Abstract
This study aimed to elucidate the biological or mechanical causes of stent edge restenosis (SER) via intravascular ultrasound (IVUS). A retrospective assessment was conducted on 126 SER lesions that underwent IVUS prior to revascularization. The primary mechanisms of SER were categorized. (1) neointimal hyperplasia (NIH); (2) neoatherosclerosis; (3) uncovered lesion; (4) stent underexpansion; or (5) a protruding calcified nodule (CN). The predominant biological or mechanical causes of SER were NIH in 42.9% (n = 54) of lesions, neoatherosclerosis in 32.5% (n = 41), uncovered lesion in 14.3% (n = 18), stent underexpansion in 7.9% (n = 10), and protruding CN in 2.4% (n = 3). The 2-year device-oriented clinical endpoints (DoCE) incidence was 7.1% (n = 9). The group with biological causes treated via drug-coated balloons (DCB) exhibited a comparable DoCE rate (9.5%) to those with biological causes treated with drug-eluting stents (DES) and mechanical causes managed with or without restenting (6.0%, HR 2.78, 95% CI: 0.91–9.21; p = 0.161). The majority of the analyzed SERs were attributed to biological causes, including NIH, neoatherosclerosis, and uncovered lesions. The 2-year DoCE rate within patients receiving DCB for mechanically or biologically induced SER was similar to that observed in patients receiving new DES.
Similar content being viewed by others
Introduction
Drug-eluting stent (DES) has totally transformed the area of percutaneous coronary intervention (PCI) as the incidence of in-stent restenosis (ISR) decreased dramatically1. The available next-generation DES bear thinner and thinner metal struts as well as improved polymers, leading to significantly fewer ISR and thrombotic events than first-generation DESs, however, treating ISR through PCI remains a formidable challenge2. Stent edge restenosis (SER), a specific kind of ISR that develops at the margins of the implanted stent, has emerged as a major limitation following stent placement3. The incidence of SER has been documented to range from 3.5 to 10.1% 4,5,6,7, surpassing the overall 2-year restenosis rates ranging between 2.9 and 6.4%. associated with modern DES8,9. The mechanism of SER is multifactorial, encompassing excessive vascular response and injury resulting from damage to the adjacent coronary artery during balloon inflation or stent deployment, inadequate coverage of residual plaque (longitudinal geographic miss), discrepancies between balloon and artery sizes (axial geographic miss), and mechanical stresses at the interface between the stent platform and the marginal segment, which may appear at the stent edge due to severe angulation, large stent struts, and small lumen area in the stent edge zones4,10. Furthermore, mechanical stresses attributed to hinge motion, residual plaque burden, and lipid arc have been identified as potential risk factors for SER incidents11. Despite these findings, several knowledge gaps remain. Most existing research has relied on angiography or optical coherence tomography (OCT) for SER evaluation, whereas intravascular ultrasound (IVUS) provides a more comprehensive assessment of plaque burden, vessel remodeling, and mechanical stress at the stent edge. However, systematic classification distinguishing biological (e.g., neointimal hyperplasia, neoatherosclerosis, uncovered lesion) from mechanical (e.g., stent underexpansion, calcified nodule) causes of SER is lacking, despite its crucial role in guiding treatment decisions. Additionally, while drug-coated balloon (DCB) has been shown to be effective in treating ISR, its comparative efficacy against DES in different SER subtypes remains unclear. Furthermore, the long-term clinical durability of DCB vs. DES in SER treatment has not been thoroughly investigated. This study aims to systematically categorize SER mechanisms using IVUS, evaluate their impact on revascularization outcomes, and compare long-term (2-year) clinical results between DCB and DES, providing new insights into optimized treatment strategies for SER.
Methods
Study population
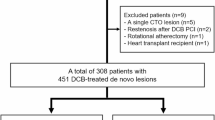
In the single-center, retrospective observational registry, patients with coronary artery disease (CAD) who underwent IVUS-guided treatment for SER lesions from January 1, 2010 to December 31, 2021, were consecutively enrolled. We applied a set of stringent inclusion criteria to ensure the integrity and relevance of our study cohort: (1) patients presenting with symptomatic SER underwent subsequent IVUS-guided vascular intervention for lesion management, and (2) the presence of high-quality IVUS images suitable for analysis prior to any intervention or after predilation using a balloon with a diameter of ≤ 2.5 mm. For patients with multiple repeat revascularizations related to SER lesions, only the initial event was included in the assessment. The exclusion criteria comprised suboptimal IVUS image quality (n = 7), contraindications to antithrombotic medications (n = 3), unsuccessful PCI defined as the inability to cross the lesion with a guidewire or device or a residual stenosis of greater than or equal to 70% (n = 12), and the absence of follow-up data (n = 6) (Fig. 1). The study protocol was reviewed and approved by the Research and Ethics Committee of Xiangtan Central Hospital and adhered to the principles outlined in the Declaration of Helsinki (as amended in 2013). All participants, or their informants in cases of severe cognitive impairment, were informed about the study’s purpose and provided written informed consent (X201875231-1).
