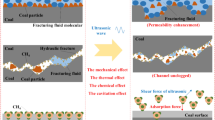
Abstract
Acid fracturing fluids can effectively improve the microporous structure of coal, thereby enhancing the permeability of coal seam and the efficiency of gas drainage. To explore the effects of acid fracturing fluids on the pore structure modification of coal samples from different coal ranks, hydrochloric acid-based acid fracturing fluids were prepared and used to soak four types of medium to high-rank coal in an experiment. High-pressure mercury intrusion and liquid nitrogen adsorption techniques results demonstrated that the acid fracturing fluid can effectively alter the pore structure of coal. However, the modification effect does not exhibit a linear relationship with coal rank. The porosity of fat coal and coking coal increased by approximately 30%, while the surface area of gas coal and fat coal increased by about 20%. The new micropores produced by the acid fracturing fluid will increase the roughness of the fracture surface, but the widening of the original fracture will reduce the tortuosity of the fracture. Only the fractal dimension of lean coal has a significant change, about 6%. Overall, acid fracturing fluid has the best effect on gas coal and coking coal. The research results provide a reference for the selection and application of acid fracturing fluid in coal seam hydraulic fracturing.
Similar content being viewed by others
Introduction
According to data from the International Energy Agency (IEA), coal is expected to remain a primary energy source for several countries in the future, especially for major energy consumers such as China and Indonesia1,2. However, a considerable portion of China’s coal seams contain substantial amounts of gas (coalbed methane), which can lead to safety incidents, such as coal and gas outbursts, rendering the safe mining of coal challenging3,4. Moreover, the primary component of coalbed methane is CH4, which is also a focus of new energy research5,6. Additionally, the greenhouse effect of coalbed methane is approximately 25 times that of carbon dioxide, making it one of the significant contributors to greenhouse effects7,8. Consequently, the extraction of coalbed methane offers advantages such as facilitating coal mining, producing new energy sources, and reducing carbon emissions.
Coal seams generally have low permeability, leading to poor gas extraction results, which necessitates artificial construction measures to enhance the permeability. Currently, hydraulic slotting and hydraulic fracturing technologies are commonly used, with hydraulic fracturing demonstrating particularly notable effectiveness9. Hydraulic fracturing can create extensive fractures within the coal seam, providing pathways for gas extraction. Fracturing fluids can modify the microstructure of the coal, increase its permeability, and promote the desorption and flow of gas, thereby enabling efficient extraction of coalbed methane10. The fracturing fluids used today include clean fracturing fluid, acidic fracturing fluid, and foam fracturing fluid, among which acidic fracturing fluid can react intensely with coal, effectively modification the micro-porous fractures in the coal.
Ni et al.11 investigated the impact of acidic fracturing fluid containing SDS, hydrochloric acid, and hydrofluoric acid on the physical structure of coal. They discovered that SDS effectively enhances the modification effect of the acidic fracturing fluid on coal, reducing the blockage of pores by the fluid. Zhang et al.12 noted that acidic fracturing fluid composed of HCl and CTAB could modify the functional groups of coal, escalating its pore connectivity and average pore diameter, subsequently affecting the coal’s wettability, with the ultimate effectiveness influenced by the coal rank and mineral content. Through immersion experiments, Li et al.13 compared the influence of clear water, clean fracturing fluid, and acidic fracturing fluid on coal’s physical pore structure, ascertaining that the acidic fracturing fluid exhibited the most significant improvement in micro-pore structure. Wang et al.14 developed an acidic clean fracturing fluid using erucic acid, finding this fluid to possess favorable viscosity and filtration properties while altering the functional groups and microstructure of the coal. Balucan et al.15 researched the impact of hydrochloric and hydrofluoric acids on coal permeability and fracture compressibility, they found that permeability could be enhanced by up to 4.5 times, and fracture compressibility reduced to one-third, attributing these outcomes to large crystalline minerals formed by the acid interacting with the minerals providing structural support to the fractures. He et al.16 investigated the effects of various types and concentrations of strong acidic fracturing fluids on coal pore structure, revealing a more significant impact on bituminous coal compared to anthracite, with erosion rates and pore structure changes correlating positively with the coal’s metamorphic degree. Wang et al.10 analyzed the impact of hydrochloric acid-based acidic fracturing fluid on coal’s pore and functional group structures, noting that higher concentrations of hydrochloric acid enhanced the coal’s gas adsorption capacity, and positing that a concentration range of 3–5% yielded the best overall transformative results on coal. Miao et al.17 employed nitric acid and ionic liquid to formulate a transformative fluid to bolster the permeability of coal seams. They discovered that this fluid could augment the coal’s pore structure; however, the ionic liquid