBsAg. In the rare cases where both markers are detected, the level of anti-HBs is typically very low (less than 10 mIU/mL). This may be associated with the fact that infection and immune response are governed by different determinants, a phenomenon that is observed only infrequently.
Hepatitis B has a long incubation period of 3 to 6 months, so even with low antibody levels, the immune system can still mount a protective response to prevent infection. Long-term studies, spanning up to 20 years, indicated that children who initially responded to the hepatitis B vaccine, especially those born to hepatitis B carrier mothers, did not acquire new infections and turn to be carriers10. Similar results were observed in medical students with a mean age of 18, where the majority had anti-HBs levels below 10 mIU/ml, but one booster shot induced a rapid anamnestic response12. Therefore, with the complete vaccination schedule, there is no need for booster doses at the national level. For those born before the introduction of hepatitis B vaccination in the national immunization program and who tested positive for anti-HBs without detectable HBsAg or anti-HBc, this immunity is likely attributable to voluntary vaccination. The widespread use and affordability of hepatitis B vaccines in Thailand have made on voluntary vaccination basis.
In addition to reducing hepatitis B cases, these interventions have contributed to a continuous decline in liver cancer rates. In Taiwan, prior to the 1980s, the majority of infants born to HBsAg-positive mothers became chronic HBV carriers27, and the incidence of liver cancer in children was 0.92 per 100,000. However, following the introduction of the universal hepatitis B vaccination program, the rate of hepatocellular carcinoma (HCC) among vaccinated children significantly decreased to 0.23 per 100,00028. A population-based cancer registry in Taiwan also reported a 16.6% reduction in the annual incidence of liver cancer in children, while a slight increase of 1.3% was observed in adults between 2003 and 2011. Remarkably, since 2011, the incidence of primary liver cancer in children has reached zero29. With Thailand’s ongoing hepatitis B and C screening and treatment initiatives, liver cancer mortality is expected to further decline by 2030.
The study population in this research included a higher proportion of children compared to adults when compared to the general population of the country. Therefore, it was necessary to estimate the prevalence of hepatitis B surface antigen (HBsAg) carriers in each age group to calculate the average HBsAg prevalence rate for the entire population. Based on the total population of Thailand, which is 64,762,276 people, the estimated number of HBsAg carriers was 1,088,992 individuals, representing a national HBsAg carrier rate of 1.68% (Table 2).
This study represented a large-scale, ongoing investigation conducted every 10 years to provide comparative data over time. However, there were limitations, as the study only included healthy individuals and did not address high-risk populations such as prisoners, people who inject drugs (PWID), healthcare workers, or patients with liver disease, chronic illnesses, or symptomatic HIV. Consequently, the reported infection rates may be lower than the actual figures. Further research is needed in high-risk groups to identify the true burden of hepatitis B infections.
Thailand has implemented effective measures to prevent hepatitis B infections through the administration of vaccines from birth and completion of the vaccine series in the first year of life. Additionally, hepatitis B screening in pregnant women, the use of HBIG, and the current policy of antiviral treatment during the late stages of pregnancy for women with hepatitis B have further reduced transmission rates. Coupled with universal blood screening using sensitive nucleic acid testing and universal precautions similar to those used for HIV, hepatitis B infection rates in Thailand have significantly declined. This data, collected over the past 30 years, forms the basis for ongoing hepatitis prevention and control measures, helping Thailand meet WHO’s 2030 target of minimizing hepatitis infections.
Materials and methods
Institutional review board statement
This research project is a national study under the project titled “Evaluation of the impact of hepatitis B vaccine immunization program as part of EPI after 30 years of implementation and seroprevalence of hepatitis A, B, and C in Thailand.” The project aims to determine the incidence of infection and immunity against hepatitis A, B, and C viruses. It is a cross-sectional study conducted among target population groups, tracking changes every 10 years, starting from the first study in 2004, followed by 2014, and this year’s study. The study focuses on the Thai population, and it has been approved by the Institutional Review Board of the Faculty of Medicine, Chulalongkorn University Ethics Committee (IRB number 787/66). Volunteers were informed about the study’s purpose and signed an informed consent form, either by the individual or their legal representative. All procedures followed the Good Clinical Practice (GCP) guidelines and the Helsinki Declaration.
Location of the study
This study aims to represent the entire country but could not feasibly cover all 77 provinces. Therefore, provinces representative of each geographical region of Thailand were selected. Geographically, Thailand is divided into four regions. For convenience in conducting the study, Uttaradit was chosen to represent the northern region, Buriram the northeastern region, Ayutthaya the central region, and Trang the southern region. Thus, the scope is limited to these four provinces as regional representatives as illustrated in Fig. 4.
Map of Thailand indicating the sampling site in 4 provinces, Ayutthaya, Buriram, Trang, and Uttaradit. Each color represents a different region of the country (pink: northern region, brown: northeastern, blue: central region and yellow: southern region). The number of samples collected for the individual district is indicated.
