Abstract
Optimizing process parameters is essential for developing high efficacy carbonaceous adsorbents. This study investigated carbon dioxide (CO2) capture using activated carbon (AC) synthesized from date-palm leaflets. Key process parameters—pyrolysis temperature, residence time, and KOH-to-carbon (KOH/C) impregnation ratio—were systematically varied to synthesize highly nanoporous AC for enhanced CO2 uptake. Instead of relying on an intuitive selection of process variables, the optimization process was implemented using Response Surface Methodology (RSM) protocol, which provided a structured and effective strategy for process optimization. Over twenty different AC samples were synthesized and evaluated for their CO2 adsorption capacities. The optimal conditions, identified as 700 °C, 1.5 h, and a 3:1 (KOH/C) impregnation ratio, yielded AC with exceptional CO2 uptake capacities of 6.71 mmol/g at 0 °C and 4.214 mmol/g at 25 °C, outperforming most previously reported biomass-derived ACs. This superior performance is attributed to the well-developed nanoporous structure and high nitrogen content of the optimized sample, as confirmed by N2 adsorption isotherms, elemental analysis, scanning electron microscopy (SEM), and Fourier transform infrared spectroscopy (FTIR). The optimized AC demonstrated excellent stability over multiple adsorption–desorption cycles. Additionally, a high isosteric enthalpy of adsorption (35 kJ/mol at 0.2 mmol/g) further confirmed preferential CO2 adsorption at energetically favorable nanopore sites. This study underscores the potential of date-palm leaflets as a sustainable and abundant precursor for synthesizing high-efficacy AC for carbon capture.
Similar content being viewed by others
Introduction
The continuous rise in the greenhouse gas (GHG) emissions, particularly CO2, is the main cause of global warming and climate change1. Addressing this urgent challenge requires coordinated efforts to reduce CO2 emissions, with carbon capture and storage (CCS) technologies playing an indispensable role2,3,4,5,6. Among the various CCS approaches, adsorption-based technologies using hierarchical porous materials have garnered substantial attention due to their cost-effectiveness, scalability, and efficiency7,8,9.
Biomass-derived activated carbon (AC) has gained prominence as a promising adsorbent for CO2 capture. Its key advantages include low production cost, hydrophobicity, high thermal and chemical stability, and the ability to utilize waste-derived precursors10,11,12. Moreover, using waste biomass for AC production not only enhances carbon capture efforts but also promotes sustainable waste management practices.
Date-palm waste biomass is an abundant and renewable resource in the Kingdom of Saudi Arabia, with an estimated annual generation exceeding 1 million metric tons13,14. This lignocellulosic biomass, rich in carbon, offers a sustainable and cost-effective precursor for producing AC. Numerous studies have explored the potential of AC derived from various lignocellulosic agricultural wastes15,16,17. For instance, corncob-derived AC activated with KOH yielded CO2 removal capacity of 4.5 mmol/g at 15 °C and 1 atm, outperforming AC produced using other activating agents such as H3PO4 and ZnCl215. Similarly, CO2-activated bamboo residue exhibited an uptake capacity of 3.4 mmol/g at 0 °C and 1 atm16. Recently, six activated carbon (AC) samples were derived from olive stones and almond shells through pyrolysis at 850 °C for two hours with varying KOH-to-carbon (KOH/C) impregnation ratios. While higher impregnation ratios resulted in greater surface areas, the highest carbon capture capacities were observed for samples with lower impregnation ratios—6.1 mmol/g for olive stones and 5.9 mmol/g for almond shells at 0 °C17.
Research on date-palm waste for carbon capture has mainly focused on date seeds. For example, Al-Wasidi et al. produced three date-seed-derived AC samples by pyrolyzing at 500 °C, 600 °C, and 700 °C for three hours with a KOH/C mass ratio (impregnation ratio) of 1:1. The highest CO2 removal capacity of 3.7 mmol/g at 0 °C was achieved with the AC prepared at 700 °C, which had a pore volume of 0.161 cm3/g18. In another study, Iranvandi et al. compared the carbon capture performance of different ACs using thermogravimetric analysis with a mixed gas stream of CO2 and N219. Increasing the CO2 partial pressure in the inlet gas was found to enhance the uptake capacity of ACs. The highest uptake, 2.67 mmol/g obtained at 25 °C, was recorded for KOH-activated date-seed-derived AC in a gas mixture of 90% CO2 and 10% N2. This capacity was significantly higher than that of its non-activated counterpart. However, the addition of nanosilica to improve fluidization hydrodynamics slightly reduced the efficacy of the AC19.
This study focuses on date-palm leaflets, which are individual leaf segments of the fronds and account for nearly one-third of the annul pruning waste on a dry weight basis20. Moreover, the leaf waste has been reported to possess higher carbon content compared to other parts of the tree, such as seeds and stems, highlighting its potential as a high-yield precursor for the synthesis of carbonaceous adsorbents21. However, current literature lacks studies on their utilization for such applications.
Recently, date-palm leaflets derived biochars were prepared at different carbonization temperature (300, 400, 500, 600 °C) and used them for carbon capture in a fluidized bed system with a mixed inlet gas stream containing low CO2 partial pressure (10% CO2 and 90% air)22. The biochar sample synthesized at 600 °C reportedly exhibited an uptake capacity of 5.7 mmol/g (equivalent to 0.25 kg CO2/kg biochar) apparently under ambient conditions. However, biochars generally exhibit substantially lower adsorption capacities than their activated counterparts17,19. Furthermore, the low partial pressure of carbon dioxide in mixed gas streams reduces the mass transport driving force, resulting in lower adsorption capacities, as predicted by isotherm models. Therefore, the reported CO2 uptake of 0.25 kg CO2/kg with date-palm leaflet biochar under a 10% CO2 gas mixture is unusually high compared to other agro-waste-derived adsorbents reported in the literature.
This study investigates the optimization of AC production from date-palm leaflet waste for enhanced CO2 adsorption. This study systematically investigated the effects of critical process parameters, including residence time, pyrolysis temperature, and the KOH-to-carbon (KOH/C) impregnation ratio. Unlike most previous studies that relied on intuitive selection of process parameters, this study employed Central Composite Design (CCD) based Response Surface Methodology (RSM) to systematically design the experimental framework. This approach encompassed over twenty runs under carefully selected process conditions. The results demonstrated excellent agreement between the RSM-predicted optimal conditions and experimental validation, underscoring the robustness and reliability of the optimization approach. Comprehensive characterization of the optimized AC provided insights into the relationship between its physicochemical characteristics, such as pore volume, surface area, elemental composition, pore size distribution, and functional groups, and its CO2 adsorption performance. Furthermore, isosteric heat of adsorption (Qst) were computed to assess temperature sensitivity, while cyclic adsorption–desorption tests confirmed the optimized AC’s operational stability and reusability for potential large-scale applications. Thus, both environmental and sustainability goals are addressed by this work by developing high efficacy CO2 capture adsorbent using abundant local agricultural waste.