filling smaller pore fractures could lead to reduced average permeability speeds. Furthermore, several scholars have researched the effects of acidic fracturing fluids on carbonate rocks. Gou et al.18 utilizing CT scanning and three-dimensional reconstruction techniques, compared the fracture morphologies after fracturing with conventional and carbonate-reactive acidic fracturing fluids, uncovering that the hydraulic fractures induced by acidic fluids could extend along natural fractures, forming more complex fracture networks without being constrained by stress. Kao et al.19 discovered through acidic fracturing fluid soaking experiments that the fluid’s etching effect on fracture surfaces facilitated the connection of adjacent microfractures and natural pores, leading to fluid loss and consequently diminishing the expansion length of the fractures. Liu et al.20 studied the combined effect of acidic fracturing fluid and pressure on the pore structure and fractures of shale, discerning that pressure could enhance the modification effects of acidic fracturing fluid on shale, with the joint action altering the extent of pore variations. Liu et al.21 also enhanced the recovery efficiency of coal by fracturing the coal layer’s gangue with acidic fracturing fluid, finding post-fracture the acidic solution could dissolve calcite and dolomite in the gangue, thereby increasing the number and complexity of the hydraulic fracture networks formed. Moreover, Che et al.22 observed that acidic mine water during its infiltration could increase the number of rock fracture pores, subsequently elevating the rock’s damage indicators and surrounding rock stability.
It can be discerned that acidic fracturing fluids possess substantial advantages in modifying the pore structure and permeability of coal seams. However, current research primarily focuses on the effects of various acidic fracturing fluids on coal pore structure, while their modification impacts on different coal ranks remain ambiguous. Because of the different mineral composition and pore structure of coal samples of different coal ranks, the modification effect of fracturing fluid on coal samples of different coal ranks will also be different. This lack of clarity hinders the development of hydraulic fracturing technology. Therefore, this article compares the effects of acidic fracturing fluids on the pore structures of middle to high-rank coals. The findings provide a reference for selecting appropriate acidic fracturing fluids, thereby facilitating the advancement of coalbed methane extraction technologies.
Experimental design
Samples preparation
Four coal samples of different metamorphic grades were collected from four different coal mines. The metamorphic grades and basic parameters of the four coal samples were measured, with the maximum reflectance of vitrinite (Ro,max) being 0.72, 1.15, 1.53, and 2.13, respectively. According to ASTM standards, S1, S2, and S3 are classified as medium-volatile bituminous coal, while S4 is classified as high-volatile bituminous coal. Additionally, an industrial analysis was conducted on the four coal samples, and the hardness coefficient and porosity were determined, as detailed in Table 1.
Experimental process
First, crush the coal samples and sieve out particles of about 3 mm. Then prepare an acidic fracturing fluid in the ratio of 0.5 wt% HCl + 0.5 wt% CTAB13,23. According to Yang’s research, this acidic fracturing fluid can chemically react with coal to increase the porosity of the coal structure1,24. Next, place 50 g of coal sample and 500 mL of fracturing fluid in a beaker, and then put the beaker in a constant temperature water bath. Set the temperature of the water bath to 40 °C. After soaking for 24 h, rinse the coal sample with clean water and dry it. Finally, analyze the changes in the coal sample’s pore structure using high-pressure mercury intrusion and electron microscopy scanning, as shown specifically in Fig. 1.
Experimental results
MIP analysis
The coal samples were first subjected to mercury intrusion porosimetry to assess changes in the pore structure of the coal samples before and after the action of fracturing fluid. The mercury intrusion and extrusion curves for different coal samples are shown in Fig. 2. It can be found that there are big differences in intrusion and extrusion curves of coal samples of different coal rank, which is mainly reflected in the low-pressure stage (< 10 psi) of the mercury inlet curve, that is, the curve has different change trends at this stage. S2 rises slowly in the low-pressure stage, indicating that there are fewer large size pores in S2 coal sample. S1, S3 and S4 grow faster in the low-pressure stage, indicating that there are more large size pores. Between 10 and 4000 psi, the growth trend of the curve is relatively slow, indicating that there are fewer pores at this stage. When it is greater than 4000 psi, the curves of the four types of coal samples all rise rapidly, indicating that there are more pores at this stage. In addition, the mercury curve in advance and retreat of coal samples changed after the action of fracturing fluid, showing an overall upward trend, indicating that the pores in coal samples increased.
Intrusion volume
In order to analyze the alterations in coal sample porosity structures more effectively, the pores are categorized based on their sizes following Hudot’s pore size classification standards. This classification categorizes the pores into micropores (< 10 nm), transition pores (10—100 nm), mesopores (100–1000 nm), and macropores (> 1000 nm)25.
