Abstract
Papillary thyroid cancer (PTC) is the most common type of thyroid cancer, accounting for approximately 80% of cases. Considering the lack of available blood diagnostic tests for detecting thyroid cancer, as well as the issue of overdiagnosis and overtreatment, our study aimed to investigate whether the concentrations of 48 selected proteins, including chemokines, interleukins, cytokines, and growth factors, could serve as indicators to distinguish patients with PTC from individuals with nodular goiter. The study group included 32 PTC patients and 26 nodular goiter patients as a comparative group. Serum protein concentrations were evaluated in venous blood collected in tubes without anticoagulant, using the multiplexed immunoassay method. The concentrations of basic FGF (bFGF), IL-9, IL-18, TNF-α, and TNF-β were significantly higher (p < 0.05) in patients with PTC compared to those with nodular goiter. The highest area under the ROC curve (AUC), equal to 0.720 ± 0.066, along with a diagnostic specificity of 92% and a positive predictive value of 89% for differentiating PTC patients from nodular goiter patients, was found for TNF-β. The combined evaluation of TNF-β and bFGF concentrations proved most useful in differentiating papillary thyroid cancer from benign nodular goiter, revealing a diagnostic specificity of 96%. Further studies should be conducted with an appropriate sample size and across other histopathological types of thyroid cancer to confirm that the combined evaluation of bFGF and TNF-β concentrations is useful in differentiating PTC patients from individuals with benign nodular goiter.
Similar content being viewed by others
Introduction
Currently, the incidence of thyroid cancer is increasing. However, only 1 in 10 nodular goiters will be diagnosed as thyroid cancer through histopathological examination1. The increasing diagnosis of thyroid diseases, leading to more surgeries, has resulted in the overdiagnosis of thyroid cancer, often leading to overtreatment without improving patient survival or well-being2,3,4. Resection of the thyroid gland or radioactive iodine therapy may lead to hypothyroidism, vocal cord paralysis, or calcium imbalance2,3,4,5. Thus, there is ongoing debate about the optimal approach to treating thyroid nodules and thyroid cancer, with particular emphasis on avoiding unnecessary interventions when the risk associated with nodular goiter is low5. This strategy also saves healthcare costs and prevents complications such as infection, bleeding, and damage to surrounding structures. Additionally, it alleviates undue anxiety and emotional suffering associated with a cancer diagnosis that may not impact a patient’s health2,3,4,5.
Chemokines, interleukins, cytokines, and growth factors drive thyroid cancer progression by affecting processes like proliferation, differentiation, apoptosis, and immune responses6,7,8. Chemokines promote tumor growth and metastasis by recruiting immune cells that create a pro-tumorigenic environment and directly influencing cancer cell behavior, thereby enhancing their invasive and metastatic potential6. Specific interleukins, such as IL-6 and IL-8, contribute to thyroid cancer progression, with IL-6 promoting cancer cell proliferation and survival, while IL-8 enhances angiogenesis and metastasis7,9. Cytokines like TNF-α and IFN-γ influence thyroid cancer development, while growth factors such as VEGF and EGF play crucial roles by promoting angiogenesis to supply the tumor with blood and driving cell division and survival, thereby contributing to cancer progression and therapy resistance8.
According to the American Thyroid Association (ATA), the biggest current problem is the lack of available blood diagnostic tests for detecting thyroid cancer10. Considering the ATA’s concerns and the issue of overdiagnosis and overtreatment of thyroid cancer, our study aimed to investigate whether the concentrations of 48 selected chemokines, interleukins, cytokines, and growth factors could serve as indicators to distinguish papillary thyroid cancer patients from individuals with nodular goiter.
Methods
Patients
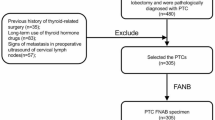
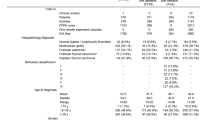
The study group included 32 patients with papillary thyroid cancer (PTC). The comparative group consisted of 26 patients with nodular goiter, of whom 25 were diagnosed with nontoxic multinodular goiter (ICD-10 code E04.2), and one had toxic multinodular goiter (ICD-10 code E05.2). Both groups underwent thyroidectomy at the General Surgical Department of the General Hospital in Wysokie Mazowieckie, Poland. The inclusion criterion for both the study and comparative groups was the result of the postoperative histopathological examination. The exclusion criteria for both groups included a history of cancer and any acute or chronic inflammatory diseases. After sample collection, each patient’s sample was codified. Further processing of the samples, specifically the evaluation of a panel of biomarkers and data analysis, was performed on anonymized samples. Demographic and clinical characteristics of the studied patients are presented in Table 1 (Table 1). Histopathological examination results for PTC patients are presented in Table 2 (Table 2). Routine laboratory parameters results for PTC and nodular goiter patients are presented in Supplemental Table S1 (Table S1).