Experimental
Materials
Local date-palm leaflets were used as the precursor for synthesizing activated carbon. Potassium hydroxide (85%), (PanReac AppliChem, Germany) was utilized as the activating agent for impregnation, while hydrochloric acid (≥ 37%) (Honeywell Fluka, US) was used for washing. High-purity nitrogen and carbon dioxide gases (Air Liquide) were used for the evaluation of CO2 capture performance.
Adsorbent synthesis
Precursor preparation
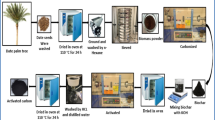
Dry date-palm fronds were sourced from 40-year-old female Khalas palm trees at the Agricultural Research and Experimentation Station of the College of Food and Agricultural Sciences at King Saud University (Dirab Station). The dried leaflets were separated from the stalks and repeatedly washed with hot water to remove dust and other impurities. They were then sun-dried for two days. After drying, the leaflets were cut, crushed into powder, and sieved to obtain particles within the size range of 75–106 µm. The powdered biomass was dried at 110 °C for 24 h in an oven (Digitheat-TFT, Selecta, Spain) to remove moisture.
Biochar production
Five grams of the dried biomass were placed in a crucible boat, inserted into a 5.5 cm ID quartz tube. The sample was heated from ambient temperature to 400 °C at a ramp rate of 5 °C per minute under a nitrogen flow of 150 mL/min for 1.5 h in a tube furnace (OTF-1200X-S, MTI, USA). This yielded 2.18 g of biochar, with an average yield of 43.6% based on multiple batches.
KOH activation
One gram of biochar was impregnated using KOH at three dry mass ratios (2:1, 3:1, and 4:1 of KOH/biochar) in 120 mL distilled water. Subsequently, the mixture was continuously stirred for 6 h at 60 °C using a Cerastir heating plate (Clifton, UK) followed by drying at 110 °C for 24 h. After drying, samples were pyrolyzed at three different temperatures (500 °C, 600 °C, and 700 °C) with a heating ramp rate of 5 °C/min under a nitrogen flow of 150 mL/min for 1.5, 2.5, and 3.5 h to obtain activated carbon samples. The resulting material was alternately washed with hot and cold deionized water and then washed with 0.1 M HCl to achieve a neutral pH. The activated carbon was dried at 110 °C for 24 h before characterization. Figure 1 presents a sequential preparation methodology for producing AC from date-palm leaflet waste.
Yield calculation
The overall yield of AC was calculated using the following equation:
Characterization
A comprehensive characterization of samples at various stages of the study was conducted to correlate process conditions with the properties of the resulting materials, as described below.
Elemental analysis
The carbon (C), hydrogen (H), and nitrogen (N) contents of the samples were determined using a PerkinElmer® CHNS/O Elemental Analyzer (2400 Series II, Waltham, MA, USA). This analysis offers insights into the elemental composition of the samples, which is crucial for understanding their adsorption properties. The activated carbon is expected to exhibit a higher carbon content compared to the precursor due to the removal of volatile components during pyrolysis. Nitrogen content is known to influence the surface functionality and chemical properties of the adsorbent.
Thermogravimetric analysis (TGA)
The thermal integrity and decomposition behavior of the samples were assessed using a DTG-60H (Shimadzu, Kyoto, Japan) by analyzing the weight reduction trend as a function of temperature and time. Approximately 5–20 mg of the activated carbon sample was placed in an aluminum pan and gradually heated from ambient temperature to 900 °C at a ramp rate of 10 °C/min. The analysis was conducted under a N2 atmosphere with a flow rate of 50 mL/min. The weight loss was continuously recorded as a function of temperature and time.
Fourier transform infrared (FTIR) spectroscopy
The surface functional groups of the samples were analyzed by measuring infrared absorption at different wavelengths, corresponding to the vibrational modes of chemical bonds. FTIR spectra were obtained using an IRPrestige-21 (Shimadzu, Japan) in the range of 4000–400 cm−1. Samples were analyzed either by mixing finely ground sample with potassium bromide (KBr) to form a pellet or directly in solid form. The spectra were interpreted to identify characteristic absorption peaks corresponding to various functional groups. The presence of hydroxyl, carbonyl, and aromatic groups indicates surface rich in oxygen-containing functional groups, which can often critically affect the carbon capture efficacy of the sample.
Surface area and porosity analysis
The textural characteristics, including porosity and surface area, of the AC samples were analyzed using a Micromeritics TriStar II Plus, employing nitrogen (N2) physisorption isotherms recorded at 77 K. The Brunauer–Emmett–Teller (BET) method was used to determine the specific surface area within a relative pressure range of 0.05–0.3 (P/P₀). At a relative pressure of approximately 0.99 (P/P₀), the total pore volume was determined, reflecting the cumulative pore volume accessible through nitrogen adsorption. The pore volume distribution was analyzed using Density Functional Theory (DFT) under the assumption of slit-shaped pores. According to the IUPAC classification, pores were categorized as micropores (< 2 nm), mesopores (2–50 nm), and macropores (> 50 nm). Prior to measurement, the AC samples were degassed at 350 °C for 10 h, while precursor was degassed at 110 °C for 10 h.
Scanning electron microscopy (SEM)
The surface morphology of the AC samples was examined using a SEM equipped with an Energy Dispersive X-Ray (EDX) analyzer (JSM-6360A, JEOL, Japan).
CO2 adsorption and regeneration
CO2 uptake capacity was evaluated using a Micromeritics ASAP 2020 with high-purity CO2 gas over a pressure range of 0.01–1.0 bar. The experimental strategy followed Micromeritics Application Note 136, which recommends using a dosing volume of 1.0 cm3/g at low absolute pressure with an equilibrium time of 45 s. To ensure stable adsorption conditions, isotherms were recorded at 0 °C, 25 °C, and 50 °C, using a water/ethylene glycol solution in an ultra-thermostatic bath (TC-502, Brookfield, USA).
Before the adsorption analysis, the samples were subjected to a two-step evacuation process. Initially, the temperature was enhanced from ambient temperature to 90 °C heating at a ramp rate of 5 °C/min, under a pressure increase of 5 mmHg/s up to 10 mmHg for 60 min. Then, the temperature was further enhanced to 350 °C at the same heating rate, under a vacuum of 4 mmHg, and this condition was maintained for 10 h. The regeneration performance of the optimal AC sample was assessed over 10 cycles of CO2 adsorption. After each cycle, the adsorption pressure was adjusted, and the reactor was evacuated to 0.01 bar. Before the initial adsorption cycle, the adsorbent was degassed at 350 °C for 10 h under a 4 mmHg vacuum using the same apparatus used for adsorption.