Figure 3 illustrates the changes in pore volume before and after the treatment of fracturing fluid. It is observed that the pore volume distribution varies across different coal ranks and pore sizes, but, on the whole, the transition pores and macropores predominate in terms of proportion. Following the treatment of fracturing fluid, there is a notable increase in the pore volume across various coal ranks; fat coal and coking coal exhibit a significant increase, approximately 30%; whereas gas coal and lean coal display a smaller increase, around 15%. The primary reason is the physicochemical reactions between the fracturing fluid and minerals in the coal, such as clay minerals and kaolinite, which result in an enlargement of pore volumes. For gas coal, the increase in the volume of transition pores and macropores post-treatment is evident, with macropores showing marked enlargement. Fat coal, which initially has the smallest pore volume, experiences an increase across all pore sizes after treatment, with the total pore volume increasing by 29%. Transition pores account for the largest proportion and the most significant increase, primarily because the abundant mineral impurities in fat coal react thoroughly with the fracturing fluid, enlarging existing pores and generating many new ones.
Coking coal has the largest pore volume, as well as the greatest change in pore volume, indicating an optimal physicochemical interaction with the fracturing fluid. Here, micropores, transition pores, and macropores are the predominant voids, with the total porosity increasing by 27.7% after treatment. The chemical reactions between the fracturing fluid and surrounding minerals enlarge pores of all sizes, resulting in a decrease in micropores, but a significant increase in transition pores, mesopores, and macropores. This is likely because the coking coal samples, having a large micro-pore volume and surface area, facilitate extensive contact with the fracturing fluid and thereby promoting chemical reactions that substantially enhance the total pore volume. For lean coal, macropores are the dominant pore type, constituting about 50% of the total pore volume. Post-fracturing fluid treatment, the overall increase in pore volume in lean coal is the least (almost 12.5%), with transition pores and macropores showing a notable increase, suggesting a less effective interaction between the fracturing fluid and the coal, mainly expanding existing voids rather than creating new micropores. The minimal impact on lean coal’s porosity arises from its lower mineral content, which limits the intensity of chemical reactions with the fracturing fluid, making it difficult to generate additional new pores.
Pore area
Figure 4 illustrates the changes in pore volume before and after the treatment of fracturing fluid. The surface area of the pores in coal samples of different ranks varies, and the alterations resulting from the fracturing fluid are also distinct. It is observable that the specific surface area of coal samples across various coal ranks initially increases and then decreases. Among these, gas coal has the smallest pore surface area, while coking coal has the largest. The surface area of the pores in different coal ranks is predominantly composed of micropores and transition pores, a characteristic informed by the nature of these specific surface areas. Additionally, it is noted that the pore surface area in both gas coal and fat coal increases by approximately 20% after treatment with fracturing fluid, whereas the surface area in coking coal and lean coal decrease by 6% and 7%, respectively. The reasons for these changes in total surface area are attributed to variations in the surface area of micropores. An increase in the number of new micropores leads to an enlargement of the micropore surface area, thereby increasing the overall surface area. If fewer new micropores are generated, and the existing micropores expand or connect to become transition pores and mesopores, this results in a reduction of the total surface area. The content of mineral impurities within the coal samples plays a decisive role in the effectiveness of the interaction between the fracturing fluid and the coal, with a higher content of impurities facilitating physicochemical reactions with the fracturing fluid. Post-treatment, micropores and transition pores in both gas coal and fat coal exhibit an increase in surface area, primarily because the treatment with fracturing fluid expands existing micropores into transition pores and generates new micropores within the coal. In fat coal, a substantial modification of micropores into transition pores contributes to a significant increase. Conversely, in coking coal and lean coal, the surface area of micropores decreases post-treatment, while the area of transition pores increases. This effect primarily results from the fracturing fluid expanding a vast number of micropores into transition pores, without generating a substantial number of new micropores in the coal.
Fractal dimension
Calculating the distribution of pore sizes can yield the fractal dimension, which in turn allows the assessment of the homogeneity of the pore structure in coal samples. For coal and rock, a higher fractal dimension indicates poorer homogeneity of the pores. Here, the Washburn equation is used to establish a double logarithmic regression equation between pressure and mercury intrusion, which is used to calculate the fractal dimension26, as shown in Fig. 5. The relationship of \(dV_{P}\) and \(dP\) is described by Eq. (1), the fractal dimension can be obtained by piecewise fitting the data.
where VP is the cumulative mercury intake when the pressure is P, mL/g; D = A + 4, where A is the slope of Eq. (1); D is the fractal dimension, and the smaller D is, the greater the homogeneity.