Material
The test material was venous blood collected in a 9 mL test tube without an anticoagulant (VACUETTE® TUBE 9 ml CAT Serum Clot Activator, Greiner BIO-ONE, Ref. number 455092). Within 30 min of venipuncture, the blood was centrifuged for 20 min at 1000 x g to separate the serum. The collected serum was then stored at -75 °C until further analysis.
Methods
Analysis of a 48-cytokine panel
Serum proteins concentrations were evaluated using the multiplexed bead-based immunoassay method (Bio-Plex Pro Human Cytokine Screening Panel, 48-Plex, Bio-Rad Laboratories, Inc., USA, Ref. number 12007283). Experiment was conducted following the manufacturer instruction.
Analysis of routine laboratory parameters
Preoperative laboratory tests: thyroid-stimulating hormone, free triiodothyronine, free thyroxine, and parathyroid hormone concentrations, complete blood count, hemostasis, and biochemical parameters, were analyzed within routine laboratory practice (For more details, refer to the Material and Methods section in the Supplemental files).
Statistical analysis
The obtained results were analyzed with the use of the GraphPad Prism 5.0 (GraphPad Software, San Diego, USA), STATISTICA 13.0 PL (StatSoft Inc., Tulsa, USA), and STATA SE 18.0 (StataCorp LP, College Station, USA) softwares. Due to the small number of cases included in both the study and control groups, statistical analysis was performed using the nonparametric Mann-Whitney U test or Kruskal-Wallis test. Spearman’s rank correlation was employed to obtain correlation coefficients. Values for continuous variables, unless stated otherwise, are presented as median with 25th and 75th percentiles (Q1 and Q3 quartiles). To assess the discriminative ability of serum bFGF, IL-9, IL-18, TNF-α, and TNF-β concentration evaluation in differentiating between the PTC patients and nodular goiter patients, a Receiver Operator Characteristic (ROC) curve was constructed. The cut-off values were obtained using the Youden Index which is used in biomarker testing to identify the optimal cut-off point that maximizes the balance between sensitivity and specificity. It is the maximum distance between the diagonal of a square with a side length of 1 and a point on the ROC curve, calculated using the formula: d = sensitivity + specificity − 1. By minimizing both false positives and false negatives, the Youden Index ensures more accurate diagnostic decisions. This method is versatile and applicable across various tests, improving clinical decision-making by selecting the most effective threshold for distinguishing conditions11.
To assess the combined discriminatory ability of two molecules, logistic regression models were constructed to obtain their linear combination. Then, for the predictor obtained in this way, ROC curves were constructed, similar to those for a single molecule. Confidence intervals (CIs) for sensitivity and specificity were calculated at a 95% confidence level (1-α = 0.95) using the C.I. Calculator: Diagnostic Statistics software (https://www2.ccrb.cuhk.edu.hk/stat/confidence%20interval/Diagnostic%20Statistic.htm). Statistical significance was determined at a significance level of < 0.05 for two-tailed p-value. The sample size was calculated using an independent two-sided t-test, with a 95% confidence level and a margin of error of ± 5%, with α = 0.05 and a power analysis of 80% (0.8). For the calculation the Sample Size Calculator (https://clincalc.com/stats/samplesize.aspx) was utilized.
