Abstract
Acute ischemic stroke (AIS) is one of the leading causes of mortality and morbidity. This study aimed to identify risk factors, subtypes, and associated outcomes of AIS in the West Bank based on sex. We retrospectively analyzed medical records from 2018 to 2022 of stroke patients from four main hospitals in the West Bank (N = 711). The Modified Rankin Scale (mRS) assessed post-stroke disability on presentation day and thirty days later based on patient history and physical examination findings. An adjusted multinomial logistic regression model was implemented to calculate the adjusted odds ratios (OR) and the 95% confidence interval (CI). The significance level was set at P < 0.05. Out of 711 records, 118 were excluded. The final analysis included 593 AIS patients, with a median age of 63 and an interquartile range of 15. The majority of the patients (60.37%) were males and most of them (62.1%) were smokers. Males were less likely to have diabetes mellitus (DM) (P = 0.037, OR = 0.691) and atrial fibrillation (P = 0.039, OR = 0.627) compared to females. Small-volume strokes accounted for the majority of cases (60.7%). AIS had a thrombotic cause in (81.9%) of patients. On presentation, (40.4%) and (37.2%) of patients had more severe symptoms with mRS scores of 4 and 3, respectively. Males were more likely than females (P = 0.018, OR = 2.03) to present with more severe symptoms (mRS 3-4-5) on day one. An increase of one year in age resulted in a 9.8% higher risk of death (mRS 6) on day one (P = 0.016, OR = 1.098). Smoking history was associated with a seven-fold increase in mortality on day one (P = 0.049, OR = 7.396). Males developed AIS at a younger age while DM and atrial fibrillation were significantly more common in females. The majority of patients reported more severe symptoms on presentation, with notable differences observed between sexes. Male patients exhibited a higher prevalence of severe symptoms compared to female patients. Additionally, key risk factors such as smoking was significantly associated with the severity of symptoms at presentation, with variations observed across sexes. Prevention of risk factors (e.g., HTN, DM, atrial fibrillation, and smoking) is crucial, and further research is required.
Similar content being viewed by others
Background
Many risk factors contribute to the development of AIS; some are non-modifiable like age, sex, ethnicity, hereditary factors, and geographic location. Other factors are modifiable and include cardiac disease (atrial fibrillation), hypertension (HTN), diabetes mellitus (DM), hyperlipidemia, cigarette smoking, obesity, unhealthy diet, and low physical activity. Notably, the modifiable factors are widely spread among young adults aged 20 to 64 years in low and middle-income countries1,2,3.
Stroke is the second leading cause of mortality, and the leading cause of disability with 50% of survivors being chronically disabled worldwide2,4. Recent studies indicate that the overall incidence of strokes (especially ischemic stroke) is increasing, with an estimate that one in four people will experience a stroke in their lifetime1,2,3.
The prevalence of stroke is sharply rising worldwide. According to recent epidemiological data, there are 16.9 million stroke victims worldwide each year, with a global incidence of 258/100,000 per year, which indicates that the incidence of ischemic stroke in young adults is on the rise5. Globally, women experience more stroke events than men due to their longer life expectancy and the higher incidence of stroke at older ages6. However, a systematic review in the Middle East showed high male-to-female ratio in 75% of research that examined sex differences in Qatar, Saudi Arabia, and Iraq, which might be attributed to the demographic makeup of these nations7.
Sex disparities in AIS are significant, with regional variations observed. Globally, women generally experience higher lifetime risk, greater post-stroke disability, and higher mortality rates compared to men8,9. These differences are partly attributed to unique risk factors such as hormonal changes, pregnancy, and a higher prevalence of hypertension and atrial fibrillation in women10,11. Additionally, women often present with more severe strokes and atypical symptoms, which can delay diagnosis and treatment8,9. Men, on the other hand, tend to have a higher incidence of stroke at younger ages and are more likely to have risk factors such as smoking and alcohol use10,11. Understanding these sex-specific risk factors and disparities is crucial for developing targeted prevention and treatment strategies to improve outcomes for both men and women.
Palestine has significant rates of stroke discharge disability and death12. The necessity for research in this area is highlighted by the fact that stroke, particularly ischemic stroke, has the highest rates of morbidity and mortality worldwide. Additionally, its modifiable risk factors are on the rise in low- to middle-income countries, including the West Bank of Palestine2,13. This rise in cases places a heavy burden on both healthcare providers and the community, as the costs of stroke care are substantial for patients, caregivers, and society as a whole. Many survivors are unable to return to their normal lives and require long-term institutional care. Therefore, our research strives to identify the primary predisposing risk factors, subtypes, complications, and outcomes of acute ischemic stroke in the West Bank of Palestine based on sex.