It has been observed that the fractal dimensions of coal samples across different coal ranks exhibit minimal variation. Among them, fat coal possesses the highest fractal dimension at 2.269, while gas coal, coking coal, and lean coal have similar fractal dimensions, approximately 2.0. Post-fracturing fluid treatment, the fractal dimensions of the coal pores remain largely unchanged, with a slight increase in gas coal and minor reductions in fat coal, coking coal, and lean coal. This suggests that the interaction of fracturing fluid with coal primarily expands existing pore sizes or generates new pores without significantly affecting the overall distribution and connectivity of pores of varying sizes.
LT-N2A
Pore structure
Comparative analyses of nitrogen adsorption data from different grades of coal reveal significant disparities. These variations primarily arise during the coal formation process, wherein the porous structure, size, and distribution of the pores evolve with coal rank. Generally, the higher the coal rank, the fewer the pores observed: a trend that is consistent across the four coal types studied in the experiment. Furthermore, the nitrogen adsorption/desorption isotherms exhibit notable differences among different ranks, influenced significantly by the form of the pores. According to standards set by the IUPAC16, adsorption isotherms and hysteresis loops can be classified into six types each, as demonstrated in Fig. 6, with isotherms ranging from Type I to VI, and hysteresis loops from H1 to H5. It is observable that the isotherms for different grades of coal before and after modification fall between Types III and IV, predominantly characterized by adsorption hysteresis loops in the middle range, although the rise in the low-pressure phase of the isotherm exhibits a slight protuberance that is not markedly prominent. The emergence of these hysteresis loops is primarily due to capillary condensation within diverse pore forms during the multilayer adsorption process. These loops definitively belong to Type H3, indicating the presence of pore types such as flat slit structures, cracks, and wedge-shaped configurations, which are not completely filled and do not exhibit adsorption saturation even in higher relative pressure zones.
As illustrated in Fig. 7, a comparison reveals that the adsorption isotherms of different grades of coal, before and after modification, are rather similar except that the N2 adsorption volume slightly increases, accompanied by more pronounced hysteresis loops. This change is mainly due to the chemical reactions between the acidic fracturing fluid and the mineral impurities within the coal, leading to the dissolution of minerals originally embedded within the coal structure. These reactions result in the formation of new pores and the enlargement of existing ones. Regardless of the scenario, these changes result in an increased presence of open pores within the coal, including a significant proportion of slit or parallel plate pores, ultimately altering the adsorption isotherms pre and post- treatment. For fat coal and coking coal, the smaller hysteresis loops indicate the presence of only a few slit or plate pores alongside numerous poorly interconnected pores, such as wedge-shaped pores. Particularly for coking coal, the modification induced by acidic fracturing fluid is the most pronounced: initially subtle hysteresis loops become significantly more evident post-treatment, suggesting the most effective chemical modification achieved by the fracturing fluid. Additionally, the variations in the hysteresis loops, indicated by differing inflection points near relative pressure 0.5, also imply varying quantities of ink-bottle shaped pores within the coal samples. This type of pore displays slow desorption at higher pressures, and rapid release of condensed nitrogen inside the pore when the relative pressure decreases to below 0.5, which is a contributing factor to the inflection in the desorption curve. Overall, post-fracturing fluid treatment results in minimal changes in lean coal, the largest adsorption capacity change in fat coal, and the most significant alteration in pore structural type in coking coal.
Fractal dimension
In order to quantitatively analyze the pore structure of coal, we calculated the fractal dimension of coal pores based on the Frenkel-Halsey-Hill (FHH) model27. Equation (2) is the FHH model. Moreover, according to the nitrogen adsorption–desorption curve previously mentioned, it can be seen that there are a significant number of ink bottle-shaped pores in the coal, which causes a sudden change in the desorption curve at a certain pressure point. To calculate the fractal dimensions reasonably, we will divide the fractal dimensions into two intervals based on pressure size: the results for relative pressures greater than 0.5 are marked as fractal dimension D1, and the results for relative pressures less than 0.5 are marked as fractal dimension D2. Since the pore diameter corresponding to a relative pressure of 0.5 is about 4.5 nm, the fractal dimension for pores ranging from 4.5 to 100 nm is D1, while that for pores ranging from 2.0 to 4.5 nm is D2.
where V is the nitrogen adsorption amount, mL/g; V0 is the monolayer adsorption amount of nitrogen at standard temperature and pressure (ml/g); P0 is the vapor pressure of nitrogen, MPa; P is the pressure, MPa; C is the constant and A is the slope of the fitted line. The fractal dimension is A + 3. The fractal dimension is generally between 2 and 3, and the closer to 2, the smoother the pores.
