Abstract
This study explores the efficacy of glibenclamide, a KATP channel inhibitor, for treating Cantú syndrome (CS), a genetic disorder characterized by hypertrichosis and cardiovascular abnormalities. Treatment with glibenclamide for Cantú syndrome has only been reported in a single case report. In this study, we tested this repurposed drug in both a zebrafish model and an open-label trial with CS patients. CS zebrafish embryos, created using CRISPR/Cas9, were treated with glibenclamide. Their cardiac function was assessed using high-speed imaging. In the trial part of the study, four adults with CS used 2.5 mg glibenclamide daily for 8 months. Hypertrichosis, cardiac function, and edema were evaluated and glucose levels were monitored continuously. In the zebrafish model of CS glibenclamide reversed cardiac abnormalities. However, in the clinical trial, the effects on hypertrichosis were mixed, and there were no significant changes in cardiac phenotype or leg edema. One participant reported reduced facial erythema and puffiness, which relapsed post-trial. The treatment was generally safe, with multiple instances of level 1 hypoglycemia but no severe adverse events. In conclusion, glibenclamide can reverse cardiac abnormalities in a CS zebrafish model. Its effect on hypertrichosis and cardiovascular features in humans with CS are unclear and dosage increases are challenging due to hypoglycemia, which is important knowledge for treatment considerations in this rare genetic syndrome.
Trial registration: EudraCT Number 2019-004651-36. Date of first registration 21/05/2021.
Similar content being viewed by others
Introduction
Cantú syndrome (CS) is a rare genetic disorder characterized by congenital hypertrichosis and macrosomia, coarse facial features, peripheral edema, and cardiac abnormalities. CS (OMIM#239850) is caused by heterozygous pathogenic variants in the ABCC9 or KCNJ8 genes1. These genes encode subunits of the ATP-sensitive potassium (KATP) channel, a critical regulator of cellular excitability. KATP channels are potassium-selective ion channels composed of four pore-forming Kir6.x subunits (Kir6.1 or Kir6.2 encoded by KCNJ8 and KCNJ11, respectively) and four regulatory sulfonylurea receptor SURx subunits (subfamily C: SUR1, SUR2 encoded by ABCC8 and ABCC9, respectively)2. KATP channels play a pivotal role in maintaining cellular homeostasis by regulating the flow of potassium ions across the cell membrane, thereby influencing membrane potential and cell excitability. In CS, gain-of-function variants in ABCC9 and KCNJ8 result in overactive KATP channels. The vast majority of CS patients has a pathogenic variant in ABCC9 (SUR2 subunit).
A key feature of CS is hypertrichosis, which is likely attributed to the dysregulated hair follicle development caused by KATP channel dysfunction in skin cells3. Another important feature is cardiomegaly, which stems from abnormal relaxation of the cardiac muscle due to the overactive KATP channels in cardiomyocytes. This results in an increased influx of calcium ions, impaired contractility, and ultimately, the development of a high-output cardiac hypertrophy4. Other reported cardiovascular anomalies encompass congenital structural defects, collateral vessels, tortuosity, arteriovenous malformation, and a dilated aortic root5.
CS phenotypes are recapitulated in animal models for the syndrome, mouse models for CS showed low systemic blood pressure, dilated blood vessels and cardiac enlargement [ref PMID: 30089727] and zebrafish model showed cardiac enlargement and vascular changes [ref PMID: 30355756]. The external development combined with the transparent nature of zebrafish larvae allows for real-time cardiovascular imaging in living animals, making the zebrafish CS model a rapid and cost effective model for drug testing.
Currently, no specific therapy is available for CS. However, there is evidence that sulfonylureas, such as glibenclamide, may mitigate CS-related abnormalities by closing the overactive potassium channels present in patients6. Glibenclamide is already utilized in the treatment of diabetes due to missense mutations in ABCC8 (MODY diabetes). Upon binding to the sulfonylurea receptor (SUR) subunit of the KATP channel, glibenclamide inhibits channel activity and reduces the potassium efflux and hyperpolarization. There is one report of a child with CS that was treated with glibenclamide from the age of 11 weeks7. His edema and pulmonary hypertension improved, and at the age of 13 months he had no cardiomegaly. This could be due to the glibenclamide treatment, but this is unsure since cardiac enlargement is not reported in all CS cases (64%)5. His hypertrichosis however did not clearly improve.