Methods
Study design, setting, population, and sampling
A retrospective analysis was conducted, and data were gathered from the medical records of Palestinian patients diagnosed with AIS who were hospitalized from 2018 to 2022 in three governmental hospitals: Dr. Khalil Suleiman Governmental Hospital in Jenin, Al-Watani Nablus Hospital, and Hebron Governmental Hospital (Alia), as well as one private hospital, An-Najah National University Hospital (NNUH).
We included patients of all age groups in our study, ensuring that any individual diagnosed with acute ischemic stroke (AIS) was considered. Diagnoses of AIS were based on comprehensive medical reports, including medical history, physical and neurological examinations, and imaging techniques. CT and MRI reports were essential for confirming the diagnosis and identifying the stroke subtype based on the TOAST classification. Exclusions were made for patients with hemorrhagic stroke, transient ischemic attack (TIA), cryptogenic stroke due to incomplete data, or spinal cord ischemia. This approach ensured that all patients included were within the specified time period of 2018–2022.
Operational definitions
The demographic data evaluated in this study pertains to patients from the West Bank of Palestine.
Hypertension (HTN) is considered when systolic blood pressure was ≥ 130 and/or diastolic blood pressure was ≥ 80 mm Hg, or taking any antihypertensive medication of the following classes: calcium channel blockers (CCBs), angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), or diuretics14. Diabetes mellitus (DM) is considered when fasting plasma glucose (FPG) ≥ 126 mg/dL, hemoglobin A1c ≥ 6.5, or random plasma glucose ≥ 200 mg/dL with classic symptoms of hyperglycemia (excessive thirst, frequent urination, unexplained weight loss), or with history of diabetes, and taking insulin or any oral hypoglycemic agent15. Hyperlipidemia is defined as the presence of one or more of the following lipid abnormalities: Total Cholesterol: Levels above 200 mg/dL, Low-Density Lipoprotein Cholesterol (LDL-C): Levels above 100 mg/dL, High-Density Lipoprotein Cholesterol (HDL-C): Levels below 40 mg/dL for men and below 50 mg/dL for women, and Triglycerides: Levels above 150 mg/dL16. Atrial fibrillation is considered when there are pathologic electrocardiogram (ECG) findings (absent p wave, irregularly irregular rhythm)17. Patients are considered to have coronary artery disease when they are previously diagnosed and prescribed medications, or they have any intervention: percutaneous coronary intervention (PCI), or coronary artery bypass surgery (CABG). Carotid Artery Disease (CAD) is defined as the presence of internal carotid artery stenosis of 70% or greater, as determined by duplex ultrasound. The diagnostic criteria include a peak systolic velocity in the internal carotid artery of ≥ 215 cm/s, an end-diastolic velocity in the internal carotid artery of ≥ 65 cm/s, a ratio of peak systolic velocities in the internal and common carotid arteries of ≥ 2.7, and a ratio of end-diastolic velocities in the internal and common carotid arteries of ≥ 3.718. Smoking is defined as the regular inhalation of tobacco smoke from cigarettes, cigars, pipes, waterpipes, or e-cigarettes. A smoker is identified as an individual who currently smokes any form of tobacco or has a history of smoking within the past year, or a person who has smoked at least one cigarette per day for the preceding three months or more, or used tobacco in any form, including waterpipes and e-cigarettes16. Small Vessel Stroke (Lacunar Stroke): These strokes occur due to the occlusion of small penetrating arteries, affecting deep brain structures and resulting in small, subcortical infarcts. Diagnosis is confirmed through MRI or CT scans. Large Vessel Stroke: These strokes are caused by the occlusion of major cerebral arteries, leading to significant neurological deficits. Diagnosis is typically made using CT angiography or MR angiography to visualize the blockage in the large arteries19. Thrombotic strokes occur when a blood clot forms in an artery supplying blood to the brain, often associated with atherosclerosis, and are diagnosed through MRI or CT scans that reveal the blockage’s location and extent. Embolic strokes happen when a blood clot or debris forms elsewhere in the body, typically in the heart, and travels to the brain, causing a blockage. These strokes are often linked to heart conditions like atrial fibrillation and are diagnosed using CT angiography or MR angiography to visualize the embolus and the affected artery20,21. Small volume ischemic strokes involve infarct sizes under 70 mL, while large volume strokes exceed 70 mL. Stroke volume estimation is typically performed using MRI, particularly diffusion-weighted imaging (DWI) and computed tomography perfusion (CTP), which help assess the extent of brain tissue damage22. Hemorrhagic transformation is considered when areas of cerebral infarction appear as cerebral hemorrhage on radiological images (CT or magnetic resonance imaging (MRI) after > 24 h of onset23. Hemoglobin (Hgb) reading is considered normal when it is 14–18 g/dL for males, and 12–16 g/dL for females24.