From the data-fitted structures yielding fractal dimensions D1 and D2, it is evident that for a given coal sample, D1 is noticeably greater than D2, a pattern consistent across all samples (Fig. 8). As fractal dimensions typically range between 2 and 3, with D1 values commonly around 2.5, this dimension serves to assess the roughness of the pore surfaces and the complexity of the pore structures. However, more than half of the D2 values fall below 2, thereby rendering it unsuitable for quantitative assessments of porosity. Furthermore, the R2 values for D1 are predominantly above 0.9, indicating a high level of reliability in these measurements, hence only D1 is analyzed here.
Post-treatment with acidic fracturing fluid, there is a slight increase in D1 values across all coal samples. This implies that the acidic fracturing fluid enhances the roughness of the pore surfaces and the complexity of the pore structures, particularly in the larger pores. We theorize that the mineral particles embedded within the coal contribute to its dense appearance and low porosity. The chemical reactions between the acidic fracturing fluid and these mineral particles expose the originally filled pores, primarily driving the increased complexity in the pore structures of the coal samples.
SEM
To observe the pore structure of coal samples of different coal ranks more intuitively after treatment, scanning electron microscopy (SEM) was performed on coal samples before and after treatment. The specific results are shown in Fig. 9. The SEM images represent only a very small area of the coal sample, which might lead to some discrepancies with the results obtained by other testing methods. However, these images do provide a certain degree of insight into the experimental results. It can be observed that there are significant differences in the surface and pore structures of the raw coal samples from different coal ranks. These differences are mainly due to the varying degrees of coalification during the coal formation process. For the raw coal samples, the S1 sample, which is of a lower coal rank, contains more mineral impurities and has a more complex surface structure compared to the other three samples, which have relatively smoother surfaces. However, the S4 sample exhibits noticeable layering on its surface, indicating the presence of partial crystallization and a higher degree of coalification. After the action of the fracturing fluid, the overall pore structures of the coal samples show varying degrees of change, with an increase in both the number and size of the pores. Before treatment, the coal sample surfaces had a certain amount of mineral particle attachments and fewer pores. After treatment with acidic fracturing fluid, the number of attached particles decreased significantly, and the number of surface pores increased noticeably. Regarding the specific effects of the fracturing fluid, the pore structure of the coking coal showed relatively larger changes, while the lean coal exhibited smaller changes. The coking coal and fat coal showed a considerable number of newly formed pores on their surfaces, whereas fewer new pores were observed in the gas coal and lean coal, which is somewhat consistent with the results of the MIP experiment.
Discussion
Changes of pore structure of coal samples
During the coalification process, influenced by temperature and stress, the internal and molecular structures of coal undergo modification resulting in changes in porosity. In the experiments conducted on four types of coal samples, all demonstrated an increase in pore volume post-fracturing fluid treatment, with coking coal (Rmax = 1.5) exhibiting the largest pore volume. Due to alterations in pore sizes during coal formation and influenced by the laws of specific surface area and pore volume, coking coal (Rmax = 1.5) also presented the largest pore surface area. After treatment with fracturing fluid, total pore surface area increased in gas coal and fat coal, while it decreased in coking coal and lean coal. This is largely attributed to changes in the dimensions of the coal’s pores post-fracturing fluid treatment, particularly noticeable in coking coal and lean coal where the average pore size significantly increased, thus reducing overall pore surface area despite an increase in pore volume. Additionally, the average pore size in coal tends to decrease and then increase as the rank progresses.
The permeability in mercury injection data is calculated according to pore distribution, capillary force, pore throat, etc. There is a certain error with the real value, but it can also indicate the change of coal sample permeability to a certain extent. As depicted in Fig. 10, post-fracturing fluid treatment, permeability in all coal samples increased to varying degrees, gas coal and fat coal experienced smaller increments (about 50%), whereas coking coal saw a threefold increase. As Rmax increases, the tortuosity and fractal dimensions of both unaltered and modified coal samples exhibited an initial increase followed by a decrease. Tortuosity represents the ease of passage through channels, indicating the resistance within these pathways. Coking coal showed the highest initial tortuosity, which decreased after fracturing fluid treatment, with the largest reduction observed in coking coal, and the highest post-treatment tortuosity noted in fat coal, likely due to changes in coal pore sizes. In terms of fractal dimensions, which describe the complexity of the pore structures, there was minimal change before and after treatment, with only fat coal showing an increase, while the others decreased. Among these, fat coal consistently had the highest fractal dimension. Based on MIP data, it can be found that coking coal (Rmax = 1.5) has the greatest impact on permeability and total pore volume, that is, this coal rank is more suitable for acid fracturing fluid modification.