The main safety concern with glibenclamide treatment is hypoglycemia. Glibenclamide has inhibitory action on pancreatic Kir6.2/SUR1-dependent KATP channels, whereas the CS genes encode for Kir6.1/SUR2 channels. There is a potential risk that glibenclamide treatment for hypertrichosis and cardiovascular problems will be limited by a larger effect of the drug on blood glucose levels. Therefore, this bench-to-bedside exploratory study is also designed to assess the feasibility, safety, and justifiability of conducting a large, randomized placebo-controlled trial to evaluate the efficacy of glibenclamide in CS patients. With careful monitoring and instructions to minimalize hypoglycemia risk, the present study aims to provide insights into the potential benefits of glibenclamide in CS patients.
Materials and methods
Part 1 – Bench: treatment with Glibenclamide in zebrafish model of Cantú syndrome
CS zebrafish were generated using CRISPR/Cas9-based nucleotide editing as previously described8.
In brief, CRISPR-Cas9 genome editing was combined with a short template oligonucleotide to introduce a single nucleotide mutation into abcc9. The experimental protocols was approved by the local ethical committee (UMC Utrecht) and the experiments were performed in accordance under the guidelines of the animal welfare committee of the Royal Netherlands Academy of Arts and Sciences (KNAW) and the Washington University Institutional Animal Care and Use Committee. The methods are reported, also in the supplementary appendix and our previous publication, in accordance with ARRIVE guidelines. The zebrafish were Tüpfel long fin (source: European Zebrafish Resource Center, ZDB-GENO-990623-2). The created zebrafish line was abcc9-associated (Sur2[G989E]). All embryos analyzed originated from group matings of adult heterozygous Sur2[G989E]: GCaMP6f CS zebrafish. Drug delivery was achieved by soaking with glibenclamide. We exposed CS fish to glibenclamide (dissolved in dimethyl sulfoxide, DMSO) for a period of 96 h starting at 1 day post fertilization (dpf). Drug stocks were made in medium with glibenclamide 50 µM. Final KATP blocker concentrations were determined by establishing concentration-dependent survival curves in wildtype fish over 4 days of exposure. Drug concentrations that resulted in > 70% of healthy fish were selected for treatment of CS fish. Zebrafish treated with DMSO (glibenclamide 1%) were used as a vehicle control, to ensure no cardiac modifications were being caused by DMSO alone. Untreated zebrafish were used to confirm that the vehicle solvent did not have an adverse effect.
Thanks to its optical clarity and simple yet functionally relevant heart structure, cardiac function is easily monitored in zebrafish larvae. After treatment, cardiac function of 5 dpf zebrafish was examined using high-speed imaging)8.
Five dpf larvae were randomly selected from control and treatment group and subjected to live high-speed video imaging to capture at least three cardiac cycles of the beating zebrafish larval heart. Cardiac output (CO), ejection fraction (EF) and diastolic end volume (DEV) were assessed and applied as readout for drug efficacy. We also assessed the effects on glucose of chronic glibenclamide exposure in the CS larvae and control larvae. We used a highly sensitive glucose assay to measure absolute glucose levels in larval lysates as a proxy for blood glucose. For detailed methods, see the supplemental appendix.
Part 2 – Bedside: treatment with Glibenclamide in humans with Cantú syndrome
This exploratory study was an open-label, single-arm, non-randomized, uncontrolled intervention study, given the rarity of CS. Participants served as their own controls, and no control group was included. The study lasted for 8 months. Baseline measurements were conducted before the start of glibenclamide treatment. This study was conducted in accordance with the relevant guidelines and principles of the Declaration of Helsinki. The study protocol was approved by our local Ethical Committee (Amsterdam UMC). All participants gave informed consent, in writing and dated, before enrollment in the study.
Study population
Participants were recruited from a cohort of Dutch CS patients who have attended annual research clinics and are enrolled in the International Registry for CS5. Eligible participants were required to have a confirmed molecular diagnosis of CS, with an ABCC9 (likely) pathogenic variant, be 16 years or older, and without history of sulfonamide allergy. The participants could not be (trying to become) pregnant during the trial.