The Modified Rankin Scale (mRS) assesses post-stroke disability on day one and day thirty, with scores ranging from 0 (no disability) to 6 (death), based on patient presentation, history, and physical exam findings25. We classified the mRS to three categories: 2 or less, 3 or more and 6. These categories represent the functional outcomes as good, poor, and death, respectively26.
Statistical analysis
The data analysis was conducted using IBM SPSS Statistics for Mac, version 27 (IBM Corp., Armonk, NY, USA). Percentages were used for categorical variables, while means and standard deviations described normally distributed data, and median and interquartile range (IQR) for non-normally distributed data. Univariate analyses were performed to assess sex-based differences in AIS, in-hospital evaluation and management of AIS patients, and in-hospital complications of diagnosed AIS patients. An adjusted multinomial logistic regression model was utilized to calculate the odds ratios (OR) and 95% confidence intervals (CI) for risk factors with mRS at day 1 to evaluate the functional outcome status in AIS patients. Finally, significant differences between groups were compared, and a significance level of P value < 0.05 was used.
Results
Sociodemographic characteristics of AIS patients
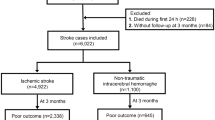
Out of 711 reviewed records, 118 reports were excluded, and 593 medical records for patients who have experienced AIS in the years 2018–2022 fulfilled the inclusion criteria. Out of the included 539 358 (60.37%) of patients were males with a median age and (IQR) 63 (15) and 235 females with mean age and SD was 69.2 (13.1) (Table 1). Most of the patients (61.7%) were in the age group category 55–74 years old. Most of the males were smokers (62.1%) while 9.8% of females were smokers.
Sex-based differences in AIS patients
Among AIS patients, there were 376 individuals with diabetes (215 men and 161 women) and 93 individuals with atrial fibrillation (47 men and 46 women). Males were less likely to have DM (P = 0.037, OR = 0.691), and atrial fibrillation (P = 0.039, OR = 0.627) than females. Smoking was more prevalent among males (62.1%) than females (9.8%), (P = < 0.001, OR = 15.062). Most of the males and females were with HTN (85%) and DM (63.4%) (Table 2).
Sex-based differences in the hospital evaluation and management of AIS
The majority of cases (60.7%) were small-volume strokes. Small vessels were most frequently affected (60.8%), followed by the middle cerebral artery (MCA) at 23.9%. Thrombotic events were identified as the underlying cause of acute ischemic stroke (AIS) in 81.9% of cases. The majority of patients had normal Hgb levels on admission (59.4%) and only (2.3%) had high Hgb levels on admission. Out of the total number of patients, only 10 received treatment with Alteplase; 6 of these patients received treatment within 3 h of the onset of symptoms, and 3 patients started treatment within 3 to 4.5 h. No significant differences were observed between males and females (P > 0.05) (Table 3).
Sex-based differences in-hospital complications of patients diagnosed with AIS
A total of 12 (2%) patients experienced hemorrhagic transformations after an AIS, with the majority of them being males. Moreover, 7 patients passed away while in hospital after an AIS admission. No significant differences were observed between males and females (P > 0.05) (Table 4).
Evaluation of outcomes of patients diagnosed with AIS using the modified Rankin scale (mRS)
On day one of presentation, (12.8%) of patients presented with mild symptoms with mRS score of 2, while the majority presented with more severe symptoms, (40.4%) with mRS score of 4, and (37.2%) with mRS score of 3. At day 30, we had data only for NNUH hospital. At day 30, the majority of patients in NNUH improved, with males showing more improvement than females, as (29%) of males had an mRS score of 2, while (31.8%) of females had an mRS score of 3 (Table 5). No significant differences were observed between males and females on day 1 and day 30 (P = 0.23, 0.824 respectively).
Factors associated with mRS at day1 to evaluate the functional outcome status in AIS patients
The adjusted multinominal logistic regression revealed that males with AIS have a 2-fold higher risk than females to present with more severe symptoms (mRS 3-4-5) on day 1 (P = 0.018, OR = 2.03). A one-year increase in age will increase the risk of mortality (mRS 6) on day 1 by 9.8% (P = 0.016, OR = 1.098, 95% CI 1.017–1.185). Smoking increased mortality at day 1 by 7-fold compared to the reference category (P = 0.049, OR = 7.396, 95% CI )1.017–1.185) (Table 6).