Moreover, an analysis of the coal pore structure was conducted based on nitrogen adsorption data, as illustrated in Fig. 11. With increasing coal rank, certain similarities in trends were observed, albeit with notable differences, primarily due to disparities between high-pressure mercury intrusion and liquid nitrogen adsorption methodologies. The former measures pores by quantifying the mercury injected into coal pores from larger to smaller sizes, covering a range from 5 to 340,000 nm. Conversely, the liquid nitrogen adsorption technique calculates porosity based on the amount of nitrogen adsorbed, focusing first on smaller pores, with a measured range of 2–500 nm. Specifically, post-acidic fracturing fluid treatment, nitrogen adsorption in coal samples increased, with gas coal and fat coal showing more significant rises of 40% and 56%, respectively, attributed to higher mineral content in lower-rank coals. Regarding average pore size, samples displayed a decrease followed by an increase pre and post-treatment, with coking coal consistently showing the lowest values. Gas coal and fat coal exhibited a decreasing trend in average pore size, whereas lean coal showed an increase (about 14%), influenced by mineral content dictating new pore formation and thereby affecting average pore sizes. As coal rank increased, fractal dimension D1 also showed a trend of initial increase followed by decrease, with the peak at coking coal. This likely correlates with pore volume and average pore size; coking coal had a larger pore volume and smaller average pore size, indicating a greater number of very small pores, likely contributing to a more complex pore surface roughness. After acidic fracturing fluid treatment, all coal samples saw increases in fractal dimension D1, with the most substantial increase in lean coal, about 6%.
It can be found that there are some differences in the test results of LT-N2A and MIP of coal samples of different coal ranks, mainly due to the different pore sizes tested by LT-N2A and MIP. LT-N2A can more accurately describe the pores with pore size of 2–50 nm, and MIP can more accurately describe the pores with pore size of 5 nm to1000 µm. Combined with the test results of LT-N2A and MIP, it can be found that no matter what kind of coal rank, fracturing fluid can increase the pore volume of the pore. When combining LT-N2A and MIP data, the modification effects of acidic fracturing fluid were most effective in gas coal and coking coal.
The reason of pore change
Coal is composed of a mixture of elements including carbon, hydrogen, oxygen, nitrogen, and sulfur. Throughout geological movements and the coal formation process, minerals such as Calcite (CaCO3), Kaolinite (AlSi4O10(OH)8), and Dolomite (CaMg(CO3)2) have formed within coal and are prevalent throughout coal seams28. Numerous studies have shown that the mineral composition and proportions present in different coal ranks vary significantly29,30. Specifically, each coal rank exhibits unique characteristics in terms of the occurrence, abundance, and origin of its mineral matter, which is influenced to some extent by the rank of the coal itself. Mineral matter in coal comprises both crystalline minerals and non-mineral inorganic elements, reflecting a complex interplay of geological processes. And these minerals can form through a variety of detrital, biogenic, or authigenic processes. Additionally, the interaction between these minerals and hydraulic fracturing fluids with differing degrees of ease. This variability affects the overall effects that fracturing fluids have on coals of different ranks. According to basic chemical principles, the hydrogen ions present in acidic fracturing fluids engage in complex chemical reactions with minerals such as Calcite and Dolomite in coal, thereby dissolving these minerals, as described in Eqs. (3)–(6). The organic matter within coal, however, is composed of complex high-molecular-weight organic compounds that have a higher activation barrier, making them more resistant to reactions with hydrogen ions. Moreover, variations in mineral composition, porosity, and the complexity of pore structures among different ranks of coal contribute to differing impacts from the interactions with fracturing fluids.
Conclusion
Acidic fracturing fluids are capable of modifying the microporous structures in coal of different ranks, though the effectiveness of these modifications varies. Analysis via high-pressure mercury intrusion and nitrogen adsorption methods reveals the following results:
(1) Post-treatment with acidic fracturing fluids, the pore volume within coal samples of varying ranks increases, by up to 30%, though variations according to pore size distribution are observed. Overall, transition pores and macropores, which form a significant proportion and are evidently affected the most by the acidic fracturing fluids.
(2) The surface area of coal samples across different ranks is predominantly composed of micropores and transition pores. The acidic fracturing fluids facilitate the formation of new micropores, enhancing the surface area of gas coal and fat coal by approximately 20%, while reducing the surface area of coking coal and lean coal by about 5% and 7%, respectively.
(3) The new micropores created in the coal by acidic fracturing fluids increase the roughness of the fracture faces; however, the widening of original fractures reduces the tortuosity of these channels. Notably, lean coal exhibits the greatest increase in fractal dimensions for about 6%, with other ranks of coal showing minimal change.