Primary and secondary objectives
In recent years, our research group held patient sessions on this rare disorder. Patients highlighted hypertrichosis as a major concern due to its psychological impact. Therefore, our primary objective was to determine whether glibenclamide treatment can reduce hypertrichosis. Secondary objectives include assessing safety and collecting further evidence of efficacy in treating various CS-related abnormalities.
Intervention
The study drug glibenclamide, an oral glucose lowering agent, was administered to the participants. The initial dose for CS participants, who do not have diabetes, was 1.25 mg per day increasing to 2.5 mg daily if tolerated.
Safety measures
The medical history and medication use was collected prior to the start of the trial. Continuous glucose monitoring was implemented to monitor glucose values and hypoglycemia (FreeStyle Libre 2, Abbott). Dose adjustments could also be made based on renal function, for which serum creatinine was measured at each follow-up visit. Adverse events were systematically collected and participants could contact the involved physicians about adverse events during the study.
Participants were advised to set the low glucose alarm at 3.5 mmol/l during the dose escalation period. Patients were instructed on the symptoms of hypoglycemia to be able to recognize an hypoglycemic event, as well as treatment of hypoglycemia. Symptomatic hypoglycemia was defined as having symptoms of low blood glucose (i.e. palpitations, sweating, pallor, reduced consciousness), combined with a sensor glucose measurement of < 4 mmol/l with resolution of symptoms after carbohydrate ingestion (Whipple’s triad).
Statistics
Nonparametric Wilcoxon signed-rank tests were used to evaluate differences in clinical outcomes between baseline and the end of the study. The sample size was not based on power analysis and was determined by feasibility for this first proof-of-concept study.
Design of the specific measurements during the trial
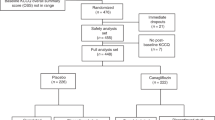
After undergoing baseline measurements (visit 1), participants started glibenclamide. The participants visited the trial site at Amsterdam UMC in total 8 times during the study (Fig. 1).
1. Dermatology and pathology: evaluation of hypertrichosis and skin
Skin and hair were evaluated at multiple time points during the study by a dermatologist. Dermoscopy pictures were taken and evaluated. Scalp/forehead biopsy was performed as described in Ohko et al.9.
Cardiology
All participants underwent echocardiographic examination, cardiac magnetic resonance imaging (CMR), and a resting and cardiac exercise stress ECG. Heart rate and blood pressure were measured at every visit. Using echocardiography (GE System, Vivid E95, 1.6- to 3.2-MHz transducer), left ventricular end diastolic volume (LVEDV), left ventricular ejection fraction (LVEF), and left ventricle global longitudinal strain (GLS) were measured. Right ventricular systolic pressure (RVSP), diastolic function, and cardiac output (CO) were measured. All CMR exams were acquired using a 1.5T MRI scanner (MAGNETOM Avanto Fit, Siemens Healthineers). Acquisition included standard clinical sequences for assessment of cardiac volumes and function, vascular anatomy and T1-mapping and late gadolinium enhancement. Abnormal native T1 values were defined as greater than 950–1050 ms, based on previously derived local institutional sequence-specific cutoffs of 2 SDs above the mean in a healthy population.
Edema
Before glibenclamide treatment and during every visit patients were asked about possible treatment they receive for leg edema. Edema was measured using an adapted method of Kuhnke10, where measurement of leg circumference by tape is taken at the malleolar level and every 10 cm for eight leg segments.
Questionnaires
Quality of life was assessed using the 5-level EQ-5D version (EQ-5D-5 L) questionnaire at each visit11. This questionnaire evaluates five parts of health (mobility, self-care, daily activities, pain/discomfort, and anxiety/depression) and asks for participants to rate their overall health using a vertical visual analog scale marked from 0 to 100. A higher score means a better overall health rating. They were compared to the scores from the Dutch population12. A questionnaire about the emotional impact of hypertrichosis had been made for this study (supplementary appendix) and was filled in at each visit.
Results
Part 1 – Bench: treatment with Glibenclamide in zebrafish model of Cantú syndrome
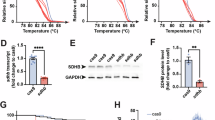
CS zebrafish (Fig. 2A) were generated using CRISPR/Cas9-based nucleotide editing. We exposed CS fish to glibenclamide. The Sur2wt/GE fish showed significantly decreased EF (~ 25%) and cardiac output (CO) (~ 25%) in the presence of glibenclamide due to equally lowered stroke volume with stable heart rate, almost restoring wildtype condition (Fig. 2B-E). Exposure to 1% DMSO vehicle did not modify cardiac function and resulted in similar values to those in untreated fish.