Population study
The study population includes Thai individuals aged between 6 months and 80 years, residing in selected provinces and districts that represent different geographical regions of Thailand. In each province, we selected both urban and rural areas in an equal ratio. The urban area was represented by the main district (Mueang district), while rural areas were represented by two outer districts. The population was distributed equally between urban and rural areas. A total of approximately 1,500 participants from each province were recruited, totaling 6,000 people.
HBV vaccine used in the EPI
The hepatitis B vaccine used in the Expanded Program on Immunization (EPI) is a yeast recombinant DNA vaccine. Each year, the vaccine used is procured through a bidding process conducted by the Department of Disease Control or the National Health Security Office (NHSO). The dosage used is the pediatric formulation, as recommended by the vaccine manufacturers.
Specimen collection
Blood samples (3 to 7 ml clotted blood) were collected and centrifuged to separate serum within 12 h. The serum was stored at − 20 °C until laboratory testing.
Laboratory testing
Laboratory testing was conducted at the Center of Excellence in Clinical Virology, Faculty of Medicine, Chulalongkorn University. Collected samples were anonymized using sample codes. Hepatitis B markers were tested using Chemiluminescent Microparticle Immunoassay (CMIA) on an automated Architect system (Abbott, Wiesbaden, Germany) for HBsAg, anti-HBs, and anti-HBc. Positive or reactive results were interpreted using the cut-off point provided by the manufacturer. Quantitative measurement of anti-HBs was performed, with seropositive defined as anti-HBs ≥ 1 mIU/ml, and seroprotection defined as anti-HBs ≥ 10 mIU/ml.
Data and statistical analysis
Collected data is presented in graphs and tables, with seroprevalence displayed as a percentage for each age group. For anti-HBs, the results were divided into seropositive and seroprotective categories. GMT (geometric mean titer) was calculated from individuals with anti-HBs > 1 mIU/ml and presented in graphs as log10 scale. The number of hepatitis B carriers (HBsAg positive) across Thailand was estimated by multiplying the overall percentage of HBsAg-positive individuals in each age group by the current population of Thailand, obtained from official statistics registration systems, the Department of Provincial Administration, the Ministry of Interior of the Kingdom of Thailand. This study also compared the results with the findings from the National Survey on viral hepatitis in 2004 and 201418,19.
Data availability
Data is provided within the manuscript.
References
World Health Organization. Hepat. B (2024). https://www.who.int/news-room/fact-sheets/detail/hepatitis-b
Grossman, R. A. et al. An epidemiologic study of hepatitis B virus in Bangkok, Thailand. Am. J. Epidemiol. 101, 144–159 (1975).
Poovorawan, Y. et al. Protective efficacy of a recombinant DNA hepatitis B vaccine in neonates of HBe antigen-positive mothers. JAMA 261, 3278–3281 (1989).
Stevens, C. E., Beasley, R. P., Tsui, J. & Lee, W. C. Vertical transmission of hepatitis B antigen in Taiwan. N Engl. J. Med. 292, 771–774 (1975).
Beasley, R. P., Trepo, C., Stevens, C. E. & Szmuness, W. The e antigen and vertical transmission of hepatitis B surface antigen. Am. J. Epidemiol. 105, 94–98 (1977).
Beasley, R. P. & Hwang, L. Y. of referencing Epidemiology of Hepatocellular Carcinoma (ed. Vyas, G.N., Dienstag, J.L. & Hoofnagle.) 209–224Viral Hepatitis and Liver Disease, (1984).
Poovorawan, Y. et al. Comparison of a recombinant DNA hepatitis B vaccine alone or in combination with hepatitis B immune globulin for the prevention of perinatal acquisition of hepatitis B carriage. Vaccine 8, S56–59 (1990).
Poovorawan, Y. et al. Long term efficacy of hepatitis B vaccine in infants born to hepatitis B e antigen-positive mothers. Pediatr. Infect. Dis. J. 11, 816–821 (1992).
Jourdain, G. et al. Tenofovir versus placebo to prevent perinatal transmission of Hepatitis B. N Engl. J. Med. 378, 911–923 (2018).
Poovorawan, Y. et al. Evidence of protection against clinical and chronic hepatitis B infection 20 years after infant vaccination in a high endemicity region. J. Viral Hepat. 18, 369–375 (2011).
Van Damme, P. Long-term protection after Hepatitis B vaccine. J. Infect. Dis. 214, 1–3 (2016).
Posuwan, N. et al. Implementation of hepatitis B vaccine in high-risk young adults with waning immunity. PLoS One. 13, e0202637 (2018).
Chunsuttiwat, S. et al. Integration of hepatitis B vaccination into the expanded programme on immunization in Chonburi and Chiangmai provinces. Thail. Vaccine. 15, 769–774 (1997).
World Health Organization. Hepatitis B vaccination coverage. (2024). https://immunizationdata.who.int/global/wiise-detail-page/hepatitis-b-vaccination-coverage?CODE=THA&ANTIGEN=HEPB_BDALL&YEAR=
Chunsuttiwat, S., Biggs, B. A., Maynard, J. E., Thammapormpilas, P. & O-Prasertsawat, M. Comparative evaluation of a combined DTP-HB vaccine in the EPI in Chiangrai Province, Thailand. Vaccine 21, 188–193 (2002).