(4) Acidic fracturing fluids alter both the size and shape of coal’s pore structures, although the modification effects do not display a linear relationship with coal rank. Overall, acidic fracturing fluids yield the most favorable outcomes in modifying gas coal and coking coal.
Data availability
The datasets used and analysed during the current study available from the corresponding author on reasonable request.
References
Gong, S. H. et al. Rheology of high-temperature resistant fracturing fluids formed by VES, acids and oxidants for enhancing CO2 injection in deep coal seams. J. Mol. Liquids 396, 1. https://doi.org/10.1016/j.molliq.2024.123986 (2024).
Wen, Z. J., Jing, S. L., Meng, F. B. & Jiang, Y. J. Control technology for floor heave of Jurassic soft rock in the Erdos Basin of China: A case study. J. Cent. South Univ. 29(12), 4051–4065. https://doi.org/10.1007/s11771-022-5173-8 (2022).
Ye, M. L. et al. Study on crack propagation mechanism of coal under the different orientation between prefabricated slots and true triaxial stress. Energy 298, 1. https://doi.org/10.1016/j.energy.2024.130988 (2024).
Zuo, S. J., Peng, S. Q., Zhou, D. P., Wang, C. W. & Zhang, L. An analytical model of the initiation pressure for multilayer tree-type hydraulic fracturing in gas-bearing coal seams. Geomech. Geophys. Geo-Energy Geo-Resour. 8(6), 1. https://doi.org/10.1007/s40948-022-00509-9 (2022).
Yang, M. M., Zhang, J. Y., Zhang, L. & Gong, S. H. Physicochemical Effect on Coal Pores of Hydrocarbon Chain Length and Mixing of Viscoelastic Surfactants in Clean Fracturing Fluids. Acs Omega 9(17), 19418–19427. https://doi.org/10.1021/acsomega.4c00692 (2024).
Zhu, X. Y. et al. Fracture damage characteristics of hard roof with different bedding angles induced by modified soundless cracking agents. Eng. Fract. Mech. 289, 1. https://doi.org/10.1016/j.engfracmech.2023.109387 (2023).
Zhou, Z. et al. Mechanical properties and failure characteristics of supercritical carbon dioxide soak in water-bearing coal rocks. Energy 286, 1. https://doi.org/10.1016/j.energy.2023.129599 (2024).
Zuo, S. J. et al. The effect of temperature and ultrasonic power on the microstructure evolution of coal modified by clean fracturing fluid: An experimental study. Energy 1, 306. https://doi.org/10.1016/j.energy.2024.132436 (2024).
Zuo, S. J., Zhang, L. & Deng, K. Experimental study on gas adsorption and drainage of gas-bearing coal subjected to tree-type hydraulic fracturing. Energy Rep. 8, 649–660. https://doi.org/10.1016/j.egyr.2021.12.003 (2022).
Wang, Z. P. et al. Effects of acid-based fracturing fluids with variable hydrochloric acid contents on the microstructure of bituminous coal: An experimental study. Energy 244, 1. https://doi.org/10.1016/j.energy.2021.122621 (2022).
Ni, G. H. et al. The effect of anionic surfactant (SDS) on pore-fracture evolution of acidified coal and its significance for coalbed methane extraction. Adv. Powder Technol. 30(5), 940–951. https://doi.org/10.1016/j.apt.2019.02.008 (2019).
Zhang, Y. et al. Experimental study on the influence of acid fracturing fluid on coal wettability. Fuel 343, 1. https://doi.org/10.1016/j.fuel.2023.127965 (2023).
Li, L. W., Sun, H. T., Wu, W. B., Zuo, S. J. & Sun, P. Evolution characteristics of the microstructure with different fracturing fluids: An experimental study of Guizhou bituminous coal. Acs Omega 8(32), 29803–29811. https://doi.org/10.1021/acsomega.3c04351 (2023).
Wang, C. M., Zhou, G., Jiang, W. J., Niu, C. X. & Xue, Y. F. Preparation and performance analysis of bisamido-based cationic surfactant fracturing fluid for coal seam water injection. J. Mol. Liquids 332, 1. https://doi.org/10.1016/j.molliq.2021.115806 (2021).
Balucan, R. D., Turner, L. G. & Steel, K. M. Acid-induced mineral alteration and its influence on the permeability and compressibility of coal. J. Nat. Gas Sci. Eng. 33, 973–987. https://doi.org/10.1016/j.jngse.2016.04.023 (2016).