Results
Papillary thyroid cancer (PTC) patients versus nodular goiter patients
Of the 48 analyzed proteins, the concentrations of five molecules differed significantly between the group of patients with PTC and the group of nodular goiter patients. The median bFGF concentration was significantly higher (p = 0.0246) in patients with PTC (26.15 pg/mL, Percentiles: 23.99–28.23 pg/mL) compared to patients with nodular goiter (22.88 pg/mL, Percentiles: 19.98–26.67 pg/mL). The median IL-9 concentration was significantly higher (p = 0.0246) in patients with PTC (295.9 pg/mL, Percentiles: 247.1-319.5 pg/mL) compared to patients with nodular goiter (263.9 pg/mL, Percentiles: 227.6-297.7 pg/mL). The median IL-18 concentration was also significantly higher (p = 0.0228) in patients with PTC (57.72 pg/mL, Percentiles: 38.06–82.86 pg/mL) compared to patients with nodular goiter (35.18 pg/mL, Percentiles: 24.20–58.50 pg/mL). The median TNF-α concentration was significantly higher (p = 0.0101) in patients with PTC (69.62 pg/mL, Percentiles: 58.56–74.65 pg/mL) compared to patients with nodular goiter (61.47 pg/mL, Percentiles: 38.71–68.84 pg/mL). The median TNF-β concentration was significantly higher (p = 0.0037) in patients with PTC (555.7 pg/mL, Percentiles: 477.1-588.7 pg/mL) compared to patients with nodular goiter (487.4 pg/mL, Percentiles: 415.8-540.2 pg/mL). The concentrations of the remaining analyzed proteins did not differ significantly between the analyzed patient groups (Fig. 1A-E, Supplemental Table S2).
(A–E) Serum bFGF, IL-9, IL-18, TNF-α, and TNF-β concentrations comparison between papillary thyroid cancer (PTC) patients and nodular goiter patients. Statistical significance: * p < 0.05, ** p ≤ 0.01. bFGF basic fibroblast growth factor, IL-9 interleukin-9, IL-18 interleukin-18, TNF-α tumor necrosis factor α, TNF-β tumor necrosis factor β.
We also analyzed bFGF, IL-9, IL-18, TNF-α, and TNF-β concentrations depending on the type of thyroidectomy, location of the lesion within the thyroid gland, the presence of the vocal cord mobility disorders or paresis, smoking history, the presence of angioinvasion, the presence of glazing, the CK19 staining result, the presence of the satellite lesions, and presence of the capsular infiltrations. We only found the statistical difference for TNF-α between patients which have angioinvasion based the histopathological examination (603.0 pg/mL, Percentiles: 557.3-612.7 pg/mL) compared to those without angioinvasion (518.9 pg/mL, Percentiles: 466.2-564.6 pg/mL) (p = 0.0073). Any other significant differences were not reported (Supplemental Tables S3a-i).
Correlation coefficient analysis
In both the group of patients with PTC and the comparison group of patients with nodular goiter, the concentrations of bFGF, IL-9, IL-18, TNF-α, and TNF-β were correlated with age. None of the above-mentioned proteins, regardless of the group analyzed, showed any correlation with age.
In the next step of analysis, the concentrations of bFGF, IL-9, IL-18, TNF-α, and TNF-β were correlated with each other. In the group of PTC patients, IL-9 concentration positively correlated with TNF-α (r = 0.617, p < 0.001) and TNF-β concentrations (r = 0.872, p < 0.001). Moreover, TNF-α concentration positively correlated with TNF-β levels (r = 0.558, p < 0.001) (Table 3; Fig. 2A). In the group of nodular goiter patients, bFGF concentration positively correlated with IL-9 (r = 0.522, p = 0.0062), IL-18 (r = 0.454, p = 0.0199), TNF-α (r = 0.767, p < 0.001), and TNF-β (r = 0.423, p = 0.0314) concentrations. IL-9 concentration positively correlated with TNF-α (r = 0.776, p < 0.001) and TNF-β (r = 0.860, p < 0.001) levels. IL-18 concentration positively correlated with TNF-α levels (r = 0.462, p = 0.0175). Moreover, TNF-α concentration positively correlated with TNF-β levels (r = 0.744, p < 0.001) (Table 4; Fig. 2B). No other correlations were observed, both in the study and comparison groups.
(A,B) The heat map of the complex relationship between serum bFGF, IL-9, IL-18, TNF-α, and TNF-β concentrations in PTC patients (A) and nodular goiter patients (B) patients. The utilized color scale and the R values found in individual fields denote the strength and direction (no minus sign for positive correlation, with a minus sign for negative correlation) of the linear relationship between the two variables. Statistically significant dependencies for the examined parameters have been elucidated in the results section.