Tharmaphornpilas, P., Rasdjarmrearnsook, A. O., Plianpanich, S., Sa-nguanmoo, P. & Poovorawan, Y. Increased risk of developing chronic HBV infection in infants born to chronically HBV infected mothers as a result of delayed second dose of hepatitis B vaccination. Vaccine 27, 6110–6115 (2009).
Wanlapakorn, N. et al. Immunogenicity of the pentavalent DTwP-HB-Hib vaccine (Shan-5) used in the Thai expanded program on immunization compared to the hexavalent DTaP-HB-Hib-IPV and DTwP-HB-Hib (Quinvaxem) vaccines administered to infants at 2, 4, 6 months of age. Vaccine 41, 3855–3861 (2023).
Poovorawan, Y. et al. Impact of hepatitis B immunisation as part of the EPI. Vaccine 19, 943–949 (2000).
Chongsrisawat, V. et al. Hepatitis B seroprevalence in Thailand: 12 years after hepatitis B vaccine integration into the national expanded programme on immunization. Trop. Med. Int. Health. 11, 1496–1502 (2006).
Posuwan, N. et al. The success of a universal Hepatitis B immunization program as part of Thailand’s EPI after 22 years’ implementation. PLoS One. 11, e0150499 (2016).
World Health Organization. Combating hepatitis B and C to reach elimination by 2030. (2024). https://www.who.int/publications/i/item/combating-hepatitis-b-and-c-to-reach-elimination-by-2030
World Health Organization. Prevention of mother-to-child transmission of hepatitis b virus: guidelines on antiviral prophylaxis in pregnancy. (2024). https://iris.who.int/bitstream/handle/10665/333391/9789240002708-eng.pdf
Posuwan, N. et al. Towards the elimination of viral hepatitis in Thailand by the year 2030. J. Virus Erad. 6, 100003 (2020).
Jiamsiri, S. et al. Implementation of strategies to prevent mother-to-child transmission of Hepatitis B virus infection, Thailand, 2016–2017. Outbr Surv. Invest. Response J. 17, 28–35 (2024).
World Health Organization. The Triple Elimination of Mother-to-Child Transmission of HIV, Hepatitis B and Syphilis in Asia and the Pacific, 2018–2030. (2024). https://iris.who.int/bitstream/handle/10665/274111/9789290618553-eng.pdf?sequence=1
Chimparlee, N., Oota, S., Phikulsod, S., Tangkijvanich, P. & Poovorawan, Y. Hepatitis B and Hepatitis C virus in Thai blood donors. Southeast. Asian J. Trop. Med. Public. Health. 42, 609–615 (2011).
Chang, K. C. et al. Survey of Hepatitis B virus infection status after 35 years of universal vaccination implementation in Taiwan. Liver Int. 44, 2054–2062 (2024).
Hsu, H. Y., Chang, M. H., Chen, D. S., Lee, C. Y. & Sung, J. L. Baseline seroepidemiology of hepatitis B virus infection in children in Taipei, 1984: a study just before mass hepatitis B vaccination program in Taiwan. J. Med. Virol. 18, 301–307 (1986).
Chang, M. H. et al. Long-term effects of hepatitis b immunization of infants in preventing liver cancer. Gastroenterology 151, 472–480 (2016).
Hung, G. Y., Horng, J. L., Yen, H. J., Lee, C. Y. & Lin L.Y. changing incidence patterns of hepatocellular carcinoma among age groups in Taiwan. J. Hepatol. 63, 1390–1396 (2015).
Acknowledgements
The authors would like to thank the Health Systems Research Institute (HSRI) and the MK Restaurant Group, Aunt Thongkham Foundation for funding support, the Thailand Ministry of Public Health study coordinators, Uttaradit, Buriram, Ayutthaya, and Trang provincial health officers, and staff of the health promotion center for their dedication, cooperation, and support. In addition, the authors sincerely appreciate the staff of the Center of Excellence in Clinical Virology, Chulalongkorn University laboratory, and the Education and Public Welfare Foundation for their support in this study. Additionally, Pornjarim Nilyanimit received support from the Second Century Fund (C2F) fellowship at Chulalongkorn University.
Author information
Authors and Affiliations
Contributions
Y.P., W.B., P.N., N.W., P.A., and C.S. conceptualization. N.T., P.P., S.P., P.L., W.T., T.L., S.V., S.C., P.M., and C.P. samples and data collection. P.V., L.W., and S.K. methodology. N.S., and P.N. formal analysis. P.N., N.W., and Y.P. writing and editing manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Nilyanimit, P., Wanlapakorn, N., Vichaiwattana, P. et al. Significant reduction in Hepatitis B virus infections following 32 years of universal Hepatitis B vaccination as part of EPI, Thailand. Sci Rep 15, 1167 (2025). https://doi.org/10.1038/s41598-024-84854-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-84854-7