Discussion
In this retrospective study, a total of 593 Palestinian patients with acute ischemic stroke (AIS) were included. Globally, males account for 47% of all strokes yearly, while females account for 53%6. This study found that most of the patients were male (60.37%), which is consistent with findings from another study conducted in Palestine27. This male predominance has also been observed in a retrospective study conducted in Jordan28. Additionally, research in the Middle East, including studies conducted in Qatar, Saudi Arabia, and Iraq, has reported a high male-to-female ratio, possibly attributed to the demographic composition of these nations7. In populations with greater ethnic diversity, stroke incidence by gender can vary significantly. For example, in the United States of America., Black men and women have a higher stroke risk compared to White or Hispanic populations, with studies showing black individuals experienced a 2.4-fold increase in stroke incidence, while Hispanic individuals had a twofold increase compared to white individuals29. In contrast, East Asian populations often exhibit a more balanced gender ratio in stroke incidence, with studies in China reporting male predominance at approximately 54%, which is closer to the global trend30. These differences emphasize how local demographic, genetic, and socioeconomic factors shape stroke epidemiology. Our study highlights the unique male predominance in the West Bank, while global comparisons underscore the value of investigating diverse ethnic backgrounds to fully understand stroke patterns across different populations. The higher incidence of AIS among males in our results could be attributed to sex hormones31,32. Physiologically, estrogen has strong dilatory effect on the vascular endothelium, while testosterone has opposite effect31,32. Males have high testosterone levels, and negligible estrogen levels compared to females, they are more susceptible to vasoconstriction, the first step of hemostasis31,33. Moreover, smoking was linked to HTN, DM, and higher resting heart rates, all of which are stroke risk factors that increase the risk for developing AIS31,34. In this study, males were more likely to be smokers than females, and the smoking percentage among AIS patients (41.3%) is nearly similar to a previous study in Palestine (45%)12. In an Insulin Resistance Intervention after Stroke (IRIS) trial cohort, quitting smoking after an AIS was linked to substantial health benefits over a period of 4.8 years35. Therefore, assisting individuals to quit smoking should be a top concern for healthcare practitioners. Further studies are needed to investigate the difference in male predominance in AIS among Palestinians.
In agreement with a previous local study, the majority of patients were elderly (< 65 years old)12. This finding also agrees with other studies in Jordan and Egypt28,36. Elderly people are more susceptible to certain medical conditions that enhance the risk of AIS, like metabolic syndrome, heart diseases e.g. atrial fibrillation and myocardial infarction, past medical and drug history37,38. Aging is indeed a major risk factor for AIS and Complex Aortic Plaque which is one of the potential causes of this disease39.
DM causes blood vessel damage and diabetic vasculopathy, which raises the risk of AIS due to persistently elevated blood glucose levels40. In addition, diabetic patients who have AIS, typically have worse outcomes41. Moreover, DM and hyperglycemia can exacerbate the risk of stroke by increasing the permeability of the blood-brain barrier and the volume of the infarct following a stroke40. In agreement with previous studies, most of the AIS patients were diabetics41,42. Males were less likely to be diabetic than females in this study which agrees with a pervious study43. This implies the importance of screening and proper management of DM. Females were also at higher risk than males to have atrial fibrillation. These results agree with the results of a previous study10. Females with atrial fibrillation at increased risk to have AIS with more disabling outcomes compared to males44. Given the common association between atrial fibrillation and cardioembolic stroke, it becomes crucial to accurately identify and manage this condition with precision45. Hence, evaluating the likelihood of acute ischemic stroke (AIS) in atrial fibrillation and promptly starting treatment to decrease the risk of stroke related with atrial fibrillation is an essential aspect of managing this cardiac arrhythmia46.
AIS patients were primarily thrombotic (81.9%), while 18.1% were cardioembolic similar to previous studies20,21. Regarding stroke volume, small-volume strokes were more common than large-volume ones, with lacunar vessels involvement in the majority of cases (60.8%). This is higher than what was reported in previous studies47,48. Internationally, lacunar vessel strokes account for 30% of ischemic strokes48. Epidemiological research in the Middle East estimated that the frequency of lacunar ischemic strokes varied from 8.9 to 59.7%49. The underlying pathology of lacunar strokes is thought to be primarily caused by lipohyalinosis and microatheroma that obstructs small penetrating arteries50. Risk factors of lipohyalinosis and microatheroma include HTN and DM. Since HTN and DM have high prevalence among AIS patients in this study, this may explain the higher incidence of lacunar strokes50. Therefore, we recommend proper control and management to decrease the burden of HTN and DM among Palestinians.