48-panel cytokine analysis based on FNAB results in PTC patients
The statistical analysis did not reveal any significant changes based on FNAB results. The data are included in the Supplemental Files, Table S5.
Diagnostic utility results
The highest area under the ROC curve (AUC), equal to 0.720 ± 0.066, as well as diagnostic specificity (92%) and positive predictive value (89%) to distinguish patients with PTC from individuals with nodular goiter was found for TNF-β (Table 5, Supplemental Figure S1). Thus, in the next step, we analyzed the diagnostic usefulness of TNF-β in combination with other proteins. We found that the combined evaluation of TNF-β and bFGF concentrations was the most useful in differentiating papillary thyroid cancer from benign nodular goiter, revealing a diagnostic specificity of 96% (Table 6, Supplemental Tables S4a-d). We also tested other possible protein combinations, but none revealed higher specificity (data not shown).
Power analysis and sample size calculation
Our study is the first that evaluated serum bFGF, IL-9, IL-18, TNF-α, and TNF-β concentrations using a multiplex method in PTC and nodular goiter patients. Therefore, we were not able to conduct a priori power analysis and sample size calculations. We conducted post-hoc power analysis and sample size calculation. Post-hoc power analysis (with the α established at 0.05) yields 35.4% for bFGF, 48% for IL-9, 51.2% for IL-18, 100% for TNF-α, and 73.3% for TNF-β. Sample size calculation indicated that a minimum group size was established for each group as follows: 53 for bFGF, 18 for IL-9, 53 for IL-18, 22 for TNF-α, and 21 for TNF-β. With our study group consisting of 32 PTC patients and 26 nodular goiter patients, we meet the sample size requirements for IL-9, TNF-α, and TNF-β.
Discussion
Only a few studies in the available literature have assessed the circulating levels of cytokines, interleukins, and growth factors in patients with thyroid cancer7,12,13,14,15. Our study is the first to evaluate the levels of 48 proteins in patients with PTC and benign nodular goiter.
Basic fibroblast growth factor (bFGF), also known as FGF-2, plays a significant role in cell proliferation, differentiation, angiogenesis, and wound healing. In the context of cancer, this indicate its potential involvement in tumorigenesis, angiogenesis, and metastasis. Studies by Xu et al.16 showed that miR-27b-3p could inhibit bFGF overexpression in PTC cell lines, resulting in reduced cell proliferation, migration, and invasion. It was also found that bFGF is strongly expressed in human PTC, primarily localized to the cytoplasm of cancer cells17, and the rate of bFGF expression in thyroid cancer tissues is significantly higher compared to adjacent normal thyroid tissues18. Further studies, conducted by Jia et al.19, indicated that the number of bFGF-positive cells was significantly higher in the PTC group than in the benign goiter group. Moreover, the number of bFGF-positive cells in the invasive PTC group was significantly higher compared to the non-invasive PTC group. These findings suggests that bFGF expression is associated with the malignant behavior of thyroid cancer. Detecting bFGF expression in postoperative thyroid goiter samples can help evaluate the invasiveness of PTC, providing insight into its risk and offering a more reliable basis for diagnosis and identification19.
Studies conducted by Ria et al.12 indicated that the levels of bFGF were significantly higher in the serum of patients with both neoplastic and benign goiter compared to the control group. Additionally, bFGF concentrations significantly decreased after total thyroidectomy. However, in their study, the mean preoperative serum levels of bFGF did not differ between benign and malignant cases12. This contrasts with our current findings, which suggest that the assessment of circulating bFGF may be helpful in differentiating PTC from the benign goiter group. Such a discrepancy could result from differences in methodology (multiplex versus ELISA) and the smaller number of males included in our study groups. It should also be noted that in our study, the diagnostic specificity for bFGF was only 54%. However, the tested protein demonstrated much higher diagnostic sensitivity (81%), meaning that the evaluation of bFGF is more useful for correctly identifying individuals with PTC than for identifying those without it. This finding could support the hypothesis of Jia et al.19, who indicate that bFGF is a factor associated with thyroid cancer malignancy.