Using the Clausius-Clapeyron approach, the isosteric heat of adsorption (\({Q}_{st}\)) was assessed as follows:
where \({Q}_{st}\) (kJ/mol) is computed from the slopes of the straight lines obtained through linear regression by plotting \(\text{ln}{P}_{{CO}_{2}}\) against \(1/T\) at a fixed adsorbed amount of CO2. Here, \(R\) is the universal gas constant (8.314 J/mol·K).
Preliminary analysis of the effect of the independent process variables on CO2 adsorption
The CO2 adsorption capacity of activated carbon (AC) is critically dependent on three independent synthesis parameters; pyrolysis temperature, residence time, and the KOH-to-carbon (KOH/C) impregnation ratio10,11. This initial investigation helped define appropriate ranges for the variables in the subsequent experimental design 2. AC samples were synthesized by varying one parameter at a time while holding others constant.
Effect of activation temperature
The influence of activation temperature was examined between 500 °C and 900 °C. At a fixed residence time of 1.5 h, CO2 uptake increased with temperature from 500 °C to 700 °C for all tested KOH/C ratios (2, 3, and 4), as shown in Fig. 2a. The highest uptake (4.214 mmol/g) was achieved at 700 °C with a 3:1 KOH/C ratio. However, further increasing the temperature to 900 °C (tested for 3:1 ratio) led to a noticeable decline in CO2 uptake. This decrease suggests high temperatures may cause structural degradation, such as the collapse of micropores or their widening into less effective meso- and macropores, compromising CO2 adsorption. As discussed later in Sect. 3.3.6, the high reproducibility of adsorption at 700 °C and 3:1 ratio was validated through ten replicate runs, yielding an average uptake of 4.133 ± 0.032 mmol/g, confirming both performance stability and data reliability.
Effect of process parameters on CO2 uptake (at 25 °C and 1 bar) for different AC samples: a Activation temperature and KOH/C mass ratio (residence time fixed at 1.5 h); b Residence time and activation temperature (KOH/C mass ratio fixed at 3:1); c Residence time and KOH/C mass ratio (activation temperature fixed at 600 °C).
Effect of residence time
The effect of activation time was investigated at different temperatures, holding the KOH/C ratio constant at 3:1 (Fig. 2b). The CO2 adsorption capacity was nearly constant as the residence time increased from 0.5 to 2.5 h at 700 °C, followed by a decline at 3.5 h. Conversely, at 600 °C, the highest uptake was observed at 3.5 h, while at 500 °C, the uptake increased steadily with the residence time. These observations suggest an interaction between temperature and time: longer durations may be necessary for sufficient pore development at lower temperatures, but at higher temperatures (like 700 °C), shorter times (around 1.5 h) are sufficient, and extended times might lead to over-activation and damage the microporous structure crucial for CO2 capture.
Effect of KOH/C impregnation ratio
The impact of the chemical activating agent was assessed by varying the KOH/C mass ratio at a fixed activation temperature of 600 °C for different residence times (Fig. 2c). A consistent increase in adsorption occurred as the ratio increased from 2:1 to 3:1. However, further increasing the ratio to 4:1 consistently led to a decline in CO2 uptake, regardless of the residence times. This indicates that while sufficient KOH is necessary for effective pore creation, an excessive amount can be detrimental, likely causing excessive etching, widening of micropores, or collapse of the porous carbon structure. To assess data reliability, six replicate experiments were conducted at 600 °C for 2.5 h using a 3:1 KOH/C ratio. As summarized in Table 2 (S.N. 15–20), the CO2 uptake values showed strong consistency, with an average of 3.786 ± 0.076 mmol/g, confirming the reproducibility of the experimental results in this study.
Overall trends
The AC sample, synthesized at 700 °C with a residence time of 1.5 h and 3:1 KOH/C mass ratio, demonstrated the highest carbon capture capacity, achieving 4.21 mmol/g at 25 °C and 1 bar. Among the three independent process variables studied, the activation temperature and KOH/C ratio appeared to have a more pronounced effects on CO2 removal compared to the residence time, particularly near the optimal temperature23. These initial findings guided the selection of variable ranges for the RSM optimization detailed in Section "RSM designed optimal AC preparation strategy".
RSM designed optimal AC preparation strategy
To determine the optimal operating conditions for developing high efficacy activated carbon (AC) for CO2 capture, we used RSM with CCD, employing Design Expert 13 software. Based on preliminary experiments, the levels and ranges of three key process variables—temperature, residence time, and KOH/C mass ratio—were chosen, as outlined in Table 1. A second-order polynomial model was utilized to analyze the influence of independent process variables on the predicted model response, specifically the carbon capture capacity of the activated carbon. Mathematically, the model can be represented as:
where \(Y\) is predicted response (i.e., CO2 uptake), \({X}_{i}\) and \({X}_{j}\) are the coded independent variables as shown in Table 1, and the \(\beta\) terms are the coefficients of the above quadratic equation. Here, \({\beta }_{0}\) is the intercept, \({\beta }_{i}\) are the linear coefficients corresponding to the main effects of the variables \({X}_{i}\), \({\beta }_{ij}\) are the interaction coefficients for the interaction terms \({X}_{i}{X}_{j}\), and \({\beta }_{ii}\) are the quadratic coefficients for the squared terms \({X}_{i}^{2}.\) In the present case, the above equation simplifies as:
Results and discussion
Process optimization
The primary objective was to enhance the carbon capture efficacy of the adsorbent by maximizing the CO2 uptake per unit mass through optimization of critical process variables: temperature (X1), residence time (X2), and KOH/C mass ratio (X3). Using RSM with CCD, the effect of these process variables on the predicted model response (Y), i.e., CO2 uptake, was systematically studied. As detailed in Table 2, 20 experiments with different values of the process variables were carried out.
The quadratic equation (Eq. 3), representing the effects of different process variables and their interactions, was fitted to the data. The actual experimental values, predicted responses, and corresponding errors are presented in Table 2. The final quadratic equation with the best-fitted coefficients is:
Good agreement was observed between the predicted and actual responses, as depicted in Fig. 3. The coefficient of determination (\({R}^{2}\)) was calculated to assess the accuracy of the model fit. A high \({R}^{2}\) value of 0.97, along with an adjusted \({R}^{2}\) of 0.95 indicates that the model correctly describes the relationship between the independent variables and CO2 adsorption capacity.