Hypoglycemia as side effect of glibenclamide treatment in zebrafish
High doses of glibenclamide, as are required to correct CS-related phenotypes, would not only block CS KATP channels but also pancreatic channels, leading to elevated insulin secretion and decreased blood glucose. Consequently, hypoglycemia is predicted as the most likely adverse effect of glibenclamide when applied in non-diabetic patients, potentially limiting clinical application for CS. Thus, we assessed the effects of chronic glibenclamide in zebrafish. Glibenclamide exposure resulted in an obvious but not significant decrease in whole-body glucose (WBG) that failed to fully normalize after day 4 of treatment although an upwards trend was visible (Fig. 2E). Control larvae revealed WBG levels in accordance with previously published studies demonstrating a peak at 1 dpf followed by a significant decrease at 3dpf due to the formation of pancreatic β-cells islets and an associated increase in pancreatic insulin expression13,14. We cannot exclude that the hypoglycemia itself has an effect on the cardiac phenotype in the zebrafish study.
Glibenclamide corrects CS-related cardiac phenotype in Sur2wt/GE larvae. (A) Sur2wt/GE larvae were exposed to various KATP inhibitors over a period of 96 h starting at 1dpf. Larvae treated with DMSO were used as a vehicle control. (B-D) CS-associated cardiac features, namely ejection fraction, cardiac output and end-diastolic volume were assessed in Sur2wt/GE larvae after GLB treatment using high-speed video imaging. For all graphs, significance was determined by one-way ANOVA and subsequent post-hoc Tukey’s test for pairwise comparison: * p<0.05; ** p<0.01; *** p<0.001; **** p<0.0001. Data shown as mean ± SEM. Sample size: (B-D) wt/wt(-), n = 10; wt/wt(+), n = 16; wt/GE(-), n = 19; wt/GE(+), n = 41; All embryos analyzed originated from group matings of adult Sur2wt/GE zebrafish. (E) Chronic exposure to glibenclamide reduces whole-body glucose levels in CS zebrafish larvae. Absolute glucose levels in larval lysates consisting of a pool of 10 larvae each were measured applying a highly sensitive glucose assay kit. Glucose was measured in WT fish and fish prior to glibenclamide exposure at 1 dpf and after at 3 and 5 dpf. Mean whole-body glucose measurements from WT and Sur2wt/GE control and GLB treatment group. Additionally, absolute glucose levels of WT fish grown in E3 medium are shown.
Part 2 – Bedside: treatment with glibenclamide in humans with Cantú syndrome
Participant data
The trial was conducted from January 2023 until September 2023. We currently know 12 patients with CS in The Netherlands. The original recruitment target was set at a maximum of 7 participants that could meet the inclusion criteria in our Dutch cohort, as five of our CS patients are younger than 18 years old. Of the seven patients asked to participate, five were willing to be enrolled. Four patients were eventually enrolled and met the inclusion criteria; one patients was excluded because of pregnancy. Patient characteristics and baseline measurements are shown in Table 1.
Hypertrichosis and skin
There were no clear changes in hypertrichosis in the participants on clinical observation. The self-reported evaluation of the participants at the last visit differed. One participant noted no change in hair (growth/thickness) at all. One participant (case 2) noted that her hair became thinner and lighter. On some parts of her legs there were no more hairs visible. The two other participants experienced hair loss or thinning of scalp hair, but no effect on the hairs on the arms, legs, or face. One of the participants (case 2) noticed less facial erythema and that her face appeared less ‘puffy’ at the end of the trial, which both returned after stopping the medication.
Skin biopsy results
Skin biopsies taken from the scalp at the frontal hairline of the four patients revealed no distinct differences in the samples collected before and after treatment. The results of both biopsies of one patient (case 2) are depicted in Fig. 3, as an example to show that most of the hair follicles in both samples were in the anagen phase (the active growth phase of the hair follicle). A sporadic vellus hair follicle was also observed at both time points. The biopsy before treatment additionally revealed one telogen hair follicle, but this can be considered within the spectrum of physiological variation. The observations in the biopsies before and after treatment cannot confirm the self-reported evaluations of three participants who noted that their scalp hair had become thinner. In particular, there was no observable shift in the hair cycle towards the catagen or telogen phase, nor an increase in vellus hairs after treatment. These findings are consistent with the observations of the dermatologists, who concluded that there were no clear changes in hypertrichosis in the participants at the end of the trial.