Interleukin-9 (IL-9) acts on multiple cell types and can have both pro-tumor and anti-tumor effects depending on the context and type of cancer20,21,22,23. However, research on IL-9 in thyroid cancer is limited20,23,24,25. Ying et al.23 demonstrated that the combination of IL-9 and IL-21 exerts a synergistic apoptotic effect and a direct cytotoxic impact on thyroid cancer. Additionally, this combination promotes the proliferation and differentiation of Th9 cells and CD8 + T cells, potentially inhibiting cancer progression and preventing immune escape23. Zivancevic-Simonovic et al. presented that PTC patients with Hashimoto thyroiditis (HT) had significantly higher levels of IL-9 compared to the PTC patients without HT20. Other studies conducted by the authors showed that differentiated thyroid cancer patients had significantly higher IL-9 concentrations compared to the control individuals25. We found that IL-9 concentration significantly differentiates PTC patients from thyroid goiter patients. However, both the diagnostic sensitivity and specificity for IL-9 were relatively low. Interleukin-18 (IL-18) is a proinflammatory cytokine that stimulates Th1 cells to combat malignant tumors. However, it can also activate Th2 cells, potentially undermining the immune system’s ability to recognize cancer cells26. Thus it may also contribute to the development and progression of certain cancers27. Kobawala et al.26 showed that circulating levels of IL-18 were significantly higher in thyroid cancer patients compared to the control individuals. In their study, patients with benign thyroid diseases also had statistically higher IL-18 concentrations compared to the control group. The analysis of the AUC showed that evaluating IL-18 concentration was useful in differentiating PTC and anaplastic thyroid cancer patients from those with benign thyroid diseases26, which aligns with our findings. In the current study, the diagnostic specificity for IL-18 was relatively low (58%). However, the tested protein demonstrated much higher diagnostic sensitivity (81%), indicating that, like bFGF, the evaluation of IL-18 is more useful for correctly identifying individuals with PTC than for identifying those without it. The studies by Kobawala et al.26 and our results suggest that analyzing circulating IL-18 levels could be valuable as a differentiating factor in thyroid tumorigenesis and that the rise in serum IL-18 levels may be triggered in response to the tumor.
Tumor necrosis factor-α (TNF-α) is a proinflammatory cytokine crucial for immune system regulation, inflammation, and apoptosis. It significantly influences cancer development by regulating interactions within the tumor microenvironment28. TNF-α’s role in thyroid cancer is complex, exhibiting both tumor-promoting effects at low concentrations by enhancing angiogenesis and metastasis, and tumor-suppressing effects at high concentrations through its antitumor activity29,30,31. Studies conducted by Gheorghe et al.29 demonstrated that TNF-α may exert varying antitumor effects in response to radioiodine treatment, depending on the patient’s immune profile. Lumachi et al.30 reported that TNF-α functions as a growth inhibitor in PTC cell lines. TNF-α expression was localized in the cytoplasm and was significantly higher in PTC patients compared to those with benign goiter13. Circulating TNF-α levels were shown to be significantly higher in thyroid cancer patients compared to healthy individuals13,14,15. An increased TNF-α concentration was also an indicator of lymph node metastasis14. Additionally, Kobawala et al.13 reported that TNF-α levels were significantly higher in PTC patients compared to those with benign goiter, which aligns with our current findings. The diagnostic sensitivity and specificity for TNF-α concentration evaluation were 53% and 77%, respectively, indicating that in our study the protein was more effective at correctly identifying individuals without PTC. Additionally, we demonstrated a statistically significant difference in TNF-α levels between patients with angioinvasion, as determined by histopathological examination, and those without angioinvasion. An explanation for our observation can be found in the study by Lv et al.31, who presented that PTC cell lines exposed to TNF-α induce epithelial-mesenchymal transition (EMT). EMT and angioinvasion are critical to cancer progression, as EMT promotes tumor cell migration and tissue invasion, including into blood and lymphatic vessels, enabling tumor cells to infiltrate vessels more efficiently and drive angioinvasion and metastasis32.