Low and high levels of Hgb have a significant effect on clinical outcomes of AIS patients51,52,53. A previous study demonstrated that 40% of AIS patients had low Hgb level, which is consistent with our study (38.3%)54. Low Hgb levels were more predominant among Palestinian AIS females (43.9%) than males (34.7%). Insufficient levels of Hgb result in tissue hypoxia, which induces vasodilation. This reduction in blood pressure triggers the activation of the sympathetic nervous system, leading to peripheral vasoconstriction. Consequently, the risk of thrombus formation is heightened51,54. High Hgb levels increase blood viscosity and promote thrombosis explaining the association between high Hgb levels and ischemic stroke55. Further research is needed to establish the relationship between Hgb level on admission and the severity of functional outcomes of acute ischemic stroke in Palestine52,54.
A total of 10 participants (1.7%) in the current study received Alteplase. This percentage aligns with prior research findings, which indicate that the administration of Alteplase in low-middle income nations, such as West Bank-Palestine, is low, ranging from 1 to 3%. The expensive cost of Alteplase serves as a significant obstacle in these cases12,56. The absence of well-structured stroke facilities in Palestine necessitates the admission of stroke patients to medical or other hospital units. Undoubtedly, this phenomenon results in the division of healthcare delivery and worse clinical results57. Future research conducted in low and middle-income countries should include the limited number of patients receiving alteplase treatment. To address this, researchers should consider expanding the sample size of their study by including more hospitals or extending the study duration, reflecting the significance of this therapy.
One of the in-hospital complications is hemorrhagic transformation, which has devastating effect on the brain and increases mortality rate23. This study showed that 2% of AIS patients developed hemorrhagic transformations which is consistent with previous local study 3%, and a previous systemic review (2.6–5.6%)12,58. Moreover, the mortality rate in-hospitals were 1.18% which is less of what reported in other study conducted in Germany (7.13%)59.
A greater death rate was linked to history of atrial fibrillation, prior strokes, and cardiovascular problems during hospitalization60. The best way to improve the prognosis of ischemic stroke patients is to prevent these diseases and treat them appropriately59.
On day 1 of the presentation, 40.4% of patients had a modified Rankin Scale (mRS) score of 4 indicating moderately severe disability; unable to walk or attend to bodily functions without the assistance of another individual25. These results correspond with previous studies in Palestine that explains the burden of this condition on the families and medical staff12. Long hours of caring, emotional stress, and financial concerns are all elements that put stress on families and caregivers. In order to reduce the stress on them and promote patient recovery, stroke rehabilitation programs should address these challenges. Examples of these include counseling sessions and training in practical nursing skills. On day 1, males had a 2-fold higher risk than females to present with more severe symptoms. Moreover, increasing one year age had increased the risk of mortality on day 1 by 9%. On the other hand, smokers were 7 times more likely to have higher mortality rate than non-smokers. To enhance the successes of smoking cessation, it is necessary to implement appropriate behavioral and pharmacological interventions, together with expert counselling and guidance. By day 30, most of NNUH improved, with males showing more improvement (mRS = 2) than females (mRS = 3). It is worth mentioning that one of the limitations of this study is the fact that results for day 30 were only available for NNUH patients. This limited availability of data could potentially affect our ability to fully discuss the results for this period of the study. However, despite this limitation, we can still draw meaningful conclusions from the available data. These data indicate that AIS patients at NNUH showed better improvement at day 30 compared to day 1 of presentation.
This study has some limitations. Access to the medical records of patients with AIS was difficult because there was no proper use of the ICD-10 coding system of the Palestinian Ministry of Health. This study was a retrospective study which depends on review of medical records that were originally not designed to collect data for research; some information is bound to be missing. Moreover, results for day 30 were only available for the private hospital patients. Despite these limitations, this study continues to be valuables, as it provides sex-based comparison for risk factors of AIS in West Bank and contributes to our understanding of AIS management and outcomes. These findings can be used by researchers in addressing these limitations and developing further investigations into this important area of medical care.