The effects in the skin biopsies. Histology of skin biopsies before (I) and after (II) treatment, taken from the scalp at the hairline of case 2 (H&E stained, 100x), reveals essentially identical features, as assessed by the pathologist at our center, who has experience in evaluating such skin biopsies, in collaboration with the involved dermatologists. The hair follicles in both samples are predominantly in the anagen phase (A). In the biopsy before treatment (I), one hair follicle in the telogen phase (T) and one vellus hair follicle (V) are also visible.
Safety assessment
No severe adverse events occurred. The adverse events are listed in the supplementary appendix. One participant (participant 2) needed dosage adjustment (from 2.50 mg to 1.25 mg once daily), because of symptomatic hypoglycemia. For one participant (participant 1) the medication schedule was changed from 2.50 mg daily to 1.25 mg twice a day because of hypoglycemia after medication ingestion. After dose adjustment, no hypoglycemic event occurred. There was no uniform effect of glibenclamide treatment on weight development. Two participants gained weight (both + 0,5 kg/m2 increase in BMI), and two participants lost weight (-0,7 kg/m2 and − 0,1 kg/m2 respectively). There were no changes in renal function (mean change in estimated glomerular filtration rate of + 1 mL/min/1.73m2).
Cardiovascular effects
Upon echocardiographic evaluation all subjects showed signs of a high-output cardiac phenotype with dilated atria and ventricles. The average echocardiography LVEF in our participants was 64%. Average MRI LVEF was 60,3%. The diastolic function was normal in the participants. In line with recent observations, we found an overall increased global longitudinal strain4. Additional measurements showed a decreased post-systolic contraction of the lateral wall in all patients. Cardiovascular magnetic resonance (CMR) imaging showed increased T1 values throughout the myocardium. At the last visit, echocardiography and CMR were repeated. In general, no effect of the glibenclamide treatment was seen on the cardiovascular imaging and measurements (Table 2). No significant changes were seen for LVEDV, LVEF, RVSP, CO and in aortic root diameter. The pericardial effusion seen on CMR and ultrasound remained present for all participants.
Edema
As expected, we saw fluctuations of leg edema during the trial, which were mainly related to warm weather or increased standing time in daily life. There were no clear clinical differences in edema measurements before the glibenclamide use and at the end of the trial (mean difference of 0.5 cm).
Quality of life
No clear differences were seen in quality of life, but especially no improvement (see Supplementary Table 2). For one case, the EQ-5D-5 L scores were lower because of worse scores on mobility and pain.
Discussion While the genetic basis of CS was established almost a decade ago, targeted treatment has remained elusive. By directly targeting over-active KATP channels using KATP channel inhibitors, it may be possible to prevent or reverse consequences of KATP overactivity. In our CS zebrafish model, glibenclamide significantly corrected the cardiac phenotype. These data suggested that glibenclamide was a promising candidate for drug repurposing. During the first human trial with glibenclamide for Cantú syndrome, four patients with this rare disorder were extensively evaluated for treatment effects and safety. Three out of four participants reported changes in hair growth at the end of the trial. However, these observations could not be uniformly confirmed by clinical observations of the hair and by skin biopsies. One participant noted that her facial erythema and edema improved, but this observation could not be made in the other three participants. The study data showed no clear improvements in cardiovascular abnormalities, leg edema, or quality of life. Based on our findings, healthcare providers should meticulously weigh the therapeutic benefits of this medication in CS against its potential adverse effects.