Tumor necrosis factor-β (TNF-β), also known as lymphotoxin-α (LT-α), is another member of the TNF cytokine family and is closely related to TNF-α. TNF-β is primarily produced by T and B lymphocytes and plays a role in inflammation, immune response, and the regulation of cell death28,33,34. In addition to its established roles in the development and maintenance of lymphoid organs, TNF-β-mediated signaling has also been implicated in cancer development33,34,35. However, the mechanisms of action of TNF-β and its role in promoting thyroid cancer are still under investigation. In our study, PTC patients had significantly higher TNF-β concentrations compared to the nodular goiter group. The evaluation of TNF-β concentrations demonstrated the highest diagnostic specificity (92%) among all proteins tested to differentiate individuals with PTC from those with thyroid goiter, indicating that TNF-β is the most reliable marker for correctly identifying individuals without PTC. Further analysis showed that the combined evaluation of TNF-β and bFGF concentrations was the most useful for differentiating PTC from benign nodular goiter, revealing a diagnostic specificity of 96%. We also tested other protein combinations, but none showed higher specificity.
Lu et al., utilizing the BD™ Cytometric Bead Array (CBA) Human Th1/Th2 Cytokine Kit, which is optimized for the quantitative analysis of specific cytokines (IL-2, IL-4, IL-6, IL-10, TNF-α, and IFN-γ), demonstrated that only TNF-α among these cytokines may serve as a useful early warning biomarker for PTC. Moreover, they observed that TNF-α may be associated with lymph node metastases in patients with PTC. The authors then evaluated the diagnostic utility of TNF-α and reported an AUC comparable to our findings (0.643 vs. 0.696)14. Currently, this panel is utilized in laboratories to investigate especially the causes of recurrent miscarriages and to evaluate the severity of infections and inflammatory processes36,37. Given the lack of studies in the current literature assessing the clinical utility of the cytokines identified in our research, and the absence of validated diagnostic blood tests for thyroid cancer, we propose that our study provides a solid foundation for the development of a novel potential diagnostic kit. This kit could employ CBA technology incorporating the cytokines highlighted in our findings: bFGF, IL-9, IL-18, TNF-α, and TNF-β. However, further investigations involving larger cohorts and considering other types of thyroid cancer are essential. These studies will be crucial to confirm the diagnostic relevance of these cytokines for PTC and necessary to introduce a validated, standardized test, optimal cut-off values based on our findings.
One of the study’s limitations is the low post-hoc power for some biomarkers (bFGF, IL-9, and IL-18), which ranges from 35.4% to 51.2%. These results indicate that the power was below the desired threshold of 80%, suggesting that they should be interpreted with caution. However, it is important to note that the sample size in our study met the required group size for IL-9, TNF-α, and TNF-β (18, 22, and 21, respectively), and the statistical power for these biomarkers was sufficient (48%, 100%, and 73.3%, respectively). This suggests that the findings for TNF-α and TNF-β are more reliable, while the lower power for bFGF, IL-9, and IL-18 may limit confidence in the findings for these markers. Another limitation could be related to the fact that our thyroid cancer group was composed solely of individuals with papillary thyroid cancer. It would be interesting to determine whether there is a difference in protein concentrations in other types of thyroid cancer, such as follicular, medullary, and anaplastic thyroid cancers. For justification, we explain that thyroid cancer is relatively rare, with papillary thyroid cancer being the most common diagnosis. Thus, it was challenging to collect an adequately sized group with other histopathological types of thyroid cancer.
In conclusion, while the combined evaluation of bFGF and TNF-β concentrations shows promise as a potential tool for distinguishing PTC patients from those with nodular goiter, further validation is essential before clinical implementation. Large-scale clinical trials and standardized testing methods are necessary to confirm their diagnostic and prognostic value, as well as to assess their role in reducing unnecessary surgical interventions. Additionally, further studies should include a larger sample size and consider other histopathological types of thyroid cancer to better establish the clinical utility of these biomarkers.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author (OMKL) on reasonable request.
References
Brito, J. P., Morris, J. C. & Montori, V. M. Thyroid cancer: zealous imaging has increased detection and treatment of low risk tumours. BMJ 347, 1–6 (2013).
Krajewska, J. et al. Early diagnosis of Low-Risk papillary thyroid Cancer results rather in overtreatment than a better survival. Front. Endocrinol. (Lausanne). 11, 1–15 (2020).
Haymart, M. R. Progress and challenges in thyroid Cancer management. Endocr. Pract. 27, 1260–1263 (2021).
Horgan, D. et al. Tackling thyroid Cancer in Europe—The challenges and opportunities. Healthcare 10, 1621 (2022).