Analysis of variance (ANOVA)
To analyze the impact of key process variables on the development of a high-efficacy adsorbent, Analysis of Variance (ANOVA) was conducted. This analysis provides critical insight into the significance of each variable in influencing the response (CO2 uptake) and helps evaluate the effectiveness of the experimental design.
As shown in Table 3, the model exhibits strong overall significance, with an F-value of 40.64 and a p-value < 0.0001, with less than a 0.01% probability owing the occurrence of such a high F-value to random noise. Among the independent variables, activation temperature (X₁) is the most significant, as indicated by its F-value of 197.25 and a p-value < 0.0001. This trend is further supported by Eq. (5), where the coefficients of the temperature, 0.66 for the first-order term and 0.48 for the second-order term, were higher than those for other process variables. The KOH/C mass ratio (X₃) is the next most significant variable, with an F-value of 28.06 and a p-value of 0.0003, confirming its strong effect on the CO2 adsorption. By contrast, residence time (X2) showed a low F-value (0.4684) and a high p-value (0.5093), suggesting it has a negligible individual effect on the adsorption capacity.
Regarding interaction effects, the binary interaction between activation temperature (X1) and KOH/C ratio (X3) is significant, with an F-value of 7.35 and a corresponding p-value of 0.0219. In contrast, the interaction between time (X2) and impregnation ratio (X3) was not significant, and the correlation between temperature (X1) and time (X2) had a negligible effect, as indicated by a low F-value (0.4397) and a high p-value (0.5222). Both pyrolysis temperature (X12) and KOH/C mass ratio (X32) exhibit significant quadratic effects, with high F-values of 29.51 and 23.58, respectively and p-values below 0.001. By contrast, the quadratic effect of residence time (X22) is relatively less significant (F-value = 1.28, p-value = 0.2844), reinforcing limited influence of its variation on the CO2 adsorption capacity of the AC synthesized in this study.
An analysis of the model’s fit statistics provides further insights. The Lack of Fit test was found to be significant (F = 6.50, p = 0.0303), suggesting potential avenues for further model refinement. However, the model demonstrates strong overall performance, as reflected in a high coefficient of determination (R2 = 0.9734), confirming an excellent fit to the experimental data. The adjusted R2 (0.9494) is close to the R2, indicating that the model is not over-fitted. Moreover, the predicted R2 (0.8159) indicate reasonable predictive capability. The difference between adjusted and predicted R2 values (≈0.13) suggests good internal consistency, with perhaps slight over-prediction, which is acceptable in nonlinear systems such as adsorption. The standard deviation (0.1479) and the coefficient of variation (CV = 4.30%) indicate low residual variability relative to the mean CO2 uptake (3.44 mmol/g), signifying the precision and repeatability of the experimental data. Additionally, the Adequate Precision value of 17.9121, which measures the signal-to-noise ratio, is well above the minimum threshold of 4, further confirming and the robustness and reliability of the model.
Influence of process variables on the CO2 uptake
To gain a better understanding of how activation temperature (X1), residence time (X2), and KOH/C impregnation ratio (X3) collectively influence CO2 uptake, the experimental data were analyzed using the RSM-fitted quadratic model (Eq. 5). The resulting response surface plots and contour maps (Fig. 4) provide a clear correlation between variables and the resulting carbon capture efficacy of the adsorbents synthesized under different process conditions.
Figure 4a shows the effect of temperature (X1) and residence time (X2) at fixed impregnation ratio of 3:1. Increasing X1 from 500 °C to towards approximately 700 °C significantly enhances CO2 uptake, regardless of residence time, as reflected by the steep slope of the surface plot. This confirms the critical role of the temperature on adsorbent performance, as pointed out in Section "Preliminary analysis of the effect of the independent process variables on CO2 adsorption". A further increase in X1 leads to a decline in the adsorbent performance, suggesting optimal temperature region near 700 °C, beyond which the predicted uptake begins to decline, quantitatively supporting the hypothesis of structural degradation at excessive temperatures24. Furthermore, the model highlights the effect of the residence time; at the optimal temperature range (~ 700 °C), increasing time beyond 1.5 h shows minimal benefit and potentially a slight decrease in performance, aligning with the preliminary finding in Section "Effect of residence time" that shorter times are sufficient and potentially optimal at higher activation temperatures. The 2D contour plot highlights this optimal zone near 700 °C and shorter residence times (around 1.5 h) for a 3:1 ratio.
Figure 4b depicts the effect of the activation temperature (X1) and impregnation ratio (X3) on CO2 uptake with the residence time fixed at 1.5 h. The RSM model clearly corroborates the trend observed in Section "Effect of KOH/C impregnation ratio": CO2 uptake improves as the KOH/C ratio increases from 2:1 towards an optimum around 3:1, but drops markedly when the ratio is raised to 4:1. The model visualizes this effect across the studied temperature range, emphasizing that even under optimal thermal conditions, an aggressive chemical activation using ratio of 4:1, significantly impairs adsorbent efficacy, due to structural damage caused by over-etching25,26. The contour plot clearly indicates the highest CO2 uptake is achieved near 700 °C with an impregnation ratio between 2.5 and 3.
Figure 4c examines the interaction between residence time (X2) and KOH/C ratio (X₃) at a fixed temperature of 600 °C. Once again, the model highlights an optimal impregnation ratio near 3:1. At this lower temperature, the model shows a slightly more positive effect of increasing residence time compared to observations at 700 °C, but the impact remains less significant than that of the KOH/C ratio. This reinforces the finding from Section "Effect of residence time" that residence time plays a more critical role in structural development at lower temperatures, while its influence diminishes if the activation process is carried out at higher temperature.
Clearly, the RSM model corroborates the preliminary findings of Section "Preliminary analysis of the effect of the independent process variables on CO2 adsorption". While the earlier analysis provided insights based on varying single factors, the RSM model offers a unified perspective, mapping the complex interactions between all three variables and precisely defining the optimal region within the design space. Both analyses consistently conclude that conditions near 700 °C, a residence time of approximately 1.5 h, and a KOH/C ratio of around 3:1 yield the most favorable CO2 uptake performance for AC derived from date-palm leaflets.
Process optimization results
Optimization constraints and response
The optimization of process variables for synthesizing high-efficacy activated carbon (AC) was guided by constraints outlined in Table 4. The process variables included temperature (X1), time (X2), and KOH/C mass ratio (X3), while the response variable was CO2 uptake (Y). Each variable was assigned a range based on experimental limits, with equal weight assigned to both the lower and upper bounds. The goal for all process variables was set to "in range”, ensuring a complete exploration of the parameter space. The response variable (Y) was set to be maximized, with a lower limit of 2.22 mmol/g and an upper limit of 4.21 mmol/g, based on our experimental results. The importance level of each process variable was set to 3, reflecting their equal significance in influencing CO2 uptake. This rigorous approach enabled an effective optimization of the process while adhering to the specified constraints.