Conclusion
This retrospective study highlights the sex distribution among acute ischemic stroke (AIS) patients, with males predominating. Smoking is identified as a significant risk factor for AIS, especially among male patients. Age and diabetes mellitus (DM) are crucial factors in AIS risk, particularly among elderly individuals and those with DM, who tend to experience worse outcomes. Female AIS patients face a higher risk of atrial fibrillation and tend to have more severe AIS outcomes. Thrombotic strokes, particularly small-volume strokes involving lacunar vessels, were most common. Alteplase administration and complications were investigated, revealing challenges in low-middle income countries regarding Alteplase availability. Hemorrhagic transformations affected a small percentage of AIS patients, with an in-hospital mortality rate of 1.18%. Age and smoking were associated with higher mortality rates, while patients at NNUH showed improved outcomes by day 30 compared to initial presentation.
.
Data availability
The author confirms that all data generated or analysed during this study are included in this manuscript.
References
Shatri, G. & Senst, B. Acute Stroke. StatPearls. (2022).
Paul, S. & Candelario-Jalil, E. Emerging neuroprotective strategies for the treatment of ischemic stroke: An overview of clinical and preclinical studies. Exp. Neurol. 335, 113518 (2021).
Sweileh, W. M., Sawalha, A. F., Al-Aqad, S. M., Zyoud, S. H. & Al-Jabi, S. W. The epidemiology of stroke in Northern Palestine: A 1-Year, Hospital-Based study. J. Stroke Cerebrovasc. Dis. 17, 406–411 (2008).
Katan, M., Luft, A., Editor, G. & Schiess, N. Issue theme global health neurology. Neurol 38, 208–211 (2018).
Béjot, Y., Daubail, B. & Giroud, M. Epidemiology of stroke and transient ischemic attacks: Current knowledge and perspectives. Rev. Neurol. (Paris). 172, 59–68 (2016).
Feigin, V. L. et al. World stroke organization (WSO): Global stroke fact sheet 2022. Int. J. Stroke. 17, 18–29 (2022).
El-Hajj, M., Salameh, P., Rachidi, S. & Hosseini, H. The epidemiology of stroke in the middle East. Eur. Stroke J. 1, 180–198 (2016).
Asdaghi, N. et al. Sex disparities in ischemic stroke care: FL-PR CReSD study (Florida-Puerto Rico collaboration to reduce stroke disparities). Stroke 47, 2618–2626 (2016).
Förster, A. et al. Gender Differences in Acute Ischemic Stroke Etiology, Stroke Patterns and Response to Thrombolysis. (2009). https://doi.org/10.1161/STROKEAHA.109.548750
Rathfoot, C. et al. Gender differences in comorbidities and risk factors in ischemic stroke patients with a history of atrial fibrillation. BMC Neurol. ;21. (2021).
Song, J. W. et al. Sex differences in intracranial atherosclerosis in patients with hypertension with acute ischemic stroke. J. Am. Heart Assoc. 11, 25579 (2022).
Khatib, R. et al. Presentation, management, and outcomes of acute stroke in Palestine. J. Am. Heart Assoc. ;7. (2018).
Putaala, J. Ischemic stroke in young adults. Continuum (Minneap Minn). 26, 386–414 (2020).
Flack, J. M. & Adekola, B. Blood pressure and the new ACC/AHA hypertension guidelines. Trends Cardiovasc. Med. 30, 160–164 (2020).
Committee, A. D. A. P. P. 2. Classification and diagnosis of diabetes: Standards of medical care in Diabetes—2022. Diabetes Care. 45 Supplement_1, S17–38 (2022).
Ali, I. et al. The Prevalence of Dyslipidemia and Hyperglycemia among Stroke Patients: Preliminary Findings. Stroke Res Treat. ;2019. (2019).
Atrial fibrillation. diagnosis and management. Atr fibrillation diagnosis Manag. (2022).
Gorican, K., Chochola, M., Kocik, M. & Zak, A. Diagnostic criteria for the determination of clinically significant internal carotid artery stenosis using duplex ultrasound. Biomed. Pap Med. Fac. Univ. Palacky Olomouc Czech Repub. 164, 255–260 (2020).
El Mawla, Z., El Saddik, G., Zeineddine, M., Hassoun, M. & El Hajj, T. Cerebrovascular disease in patients with COVID-19 infection: a case series from Lebanon. Ann. Med. Surg. 85, 3701–3708 (2023).
Díaz Guzmán, J. [Cardioembolic stroke: epidemiology]. Neurologia 27 (Suppl 1 SUPPL), 1:4–9 (2012).
Mackman, N. Triggers, targets and treatments for thrombosis. Nature 451, 914 (2008).