Ullmann, T. M., Papaleontiou, M. & Sosa, J. A. Current controversies in Low-Risk differentiated thyroid cancer: reducing overtreatment in an era of overdiagnosis. J. Clin. Endocrinol. Metab. 108, 271–280 (2023).
Coperchini, F., Croce, L., Marinò, M., Chiovato, L. & Rotondi, M. Role of chemokine receptors in thyroid cancer and immunotherapy. Endocr. Relat. Cancer. 26, R465–R478 (2019).
Xi, C. et al. Interleukins in thyroid cancer: from basic researches to applications in clinical practice. Front. Immunol. 11, (2020).
Mohamad Pakarul Razy, N. H., Rahman, W. F. W. A. & Win, T. T. Expression of vascular endothelial growth factor and its receptors in thyroid nodular hyperplasia and papillary thyroid carcinoma: A tertiary health care centre based study. Asian Pac. J. Cancer Prev. 20, 277–282 (2019).
Matsushima, K., Yang, D. & Oppenheim, J. J. Interleukin-8: an evolving chemokine. Cytokine 153, 155828 (2022).
American Thyroid Association. https://www.thyroid.org/cancer-of-the-thyroid/.
Hu, X., Li, C., Chen, J. & Qin, G. Confidence intervals for the Youden index and its optimal cut-off point in the presence of covariates. J. Biopharm. Stat. 31, 251–272 (2021).
Ria, R. et al. Effect of thyroidectomy on Circulating angiogenic cytokines in papillary thyroid carcinoma and benign goiter: potential for new biomarkers? Surg. (United States). 169, 27–33 (2021).
Kobawala, T. P. et al. Significance of TNF- α and the Adhesion Molecules: L-Selectin and VCAM-1 in Papillary Thyroid Carcinoma. J. Thyroid Res. 1–17 (2016).
Lu, H. et al. Markers of immune activation: novel biomarkers to predict the early-warning indicator of patients with papillary thyroid carcinoma. Diagn. Pathol. 15, 1–9 (2020).
Zhang, X., Li, S., Wang, J., Liu, F. & Zhao, Y. Relationship between serum inflammatory factor levels and differentiated thyroid carcinoma. Technol. Cancer Res. Treat. 20, 1–8 (2021).
Xu, Y. et al. miR-27b‐3p is involved in doxorubicin resistance of human anaplastic thyroid Cancer cells via targeting peroxisome Proliferator‐Activated receptor gamma. Basic. Clin. Pharmacol. Toxicol. 123, 670–677 (2018).
Liang, H. et al. Assessment of biomarkers for clinical diagnosis of papillary thyroid carcinoma with distant metastasis. Int. J. Biol. Markers. 25, 38–45 (2010).
Ummah, M. S. [Clinical significance of expression of VEGF and bFGF in thyroid carcinoma]. Sustain 11, 1–14 (2019).
Jia, Z. Y., Wu, X. L., Zhang, Y. H., Ma, B. L. & Ma, F. C. The correlation between ultrasonographic features, bFGF, and the local invasiveness of thyroid papillary carcinoma. Med. (Baltim). 99, e20644 (2020).
Zivancevic-Simonovic, S. et al. Cytokine production in patients with papillary thyroid cancer and associated autoimmune Hashimoto thyroiditis. Cancer Immunol. Immunother. 64, 1011–1019 (2015).
Zheng, N. & Lu, Y. Targeting the IL-9 pathway in cancer immunotherapy. Hum. Vaccines Immunother. 16, 2333–2340 (2020).
Lee, J. E. et al. The role of Interleukin-9 in Cancer. Pathol. Oncol. Res. 26, 2017–2022 (2020).
Ying, X., Ma, X., Yang, Z. & Zhou, B. Th9 cytokines inhibit proliferation, promote apoptosis, and immune escape in thyroid carcinoma cells. Appl. Biochem. Biotechnol. https://doi.org/10.1007/s12010-023-04821-2 (2024).
Amirkhani, Z., Alavi, M., Kalani, M., Alavianmehr, A. & Farjadian, S. Immunomodulatory effects of Omega-3 fatty acids in patients with differentiated thyroid Cancer before or after radioiodine ablation. Iran. J. Immunol. 19, 71–90 (2022).