Table 5 lists the top five optimal solutions derived from the RSM-based optimization, with the complete list available in the Supplementary Materials (Table S1). The recommended optimal solution suggests: 670 °C temperature, 1.5 h of residence time, and a KOH/C impregnation ratio of 2.7. Under these conditions, the predicted CO2 adsorption is 4.21 mmol/g, with a desirability score of unity. The high desirability score reflects the effectiveness of the selected process variables in achieving maximum CO2 adsorption while adhering to the specified constraints. Although higher adsorption values were observed under conditions listed as 3 (e.g., 4.239 mmol/g), these conditions were not chosen as they involved higher residence times and temperatures, which would result in significantly higher energy costs. This optimal set of conditions provides valuable insights into efficient and sustainable synthesis of activated carbon for CO2 capture.
Model verification
Table 6 summarizes the optimal conditions for CO2 adsorption and verifies model’s accuracy by comparing predicted and experimental CO2 uptake. The highest experimentally measured CO2 uptake of 4.214 mmol/g was achieved at 700 °C with a residence time of 1.5 h and an impregnation ratio of 3. At these conditions, the model’s predicted response was 4.122 mmol/g, yielding an error of 2.18%. This close agreement confirms the model’s reliability and accuracy in predicting the adsorbent efficacy under optimal conditions.
Characterization of optimally synthesized activated carbon (AC) and its precursor
CHN elemental analysis
The CHNS analysis results, detailing the elemental composition of the precursor and the optimal AC, are presented in Table 7. Significant differences in the carbon, oxygen, and nitrogen contents are observed between the precursor and the AC. The carbon content of the AC is significantly higher compared to the precursor caused by the removal of volatile compounds and other non-carbon elements as a result of the activation process. Both oxygen and hydrogen levels in the AC are notably lower, consistent with the devolatilization and thermal treatment of the precursor15. The AC exhibits a higher nitrogen content compared to the precursor. This is a particularly encouraging feature, as nitrogen-containing functional groups are known to enhance the adsorbent’s affinity for CO2 molecules12,27,28.
Thermogravimetric analysis (TGA)
TGA was utilized to analyze the thermal stability and degradation behavior of both the biomass precursor and the optimal activated carbon (AC) by making three replicate runs. Figure 5a shows the mass loss profiles, while Fig. 5b shows the corresponding derivative mass loss (DTG) curves.
A detailed break-down of the stage-wise mass loss is summarized in Table 8. The precursor biomass undergoes an initial mass loss below 200 °C (5.9%), primarily due to moisture evaporation and the release of volatile organic compounds15. Between 200 °C and 450 °C, a substantial mass reduction of precursor takes place due to the thermal decomposition of lingo-cellulosic constituents. Specifically, hemicellulose degradation occurs in the 200–300 °C range, accounting for an 18.5% mass loss while cellulose degradation takes place between 300–450 °C, with a more pronounced 40% mass loss24. The DTG curve for the precursor (Fig. 5b) shows distinct peak between 280 °C and 380 °C, confirming the sequential decomposition of cellulose and hemicellulose. Above 450 °C, the precursor shows an additional 16.2% mass loss, likely due to the gradual breakdown of lignin, whose thermally stable aromatic structure contributes to its broader decomposition range.
In contrast, the AC demonstrates higher thermal stability, with the majority of its mass loss occurring at higher temperatures. An initial mass loss of 19.9% below 200 °C can be attributed to the removal of trapped moisture and residual activation byproducts trapped within the hierarchical porous network Between 200–300 °C and 300–450 °C, the mass losses were significantly reduced (2.7% and 9.9%, respectively), underscoring the effective elimination of hemicellulose and cellulose during chemical activation.
Notably, in the 450–1000 °C range, the AC shows a lower mass loss (16.2%) compared to the precursor (33.3%), likely due to the breakdown of more stable carbonaceous structures developed during the activation process. The lack of any intense DTG peak for the optimal AC sample (Fig. 5b) further suggests a more gradual and less distinct decomposition process, indicating enhanced structural integrity. The total mass loss for the precursor and the optimal AC was 81.2% and 65.8%, respectively, pointing to an enhanced stability of the AC. This improvement in thermal stability is critical for high-temperature applications, such as the cyclic regeneration of adsorbents in COc capture. The robustness of the AC ensures its consistent and long-term performance under thermal stress.
Fourier transform infrared (FTIR) spectroscopy
The surface functional groups of both the precursor and the biomass-derived optimal AC were identified using FTIR data, as shown in Fig. 6. The FTIR spectra reveal significant differences in surface chemistry, reflecting the transformation of the precursor into activated carbon during the thermal activation process. The precursor spectrum exhibits a rather wide absorption peak in the 3600–3200 cm−1 region, indicating O–H stretching vibrations attributed to moisture. This peak is absent in the AC spectrum, indicating effective dehydration during pyrolysis. Moreover, a weak peak around 1650 cm−1, associated with the bending vibration of O–H in water, diminishes significantly in the AC spectrum, further confirming the removal of water molecules. Two distinct peaks at around 2900 cm−1, corresponding to aliphatic C–H bonds, are present in both the precursor and AC spectra but are more intense in the precursor. Peaks in the 1000–1200 cm−1 region, corresponding to C–O stretching in ethers, alcohols, and esters, are prominent in the precursor but largely disappear in the AC spectrum. These changes highlight a substantial reduction in volatile organic compounds and oxygen-containing functional groups during the activation process. A peak at approximately 1500 cm−1, corresponding to aromatic C=C stretching, is more pronounced in the AC spectrum29,30,31. This indicates the development of a conjugated graphitic structure and an increase in the aromatic character of the carbon material due to the carbonization process.
The FTIR analysis underscores the significant chemical transformation of the precursor during activation. A marked reduction in volatile compounds and oxygen-containing functional groups, such as hydroxyl (-OH), along with a decrease in aliphatic groups due to thermal decomposition and carbonization. An enhancement in aromatic character, reflecting the formation of more thermally stable and graphitic carbon structures. These structural inevitably contribute to the enhanced thermal stability, adsorption capacity, and overall performance of the activated carbon.
Scanning electron microscopy (SEM) analysis
The surface morphology of the precursor and the optimized AC was analyzed using SEM images, as shown in Fig. 7. These images reveal distinct morphological transformations induced by the activation process.