Timpone, V. M. et al. Percentage Insula ribbon infarction of > 50% identifies patients likely to have poor clinical outcome despite small DWI infarct volume. AJNR Am. J. Neuroradiol. 36, 40–45 (2015).
Hong, J. M., Kim, D. S. & Kim, M. Hemorrhagic transformation after ischemic stroke: mechanisms and management. Front. Neurol. ;12. (2021).
Selva Nidhyananthan, S., Dharshana Shahini, R. & Hari Priya, S. Non-invasive haemoglobin measurement using photoplethysmographic technique. Lect Notes Data Eng. Commun. Technol. 33, 311–316 (2020).
Broderick, J. P., Adeoye, O. & Elm, J. The evolution of the modified Rankin scale and its use in future stroke trials. Stroke 48, 2007 (2017).
Rangaraju, S., Haussen, D., Nogueira, R. G., Nahab, F. & Frankel, M. Comparison of 3-Month stroke disability and quality of life across modified Rankin scale categories. Interv Neurol. 6, 36–41 (2017).
Marwat, M., Usman, M. & Hussain, M. Stroke and its relationship to risk factors. Gomal J. Med. Sci. 7, 1 (2009).
Alawneh, K. Z., Qawasmeh, M., Al, Raffee, L. A. & Al-Mistarehi, A. H. Ischemic stroke demographics, clinical features and scales and their correlations: An exploratory study from Jordan. Futur Sci. OA. 8, SIII–S4 (2022).
Sacco, R. L. et al. Stroke incidence among white, black, and Hispanic residents of an urban community: the Northern Manhattan stroke study. Am. J. Epidemiol. 147, 259–268 (1998).
Wang, W. et al. Prevalence, incidence, and mortality of stroke in China: results from a nationwide Population-Based survey of 480 687 adults. Circulation 135, 759–771 (2017).
Abdu, H. & Seyoum, G. Sex Differences in Stroke Risk Factors, Clinical Profiles, and In-Hospital Outcomes Among Stroke Patients Admitted to the Medical Ward of Dessie Comprehensive Specialized Hospital, Northeast Ethiopia. (2022). https://doi.org/10.2147/DNND.S383564
Krause, D. N., Duckles, S. P. & Pelligrino, D. A. Influence of sex steroid hormones on cerebrovascular function. J. Appl. Physiol. 101, 1252–1261 (2006).
Gale, A. J. Current Understanding of hemostasis. Toxicol. Pathol. 39, 273 (2011).
Pan, B. et al. The relationship between smoking and stroke: A meta-analysis. Med. (Baltim). ;98. (2019).
Epstein, K. A. et al. Smoking cessation and outcome after ischemic stroke or TIA. Neurology 89, 1723 (2017).
Soliman, R. H., Oraby, M. I., Fathy, M. & Essam, A. M. Risk factors of acute ischemic stroke in patients presented to Beni-Suef university hospital: prevalence and relation to stroke severity at presentation. Egypt. J. Neurol. Psychiatry Neurosurg. ;54. (2018).
Ciumărnean, L. et al. Cardiovascular risk factors and physical activity for the prevention of cardiovascular diseases in the elderly. Int. J. Environ. Res. Public. Health. 19, 207 (2022).
Knoflach, M. et al. Functional recovery after ischemic stroke–a matter of age: Data from the Austrian stroke unit registry. Neurology 78, 279–285 (2012).
Kong, Q. et al. Influence of age ranges on relationship of complex aortic plaque with cervicocephalic atherosclerosis in ischemic stroke. J. Stroke Cerebrovasc. Dis. 28, 1586–1596 (2019).
He, T., Geng, J. & Zhang, Z. Diabetics Stroke ;:169–198. (2017).
Lau, L. H., Lew, J., Borschmann, K., Thijs, V. & Ekinci, E. I. Prevalence of diabetes and its effects on stroke outcomes: A meta-analysis and literature review. J. Diabetes Investig. 10, 780–792 (2019).
Maida, C. D. et al. Diabetes and ischemic stroke: An old and new relationship an overview of the close interaction between these diseases. Int. J. Mol. Sci. 23, 23 (2022).
Arboix, A. Cardiovascular risk factors for acute stroke: Risk profiles in the different subtypes of ischemic stroke. World J. Clin. Cases WJCC. 3, 418 (2015).
Boriani, G., Colella, J., Imberti, J., Fantecchi, E. & Vitolo, M. Female sex and stroke in atrial fibrillation: An intriguing relationship. Intern. Emerg. Med. 15, 175–179 (2020).