The data of our four participants add to the scarce knowledge about glibenclamide therapy for CS. There is one young child reported with glibenclamide treatment, which showed no improvement of the hypertrichosis, but possibly glibenclamide-related improvement of the cardiovascular problems7. Our data show no clinically significant changes in the cardiac phenotype of our participants. A reason for this difference might be the age of the patients, as it was already suggested from murine studies that a more severe phenotype could warrant longer administration times for reversal of the cardiovascular abnormalities6. In CS, the biventricular dilation with preserved ejection fraction and without diastolic dysfunction can result in a high-output heart failure phenotype4. All of our studies subjects already showed signs of a high-output cardiac phenotype with dilated atria and ventricles. These features were milder in the case report of the treated young child7. We hoped that our younger patients would show a greater improvement (or slowed-down progression of disease) than our adult patients, but unfortunately we did not see these effects in our study. For future studies, one might suggest to increase the dosage of glibenclamide. Calculating a human equivalent dose based on drug treatment in zebrafish is not straightforward. Another study with CS mice used 1 mg/kg/day glibenclamide doses for 4 weeks which resulted in partial reversal of cardiac hypertrophy6. The human equivalent dose is 0.05–0.07 mg/kg/day, so our participants could have needed a higher dosage17 However, increasing the dosage does not seem feasible in most CS patients, as symptomatic hypoglycemic events occurred in three of our four participants, for which one participant needed to lower the daily dosage. On the other hand, it is not clear yet whether increasing the glibenclamide dosage is beneficial, as its effect on the K-channel could become saturated. This is suggested by findings from Rambiritch et al., who reported that a dosage higher than 2.5 mg does not further enhance insulin production beyond what is achieved with 2.5 mg18. Consequently, it is possible that no additional effect would be expected on our outcomes in CS patients either when given higher dosages than 2.5 mg.
With trials in rare diseases, it remains difficult to perform statistics and find significant effects for an individual patient. For a future study, we would recommend an n-of-1 study design to better evaluate the results per participant. Another limitation is that we are unsure whether the duration of the study was adequate to see results like improvement or reversal of the cardiomegaly. Participant 2 expressed a desire to continue the medication due to the positive effects she experienced. In consultation with the involved internal medicine physician, she now receives off-label glibenclamide, with regular evaluations of the effects. This off-label treatment could give us more insight on the long-term effects on glibenclamide on the cardiac phenotype. We do not know why only participant 2 experienced benefit from the treatment, even though she received the lowest dosage of glibenclamide. We are planning to perform pharmacogenetic testing to look into this further, but this was not included in the current study protocol. As glibenclamide is primarily metabolized in the liver via CYP2C919, which exhibits significant variability in the population, this could be one of the causes for the difference in effect.
Another large limitation of this study is the fact that glibenclamide does not specifically target the subunits of the potassium channels that are affected in CS. Treatment for CS may ultimately necessitate an agent possessing significantly enhanced potency and selectivity targeting the VSM Kir6.1/SUR2B channels that are affected in this syndrome. Our zebrafish data show that CS zebrafish larvae can be successfully used for testing the efficacy of further KATP channel inhibitors as potential CS therapy options..
Data availability
The data are available from the corresponding author upon reasonable request, with consideration for patient privacy. The zebrafish study was supported by the E-Rare Joint Transnational Cantú Treat program (I-2101-B26 to Van Haaften).
References
Grange, D. K. et al. in GeneReviews((R)) (eds M. P. Adam (1993).
Martin, G. M. et al. Cryo-EM structure of the ATP-sensitive potassium channel illuminates mechanisms of assembly and gating. Elife 6 https://doi.org/10.7554/eLife.24149 (2017).
Nichols, C. G., Singh, G. K. & Grange, D. K. KATP channels and cardiovascular disease: suddenly a syndrome. Circ. Res. 112, 1059–1072. https://doi.org/10.1161/CIRCRESAHA.112.300514 (2013).
Singh, G. K. et al. A unique High-Output cardiac hypertrophy phenotype arising from low systemic vascular resistance in Cantu syndrome. J. Am. Heart Assoc. 11, e027363. https://doi.org/10.1161/JAHA.122.027363 (2022).
Grange, D. K. et al. Cantu syndrome: findings from 74 patients in the international Cantu syndrome registry. Am. J. Med. Genet. C Semin Med. Genet. 181, 658–681. https://doi.org/10.1002/ajmg.c.31753 (2019).
McClenaghan, C. et al. Glibenclamide reverses cardiovascular abnormalities of Cantu syndrome driven by KATP channel overactivity. J. Clin. Invest. 130, 1116–1121. https://doi.org/10.1172/JCI130571 (2020).