The precursor (Fig. 7a, b) exhibits a unidirectional fibrous structure typical of lignocellulosic biomass. At higher magnification, the surface appears rather dense, with no visible pores, indicating minimal inherent porosity and a largely intact native structure prior to activation. In contrast, the optimized AC shows a dramatically altered morphology. Figure 7c and d illustrate a highly porous surface with a network of interconnected pores of varying sizes, uniformly distributed across the carbon matrix. This transition from a dense, fibrous structure to a porous architecture underscores the profound impact of chemical activation.
The observed porosity is primarily a result of the reactions between potassium hydroxide (KOH) and the carbonaceous material during pyrolysis. KOH initially reacts to form potassium carbonate, which further decomposes into metallic potassium and CO2 at elevated temperatures. The evolution of gaseous byproducts creates voids and channels throughout the carbon matrix, thereby generating a highly porous structure32,33,34. The enhanced porosity significantly enhances the pore volume and surface area of the AC, providing abundant active sites for the adsorption of target molecules. Moreover, the interconnected pore network facilitates diffusion pathways for gases, thereby ensuring high adsorption efficiency.
N2 adsorption–desorption isotherm analysis
Isotherm analysis was conducted at 77 K to characterize the textural properties of the high-efficacy adsorbent developed in this work. The adsorption and desorption isotherms are presented in Fig. 8a, with the corresponding numerical data provided in Table S2 in the Supplementary Material. At low relative pressures, a steep rise in nitrogen uptake is observed, indicating rapid filling of micropores. This is followed by a plateau at higher pressures, where further adsorption is minimal, suggesting that the micropores are fully occupied32. According to the IUPAC classification, this behavior corresponds to a Type I isotherm, characteristic of microporous structured materials where adsorption occurs primarily through pore-filling mechanism. A minor hysteresis loop is also evident, indicating the presence of a small proportion of mesopores35.
a N2 adsorption–desorption isotherms at − 196 °C for the optimal activated carbon. b Pore area and pore volume distribution derived from isotherm data using the Dollimore-Heal method. c Relative contribution of pores of different sizes on the overall pore volume and pore area of the optimal AC using the Dollimore-Heal method.
Using the Dollimore-Heal (DH) method, a total pore volume of 0.62 cm3/g and a specific pore area of 1506.3 m2/g were obtained (Fig. 8b). Here, 64% of the pore volume is associated with micropores, while mesopores and macropores contribute 25% and 11%, respectively (Fig. 8c). Owing to their high surface-to-volume ratio, micropores account for 91.7% of the total surface area, with 8.1% and 0.2% attributed to mesopores and macropores, respectively. This distribution highlights the microporous nature of the adsorbent, with the majority of pores having diameters smaller than 2 nm.
Pore volume was also evaluated using the non-local density functional theory (NLDFT) model, which is more accurate for microporous materials due to its ability to account for complex interactions between adsorbate molecules and pore walls. The NLDFT model estimated a pore volume of 0.752 cm3/g, which is over 20% higher than the DH method. According to the NLDFT model, 83.2% of the total pore volume is attributed to micropores, with 76% contributed by micropores smaller than 10.8 Å. The high proportion of micropores has been reported to be particularly advantageous for carbon capture30,33,34,35.
The results of the textural analysis, summarized in Table 9, underscores the effect of the activation process on the evolution of the porosity and surface area. The biomass precursor exhibited a negligible BET surface area, which increased moderately upon pyrolysis to biochar. However, KOH activation led to a dramatic improvement in both pore volume and surface area, highlighting the effectiveness of the activation process. These improvements directly correlate with the high carbon capture efficacy of the optimized AC sample developed in this study.
CO2 Uptake performance
Temperature effect and cyclical stability
The CO2 adsorption isotherms for the precursor (determined at 25 °C) and the optimal AC at 0 °C, 25 °C, and 50 °C are presented in Fig. 9a. A significant improvement in uptake capacity was observed for the optimized AC compared to the precursor. The optimal AC exhibited the highest CO2 adsorption capacity of 4.21 mmol/g at 25 °C and 1 bar, representing a substantial improvement following the activation process. This enhancement can be attributed to the high fraction of micropores in the AC and the elevated nitrogen content (~ 7%) identified via CHNS analysis (Section "CHN elemental analysis"). The CO2 adsorption capacity decreased with increasing temperature, declining from 6.7 mmol/g at 0 °C to 2.6 mmol/g at 50 °C. This temperature dependency indicates that CO2 capture is predominantly governed by physical adsorption, as higher temperatures reduce the adsorptive interactions.
The cyclic stability of the optimized activated carbon (AC) is illustrated in Fig. 9b. The adsorbent was subjected to 10 consecutive adsorption–desorption cycles at 25 °C, with desorption carried out under vacuum (0.01 mbar). The AC demonstrated excellent regeneration capability, retaining its CO2 adsorption capacity with minimal degradation. After a marginal decline of 1.5% observed in the second cycle, the uptake stabilized at approximately 4.11 mmol/g, which remained consistent through the tenth cycle. This high degree of stability underscores its robustness and reusability, making it a promising candidate for practical carbon capture systems. Parshetti et al., also reported strong cyclical stability in CO2 uptake for AC synthesized from palm oil empty fruit bunches36.
Isosteric heat of adsorption (Qst)
Qst is an indication of the strength of interaction between adsorbate molecules and the adsorbent, representing the energy released during adsorption. Higher Qst values indicate stronger interactions, providing a basis to distinguish between physisorption and chemisorption mechanisms.
We calculated Qst using the Clausius–Clapeyron equation (Eq. 2), applied to CO2 uptake data obtained at 0 °C, 25 °C, and 50 °C. As shown in Fig. 10, the analysis was performed at different degrees of surface coverage, which represents the fraction of adsorption sites occupied by CO2 molecules. The adsorption enthalpy is highest (35 kJ/mol) at low coverage (0.2 mmol/g) and gradually decreases to 25 kJ/mol at higher coverage (2.5 mmol/g). This trend is consistent with the typical behavior of Qst in porous materials, where micropores exhibit higher Qst values due to stronger interactions and confinement effects. In micropores, the close proximity of pore walls leads to overlapping potential fields, amplifying van der Waals forces and resulting in higher Qst. Conversely, as pore size increases, these interactions weakens, leading to lower Qst values for mesopores and macropores.
The observed decline in Qst with increasing coverage is related to the surface heterogeneity of the adsorbent. At low coverage, CO2 molecules preferentially bind to the most energetically favorable sites, typically within micropores. As these high-energy sites become saturated, adsorption shifts to less favorable sites with weaker interactions, resulting in a decline in Qst. This behavior suggests that adsorption initiates in micropores before shifting to larger mesopores and macropores, reflecting the heterogeneous and hierarchical nature of the activated carbon’s porous matrix37,38. Overall, Qst confirms the strong interaction between CO2 and the biomass-derived adsorbent developed in this study. This characteristic is critical for achieving high CO2 adsorption capacities observed in this investigation.