Simonovic, S. Z. et al. Cytokine production in peripheral blood cells of patients with differentiated thyroid cancer: elevated Th2/Th9 cytokine production before and reduced Th2 cytokine production after radioactive iodine therapy. Cancer Immunol. Immunother. 64, 75–82 (2015).
Kobawala, T., Trivedi, T., Gajjar, K., Patel, G. & Ghosh, N. Role of Interleukin-18 in thyroid tumorigenesis role of Interleukin-18 in thyroid tumorigenesis. (2016). https://doi.org/10.14319/ijcto.44.1
Jaspers, J. E. et al. IL-18-secreting CAR T cells targeting DLL3 are highly effective in small cell lung cancer models. J. Clin. Invest. 133, 1–11 (2023).
Buhrmann, C. et al. Evidence that TNF-β induces proliferation in colorectal cancer cells and Resveratrol can down-modulate it. Exp. Biol. Med. 244, 1–12 (2019).
Gheorghe, D. C., Stanciu, M. M., Zamfirescu, A. & Stanciu, A. E. TNF-α May exert different antitumor effects in response to radioactive iodine therapy in papillary thyroid Cancer with/without autoimmune thyroiditis. Cancers (Basel). 13, 3609 (2021).
Lumachi, F., Basso, S. M. M. & Orlando, R. Cytokines, thyroid diseases and thyroid cancer. Cytokine 50, 229–233 (2010).
Lv, N., Liu, F., Cheng, L., Liu, F. & Kuang, J. The expression of transcription factors is different in papillary thyroid Cancer cells during TNF - α induced EMT. J. Cancer. 12, 2777–2786 (2021).
Banyard, J. & Bielenberg, D. R. The role of EMT and MET in cancer dissemination. Connect. Tissue Res. 56, 403–413 (2015).
Bauer, J. et al. NF-ĸB, and cancer: the dark side of cytokines. Dig. Dis. 30, 453–468 (2012). Lymphotoxin.
Ruddle, N. H. & Lymphotoxin How it all began-A tribute to the travelers. Cytokine Growth Factor. Rev. 25, 83–89 (2014).
Fernandes, M. T., Dejardin, E. & dos Santos, N. R. Context-dependent roles for lymphotoxin-β receptor signaling in cancer development. Biochim. Biophys. Acta - Rev. Cancer. 1865, 204–219 (2016).
Lee, S. K. et al. An imbalance in interleukin-17-producing T and Foxp3 + regulatory T cells in women with idiopathic recurrent pregnancy loss. Hum. Reprod. 26, 2964–2971 (2011).
Tang, Y. et al. Evaluation of Th1/Th2 cytokines as a rapid diagnostic tool for severe infection in paediatric haematology/oncology patients by the use of cytometric bead array technology. Clin. Microbiol. Infect. 17, 1666–1673 (2011).
Acknowledgements
We are grateful to Jarosław Szymczuk, PhD, from the General Surgical Department of the General Hospital in Wysokie Mazowieckie, Poland, for his help with the blood collection from patients.
Funding
This research received no external funding. This work was supported by the Medical University of Bialystok.
Author information
Authors and Affiliations
Contributions
Conceptualization: JK, OMKL; Data curation: MCD, OMKL; Formal analysis: JK, AJM, OMKL; Funding acquisition: OMKL; Investigation: KMG, JK, OMKL; Methodology: JK, OMKL; Project administration: OMKL; Resources: JMK; Software: JK, AJM, OMKL; Supervision: OMKL; Validation: MCD, JK, OMKL; Visualization: MCD, OMKL; Roles/Writing – original draft: MCD, OMKL; and Writing – review & editing: MCD, KMG, JK, AJM, JMK, OMKL.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The research followed the guidelines set forth in the Declaration of Helsinki, and the protocol was approved by the Bioethics Human Research Committee at the Medical University of Bialystok (Permission No. APK.002.356.2022). Before participating in the study, all individuals provided informed consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ćwiklińska-Dworakowska, M., Gacuta, K.M., Kamińska, J. et al. Preliminary results suggest the potential of evaluating combined bFGF and TNF-β concentrations for differentiating papillary thyroid cancer from benign nodular goiter. Sci Rep 15, 15316 (2025). https://doi.org/10.1038/s41598-025-00255-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-00255-4