Ma, A. et al. Glibenclamide treatment in a Cantu syndrome patient with a pathogenic ABCC9 gain-of-function variant: initial experience. Am. J. Med. Genet. A. 179, 1585–1590. https://doi.org/10.1002/ajmg.a.61200 (2019).
Tessadori, F. et al. Effective CRISPR/Cas9-based nucleotide editing in zebrafish to model human genetic cardiovascular disorders. Dis. Model. Mech. 11 https://doi.org/10.1242/dmm.035469 (2018).
Ohko, K. et al. Skin and hair abnormalities of Cantu syndrome: A congenital hypertrichosis due to a genetic alteration mimicking the Pharmacological effect of Minoxidil. J. Dermatol. 47, 306–310. https://doi.org/10.1111/1346-8138.15216 (2020).
Kuhnke, E. & Asdonk, J. [Assessment of a patient with idiopathic edema]. Z. Lymphol. 10, 77–81 (1986).
Feng, Y. S., Kohlmann, T., Janssen, M. F. & Buchholz, I. Psychometric properties of the EQ-5D-5L: a systematic review of the literature. Qual. Life Res. 30, 647–673. https://doi.org/10.1007/s11136-020-02688-y (2021).
M, M. V. et al. Dutch tariff for the Five-Level version of EQ-5D. Value Health. 19, 343–352. https://doi.org/10.1016/j.jval.2016.01.003 (2016).
Jurczyk, A. et al. Dynamic glucoregulation and mammalian-like responses to metabolic and developmental disruption in zebrafish. Gen. Comp. Endocrinol. 170, 334–345. https://doi.org/10.1016/j.ygcen.2010.10.010 (2011).
Rocha, F. et al. Glucose overload in yolk has little effect on the long-term modulation of carbohydrate metabolic genes in zebrafish (Danio rerio). J. Exp. Biol. 217, 1139–1149. https://doi.org/10.1242/jeb.095463 (2014).
Lang, R. M. et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur. Heart J. Cardiovasc. Imaging. 16, 233–270. https://doi.org/10.1093/ehjci/jev014 (2015).
Kawel-Boehm, N. et al. Reference ranges (normal values) for cardiovascular magnetic resonance (CMR) in adults and children: 2020 update. J. Cardiovasc. Magn. Reson. 22, 87. https://doi.org/10.1186/s12968-020-00683-3 (2020).
Elmeliegy, M., Udata, C., Liao, K. & Yin, D. Considerations on the calculation of the human equivalent dose from toxicology studies for biologic anticancer agents. Clin. Pharmacokinet. 60, 563–567. https://doi.org/10.1007/s40262-021-00987-2 (2021).
Rambiritch, V., Maharaj, B. & Naidoo, P. Glibenclamide in patients with poorly controlled type 2 diabetes: a 12-week, prospective, single-center, open-label, dose-escalation study. Clin. Pharmacol. 4, 63–69. https://doi.org/10.2147/CPAA.S54809 (2014).
Varma, M. V. et al. Mechanism-based Pharmacokinetic modeling to evaluate transporter-enzyme interplay in drug interactions and pharmacogenetics of glyburide. AAPS J. 16, 736–748. https://doi.org/10.1208/s12248-014-9614-7 (2014).
Author information
Authors and Affiliations
Contributions
Conception and design of the project: HIR, GWvH, SES, MMvH. Zebrafish studies: HIR, GwVH, CGN, MMvH. Clinical trial: conducted by LK with the assistance of LM, supervised by MMvH, involved clinicians SES, MvdB, HACMDBB, KFvD, RNP, EHJ, PMJHK, BJB, MWB. All authors were involved in the data collection and the interpretation of results. Statistical analyses: LK, MvdB, HACMDBB. Draft manuscript preparation: LK. Unrestricted access to all data: LM, LK, MMvH. All authors discussed the results and contributed to the final manuscript (review and edit). Supervision of the project: MMvH.All authors agreed to submit the manuscript, read and approved the final draft and take full responsibility of its content, including the accuracy of the data and the fidelity of the trial to the registered protocol and its statistical analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kleinendorst, L., Siegelaar, S.E., Roessler, H.I. et al. Treatment of overactive KATP channels with glibenclamide in a zebrafish model and a clinical trial in humans with Cantú syndrome. Sci Rep 15, 17704 (2025). https://doi.org/10.1038/s41598-025-00547-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-00547-9