Performance benchmarking for carbon capture
The performance of activated carbon samples prepared from various agro-waste precursors and activated using KOH is summarized in Table 10. Using date-palm leaflets as a precursor, we achieved CO2 uptake values of 6.7 mmol/g at 0 °C, 4.2 mmol/g at 25 °C, and 2.6 mmol/g at 50 °C. These results are comparable to those of fern-leaf-derived activated carbon (pyrolyzed at 700 °C), particularly at 0 °C37. However, at ambient conditions, date-palm leaflets exhibit over 15% higher adsorption capacity compared to fern leaves. It is important to note that a combined physical and chemical activation process was employed for the production of the activated carbon from fern-leaf as against single chemical activation in this study. As previously mentioned, the unusually high CO2 uptake values of biochar in fluidized bed systems are excluded from this analysis.22. The CO2 capture performance achieved in this study ranks among the highest reported for biomass-derived activated carbons. These results highlight the potential of date-palm waste-derived leaflets as a low-cost, sustainable, and efficient adsorbent for carbon capture applications.
Conclusion
This study successfully developed high-efficacy AC from waste date palm leaflet for CO2 adsorption through the process optimization. Using response surface methodology (RSM), the influence of temperature, residence time, and KOH/C mass ratio on CO2 uptake were investigated for over 20 different AC samples. A Central Composite Design (CCD) approach was employed, examining the effects of three different temperatures (500, 600, and 700 °C), three residence times (1.5, 2.5, and 3.5 h), and three impregnation ratios (2, 3, and 4). The predicted optimal conditions, i.e., 670 °C, 1.5 h residence time, and an impregnation ratio of 2.7, yielded a CO2 uptake of 4.21 mmol/g. Experimentally, the highest CO2 uptake (4.21 mmol/g at 25 °C) was obtained with AC prepared at 700 °C for 1.5 h residence time with an impregnation ratio of 3, showing excellent agreement with the predicted response of 4.12 mmol/g under the same process conditions.
The optimized AC outperformed other agro-waste-derived ACs, including those derived from other fractions of date-palm waste, demonstrating superior CO2 capture capabilities. This superior performance is attributed to its highly microporous structure, with micropores accounting for 83.2% of the total pore volume (0.752 cm3/g). More specifically, pores smaller than 10.8 Å constituted 76% of the total pore volume, providing abundant, high-affinity binding sites for CO2 molecules. Moreover, the high isosteric heat of adsorption (35 kJ/mol at low coverage of 0.2 mmol/g) further confirmed the energetic heterogeneity and preferential micropore filing, further confirming strong adsorbent-adsorbate interaction.
In addition, the adsorbent showed excellent stability over multiple adsorption–desorption cycles, underscoring its robustness for practical applications. The temperature sensitivity of the CO2 uptake (6.7 mmol/g at 0 °C, 2.6 mmol/g at 50 °C) highlights its suitability for temperature-swing adsorption systems. This study establishes the potential of date-palm waste-derived leaflets as a sustainable precursor for high-performance activated carbon, offering an efficient and sustainable solution for CO2 capture.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Overview of Greenhouse Gases [Internet]. U.S. Environmental Protection Agency. 2025. Available from: https://www.epa.gov/ghgemissions/overview-greenhouse-gases.
Wimbadi, R. W. & Djalante, R. From decarbonization to low carbon development and transition: A systematic literature review of the conceptualization of moving toward net-zero carbon dioxide emission (1995–2019). J. Clean. Prod. 256, 120307 (2020).
Kheirinik, M., Ahmed, S. & Rahmanian, N. Comparative techno-economic analysis of carbon capture processes: Pre-combustion, post-combustion, and oxy-fuel combustion operations. Sustainability 13, 13567 (2021).
Gunawardene, O. H. P., Gunathilake, C. A., Vikrant, K. & Amaraweera, S. M. Carbon dioxide capture through physical and chemical adsorption using porous carbon materials: A review. Atmosphere 13, 397 (2022).
Mac Dowell, N., Fennell, P. S., Shah, N. & Maitland, G. C. The role of CO2 capture and utilization in mitigating climate change. Nat. Clim. Chang. 7, 243–249. https://doi.org/10.1038/nclimate3231 (2017).
Understanding carbon capture and storage [Internet]. British Geological Survey. 2025. Available from: https://www.bgs.ac.uk/discovering-geology/climate-change/carbon-capture-and-storage/#:~:text=CCS%20involves%20capturing%20carbon%20dioxide,a%20suitable%20deep%2C%20underground%20location.
Singh, G. et al. Emerging trends in porous materials for CO2 capture and conversion. Chem. Soc. Rev. 49, 4360–4404. https://doi.org/10.1039/D0CS00075B (2020).
Shao, X. et al. Renewable N-doped microporous carbons from walnut shells for CO2 capture and conversion. Sustain. Energy Fuels 5, 4701–4709. https://doi.org/10.1039/D1SE01000J (2021).
Fu, D. & Davis, M. E. Carbon dioxide capture with zeotype materials. Chem. Soc. Rev. 51, 9340–9370. https://doi.org/10.1039/D2CS00508E (2022).
Dziejarski, B., Serafin, J., Andersson, K. & Krzyżyńska, R. CO2 capture materials: A review of current trends and future challenges. Mater. Today Sustain. 24, 100483 (2023).
Sharma, A. et al. Carbon materials as CO2 adsorbents: A review. Environ. Chem. Lett. 19, 875–910. https://doi.org/10.1007/s10311-020-01153-z (2021).
Abuelnoor, N., AlHajaj, A., Khaleel, M., Vega, L. F. & Abu-Zahra, M. R. M. Activated carbons from biomass-based sources for CO2 capture applications. Chemosphere 282, 131111 (2021).
Nasser, R. A. et al. Chemical analysis of different parts of date palm (Phoenix dactylifera L.) using ultimate, proximate and thermo-gravimetric techniques for energy production. Energies 9, 374 (2016).
Alsulami, R. A. et al. Thermal decomposition characterization and kinetic parameters estimation for date palm wastes and their blends using TGA. Fuel 334, 126600 (2023).
Sarwar, A. et al. Synthesis and characterization of biomass-derived surface-modified activated carbon for enhanced CO2 adsorption. J CO2 Utilizat 46, 101476 (2021).
Khuong, D. A., Nguyen, H. N. & Tsubota, T. Activated carbon produced from bamboo and solid residue by CO2 activation utilized as CO2 adsorbents. Biomass Bioenerg. 148, 106039 (2021).