He, J. W. et al. Experimental study on erosion mechanism and pore structure evolution of bituminous and anthracite coal under matrix acidification and its significance to coalbed methane recovery. Energy 283, 1. https://doi.org/10.1016/j.energy.2023.128485 (2023).
Miao, Y. A. et al. Numerical investigation of methane seepage behaviour in coal with lattice Boltzmann approach: The synergistic effects of oxidizing acid and ionic liquid. Fuel 340, 1. https://doi.org/10.1016/j.fuel.2023.127538 (2023).
Gou, B. et al. Effect of different types of stimulation fluids on fracture propagation behavior in naturally fractured carbonate rock through CT scan. J. Pet. Sci. Eng. 201, 1. https://doi.org/10.1016/j.petrol.2021.108529 (2021).
Kao, J. W., Jin, Y., Zhang, K. P. & Ren, P. J. Experimental investigation on the characteristics of acid-etched fractures in acid fracturing by an improved true tri-axial equipment. J. Pet. Sci. Eng. 184, 1. https://doi.org/10.1016/j.petrol.2019.106471 (2020).
Liu, K. Y. et al. Experimental study on the influence of acid-pressure compound effect on multi-scale pore evolution of oil shale. Arab. J. Sci. Eng. 47(6), 7419–7432. https://doi.org/10.1007/s13369-022-06726-4 (2022).
Liu, H., Deng, G. Z. & Zheng, R. Experimental study on fracture mechanism in acid fracturing of siltstone gangue layer in fully mechanized mining face. Fresen. Environ. Bull. 30(2), 1265–1276 (2021).
Che, Y. X. et al. Damage behaviour of sandstone induced by combination of dry-wet cycles and acidic environment. Environ. Earth Sci. 82(1), 1. https://doi.org/10.1007/s12665-022-10693-2 (2023).
Bratcher, J. C., Kaszuba, J. P., Herz-Thyhsen, R. J. & Dewey, J. C. Ionic strength and pH effects on water-rock interaction in an unconventional siliceous reservoir: On the use of formation water in hydraulic fracturing. Energy Fuels 35(22), 18414–18429. https://doi.org/10.1021/acs.energyfuels.1c02322 (2021).
Yang, H., Yu, Y., Cheng, W., Rui, J. & Xu, Q. Influence of acetic acid dissolution time on evolution of coal phase and surface morphology. Fuel 286, 119464. https://doi.org/10.1016/j.fuel.2020.119464 (2021).
Gan, R. et al. Effects of different concentrations of weak acid fracturing fluid on the microstructure of coal. Nat. Resour. Res. https://doi.org/10.1007/s11053-024-10380-y (2024).
Wang, F., Cheng, Y. P., Lu, S. Q., Jin, K. & Zhao, W. Influence of coalification on the pore characteristics of middle high rank coal. Energy Fuels 28(9), 5729–5736. https://doi.org/10.1021/ef5014055 (2014).
Jing, Z. H. et al. A preliminary study of oxidant stimulation for enhancing coal seam permeability: Effects of sodium hypochlorite oxidation on subbituminous and bituminous Australian coals. Int. J. Coal Geol. 200, 36–44. https://doi.org/10.1016/j.coal.2018.10.006 (2018).
Li, H. et al. A review of laboratory study on enhancing coal seam permeability via chemical stimulation. Fuel 330, 1. https://doi.org/10.1016/j.fuel.2022.125561 (2022).
Finkelman, R. B., Dai, S. & French, D. The importance of minerals in coal as the hosts of chemical elements: A review. Int. J. Coal Geol. 212, 103251. https://doi.org/10.1016/j.coal.2019.103251 (2019).
Vassilev, S. V., Kitano, K. & Vassileva, C. G. Some relationships between coal rank and chemical and mineral composition. Fuel. 75(13), 1537–1542. https://doi.org/10.1016/0016-2361(96)00116-0 (1996).
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (No. 52304129), Guizhou Provincial Science and Technology Projects (ZK[2023] general 070), Natural Science Talents Fund Project of Guizhou University (Grant No. 2021064).
Author information
Authors and Affiliations
Contributions
Zhao Kang: Writing-Original draft preparation, Conceptualization, Methodology. Li Liangwei: Writing-review and editing, Resources, Visualization, Supervision. Li Kun: Data curation, Software, Visualization, Investigation. Zuo Shaojie: Writing-review and editing, Resources, Methodology. Jiang Zhizhong: Supervision, Investigation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical statement
This article does not deal with human and animal experimentation.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kang, Z., Liangwei, L., Kun, L. et al. Study on the effect of acid fracturing fluid on pore structure of middle to high rank coal. Sci Rep 15, 2097 (2025). https://doi.org/10.1038/s41598-024-85007-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-85007-6