Sajeev, J. K., Kalman, J. M., Dewey, H., Cooke, J. C. & Teh, A. W. The atrium and embolic stroke: myopathy not atrial fibrillation as the requisite determinant?? JACC Clin. Electrophysiol. 6, 251–261 (2020).
Alshehri, A. M. Stroke in atrial fibrillation: review of risk stratification and preventive therapy. J. Family Community Med. 26, 92 (2019).
Rojsanga, W. et al. Clinical risk factors predictive of thrombotic stroke with large cerebral infarction. Neurol. Int. 11, 12–14 (2019).
Yaghi, S. et al. Lacunar stroke: Mechanisms and therapeutic implications. J. Neurol. Neurosurg. Psychiatry. 92, 823–830 (2021).
El-Hajj, M., Salameh, P., Rachidi, S. & Hosseini, H. The epidemiology of stroke in the middle East. Eur. Stroke J. 1, 180 (2016).
Jensen, M. & Thomalla, G. Causes and secondary prevention of acute ischemic stroke in adults. Hamostaseologie 40, 22–30 (2020).
Liu, Y. et al. Predictive value of hemoglobin level on early neurological outcomes in acute ischemic stroke. Neurol. Res. 44, 684–691 (2022).
Al-Harbi, N., Alrasheedi, M. S. & Alshammari, S. T. Hemoglobin level is associated with severe stroke among stroke patients in Saudi Arabia. Int. J. Health Sci. (Qassim). 14, 18 (2020).
Naess, H., Logallo, N., Waje-Andreassen, U., Thomassen, L. & Kvistad, C. E. U-shaped relationship between hemoglobin level and severity of ischemic stroke. Acta Neurol. Scand. 140, 56–61 (2019).
Desai, A. et al. Impact of anemia on acute ischemic stroke outcomes: A systematic review of the literature. PLoS One ;18. (2023).
Rodriguez, G. J. et al. The hydration influence on the risk of stroke (THIRST) study. Neurocrit Care. 10, 187–194 (2009).
Sayed, M. J., El, Z. T. & El, T. H. Acute stroke care and thrombolytic therapy use in a tertiary care center in Lebanon. Emerg. Med. Int. 2014, 1–6 (2014).
Langhorne, P. & Ramachandra, S. Organised inpatient (stroke unit) care for stroke: network meta-analysis. Cochrane Database Syst Rev. ;2020. (2020).
Sun, J. et al. Risk factors of hemorrhagic transformation in acute ischaemic stroke: A systematic review and meta-analysis. Front. Neurol. ;14. (2023).
Heuschmann, P. U. et al. Predictors of In-Hospital mortality and attributable risks of death after ischemic stroke: the German stroke registers study group. Arch. Intern. Med. 164, 1761–1768 (2004).
Keller, K. et al. Impact of atrial fibrillation/flutter on the in-hospital mortality of ischemic stroke patients. Hear. Rhythm. 17, 383–390 (2020).
Acknowledgements
The authors would like to thank An-Najah National University (www.najah.edu) for the technical support provided to publish the present manuscript. They also would like to thank all governmental hospitals participated in this study and its Information technology team for their helping in permitting and extracting the data.
Funding
This study was not supported by any sponsor or funder.
Author information
Authors and Affiliations
Contributions
A. K. conceptualization, design, data acquisition, analysis, interpretation, reviewing, writing, and editing. O. K. conceptualization, design, data acquisition, analysis, interpretation, reviewing, writing, and editing. Z. (A) conceptualization, design, data acquisition, analysis, interpretation, reviewing, writing, and editing. (B) D. conceptualization, design, data acquisition, analysis, interpretation, reviewing, writing, and editing. A. H: conceptualization, design, data acquisition, analysis, interpretation, reviewing, writing, and editing. M. A. conceptualization, design, data acquisition, analysis, interpretation, reviewing, writing, and editing.
Corresponding author
Ethics declarations
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Ethical approval
The ethical approval was obtained from the Institutional Review Board (IRB) at An-Najah National University (Ref: Med. August. 2023/47). This study was conducted in accordance with the principles of the declaration of Helsinki standards. Patient informed consent was waived by the institutional review board at An-Najah National University and the Palestinian Ministry of Health because of its retrospective nature and minimal risk for patients.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
khraiwesh, A., Ikhdour, O., Alalyat, Z. et al. A retrospective study on sex disparities and risk factors in acute ischemic stroke in the West bank of Palestine. Sci Rep 15, 16135 (2025). https://doi.org/10.1038/s41598-025-01268-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-01268-9