Rahimi, V., Ferreiro-Salgado, A., Gómez-Díaz, D., Sonia Freire, M. & González-Álvarez, J. Evaluating the performance of carbon-based adsorbents fabricated from renewable biomass precursors for post-combustion CO2 capture. Sep. Purif. Technol. 344, 127110 (2024).
Al-Wasidi, A. S. et al. Adsorption studies of carbon dioxide and anionic dye on green adsorbent. J. Mol. Struct. 1250, 131736 (2022).
Iranvandi, M., Tahmasebpoor, M., Azimi, B., Heidari, M. & Pevida, C. The novel SiO2-decorated highly robust waste-derived activated carbon with homogeneous fluidity for the CO2 capture process. Sep. Purif. Technol. 306, 122625 (2023).
Al-Fidda, S. A., Abu Ayana, R. A., editors. Date Palm by-products, Their types and economic. Second Edition ed: Saleh Al-Rajhi Endowments; 2016.
Hussain, A. et al. Pyrolysis of Saudi Arabian date palm waste: A viable option for converting waste into wealth. Life Sci. J. 11, 667–671 (2014).
Ben Salem, I., El Gamal, M., Sharma, M., Hameedi, S. & Howari, F. M. Utilization of the UAE date palm leaf biochar in carbon dioxide capture and sequestration processes. J. Environ. Manage. 299, 113644 (2021).
Wei, H. et al. Granular bamboo-derived activated carbon for high CO2 adsorption: The dominant role of narrow micropores. Chemsuschem 5, 2354–2360. https://doi.org/10.1002/cssc.201200570 (2012).
Haghbin, M. R. & Niknam Shahrak, M. Process conditions optimization for the fabrication of highly porous activated carbon from date palm bark wastes for removing pollutants from water. Powder Technol. 377, 890–899 (2021).
Zhang, Y. et al. Synthesis of palm sheath derived-porous carbon for selective CO2 adsorption. RSC Adv. 12, 8592–8599. https://doi.org/10.1039/D2RA00139J (2022).
Deng, S. et al. Superior CO2 adsorption on pine nut shell-derived activated carbons and the effective micropores at different temperatures. Chem. Eng. J. 253, 46–54 (2014).
Aboud, M. F. A., Alothman, Z. A. & Bagabas, A. A. Storage and separation of methane and carbon dioxide using platinum-decorated activated carbons treated with ammonia. Mater. Res. Exp. 8, 025503. https://doi.org/10.1088/2053-1591/abe2e0 (2021).
Zhang, C. et al. Enhancement of CO2 capture on biomass-based carbon from black locust by KOH activation and ammonia modification. Energy Fuels 30, 4181–4190. https://doi.org/10.1021/acs.energyfuels.5b02764 (2016).
Serafin, J., Dziejarski, B., Cruz Junior, O. F. & Sreńscek-Nazzal, J. Design of highly microporous activated carbons based on walnut shell biomass for H2 and CO2 storage. Carbon 201, 633–647 (2023).
Serafin, J., Dziejarski, B. & Sreńscek-Nazzal, J. An innovative and environmentally friendly bioorganic synthesis of activated carbon based on olive stones and its potential application for CO2 capture. Sustain. Mater. Technol. 38, e00717 (2023).
Peres, C. B., Morais, LCd. & Resende, P. M. R. Carbon adsorption on waste biomass of passion fruit peel: A promising machine learning model for CO2 capture. J. CO Utilizat. 80, 102680 (2024).
Gan, Y. X. Activated carbon from biomass sustainable. Sources C 7:39. https://www.mdpi.com/2311-5629/7/2/39. (2021)
Nandi, R., Jha, M. K., Guchhait, S. K., Sutradhar, D. & Yadav, S. Impact of KOH activation on rice husk derived porous activated carbon for carbon capture at flue gas alike temperatures with high CO2/N2 selectivity. ACS Omega 8, 4802–4812. https://doi.org/10.1021/acsomega.2c06955 (2023).
Guo, Y. et al. Recent advances in potassium-based adsorbents for CO2 capture and separation: A review. Carbon Capture Sci. Technol. 1, 100011 (2021).
Thommes, M. et al. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 87, 1051–1069. https://doi.org/10.1515/pac-2014-1117 (2015).
Parshetti, G. K., Chowdhury, S. & Balasubramanian, R. Biomass derived low-cost microporous adsorbents for efficient CO2 capture. Fuel 148, 246–254 (2015).
Serafin, J., Dziejarski, B., Vendrell, X., Kiełbasa, K. & Michalkiewicz, B. Biomass waste fern leaves as a material for a sustainable method of activated carbon production for CO2 capture. Biomass Bioenerg. 175, 106880 (2023).
Huang, G.-G., Liu, Y.-F., Wu, X.-X. & Cai, J.-J. Activated carbons prepared by the KOH activation of a hydrochar from garlic peel and their CO2 adsorption performance. New Carbon Mater. 34, 247–257 (2019).
Khoshraftar, Z. & Ghaemi, A. Preparation of activated carbon from Entada Africana Guill. & Perr for CO2 capture: Artificial neural network and isotherm modeling. J. Chem. Petrol. Eng. 56, 165–180 (2020).
He, S. et al. Facile preparation of N-doped activated carbon produced from rice husk for CO2 capture. J. Colloid Interface Sci. 582, 90–101 (2021).
Pimentel, C. H., Díaz-Fernández, L., Gómez-Díaz, D., Freire, M. S. & González-Álvarez, J. Separation of CO2 using biochar and KOH and ZnCl2 activated carbons derived from pine sawdust. J. Environ. Chem. Eng. 11, 111378 (2023).
Acknowledgements
This project was funded by the National Plan for Science, Technology, and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, Award number 13-ENV2085-02.
Author information
Authors and Affiliations
Contributions
E.H.A. and M.A. wrote the main manuscript; E.H.A. carried out experimental and characterization tasks; M.A. supervised the research work and procured the project funding; M.B. assisted with project funding; W.A. facilitated characterization tasks. All authors reviewed the manuscript."
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Al-Ghurabi, E.H., Boumaza, M.M., Al-Masry, W. et al. Optimizing the synthesis of nanoporous activated carbon from date-palm waste for enhanced CO2 capture. Sci Rep 15, 17132 (2025). https://doi.org/10.1038/s41598-025-00498-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-00498-1
Keywords
This article is cited by
-
Biomass-derived activated carbon: a review on process parameters, material properties, and machine learning approaches for supercapacitor
Journal of Materials Science: Materials in Electronics (2026)
-
Eco-friendly tassel-derived activated carbon for efficient dye removal in wastewater treatment
Scientific Reports (2025)
-
Analysis of the CO2 adsorption on AC: experimentation and statistical studies
Scientific Reports (2025)