Clinical baseline, procedures and angiographic characteristic data
Patient demographics, comorbidities, and laboratory results were documented in a dedicated database at baseline. Current guidelines and local standard practices were followed by operators when determining interventional strategies, such as DCB angioplasty, second-generation DES implantation, use of adjunctive devices, and pharmacotherapy12,13. In general, DCB was preferred for focal, non-calcified lesions with adequate stent expansion, while DES was selected for restenotic lesions with larger burden, significant calcification, or suboptimal stent expansion requiring additional scaffolding. Treatment decisions were ultimately made by interventional cardiologists based on lesion complexity and procedural feasibility.Upon discharge, data on prescribed medications were collected. Dual antiplatelet therapy prescribed here were aspirin, 100 mg/day, and a P2Y12 receptor blocker (either clopidogrel, 75 mg/day or ticagrelor, 90 mg, twice daily). It was selected by the operator based on guidelines and the patient’s bleeding risk. As recommended by the current guidelines, additional secondary prevention medications, including statins, nitrates, β-blockers, as well as angiotensin-converting enzyme inhibitors, were prescribed.
The QAngio XA software (Medis Medical Imaging Systems, Leiden, the Netherlands) was utilized to conduct quantitative coronary angiography (QCA) assessment. Lesion morphology was evaluated via criteria from a previous study14. Two experienced angiographers, blinded to the research, independently conducted all quantitative measurements via offline computerized assessment. After administering 0.5 mg of intracoronary nitroglycerin, lesion images were captured in at least two orthogonal projections. The main angiographic parameters were minimum lumen diameter(MLD), lesion length, reference vessel diameter and percentage diameter stenosis.
IVUS image acquisition and assessment
IVUS images of the SER arteries were taken from a 40-MHz OptiCrossTM catheter (Boston Scientific, Marlborough, MA, US) using the automated pullback at 0.5 mm/s after nitroglycerin (0.1–0.2 mg) intracoronary. The IVUS catheter was pulled back and advanced more than 10 mm beyond the stent into the distal segment and more than 10 mm proximal to the stent. All the acquired IVUS images were digitally stored, and off-line analysis was done with the help of a CD-ROM software package (QIvus® Medis, Leiden, the Netherlands). SER was defined as percent diameter stenosis more than or equal to 50% involving lesion within 5 mm proximal or distal to stent edge15. Once an SER was determined, the reference segments for the proximal and distal reference segments (the most normal-looking cross-section, largest lumen, and smallest plaque area within 5 mm proximal or distal to the identified SER) were also determined. The reference slice with the smallest lumen area and the greatest plaque area was considered the minimum lumen area (MLA) site. Between the proximal and distal reference sites, the smallest stent area was chosen as the minimum stent area (MSA) sites. The identification of SER and the quantitative analyses were done by two separate, non-informing cardiologists. The intra- and inter-observer analysis showed fairly good level of agreement with the SER diagnosis (κ = 0.92 and 0.90, respectively). The neointimal hyperplasia (NIH) area, determined as the difference between the stent area and the lumen area, along with the percentage NIH (NIH/stent area), was calculated. Stent expansion was assessed by calculating the MSA relative to the largest reference lumen, providing a morphological evaluation of the stented segments16. Stent underexpansion was identified when the MSA was < 4 mm2 or the stent expansion rate was < 50%17. IVUS was performed in all cases both before and after PCI. Pre-procedural IVUS guided stent sizing, lesion assessment, and landing zone selection, while post-procedural IVUS evaluated stent expansion, apposition, and mechanism of restenosis.
Lesion preparation strategies
To optimize stent expansion, a lesion modification strategy was applied in cases with moderate to severe calcification or tortuous anatomy. Rotational atherectomy (RA) or intravascular lithotripsy (IVL) was performed in patients with circumferential calcium (> 270° arc) or a calcium thickness > 0.5 mm, as assessed by IVUS. Scoring or cutting balloons were used in patients with eccentric or nodular calcification. After lesion preparation, a non-compliant (NC) balloon was employed for high-pressure pre-dilatation. After stent deployment, high-pressure post-dilatation (≥ 16 atm) was performed using NC balloons to achieve optimal expansion. Stent expansion was defined as the minimum stent area (MSA) divided by the mean reference lumen area, with a target expansion ≥ 70% or MSA > 5.5 mm218. Cases with stent underexpansion (MSA < 4.0 mm2 or expansion < 50%) were further managed using additional NC balloon dilatation or repeat imaging to assess residual plaque burden.
Definitions
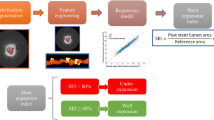
The main patterns of SER were categorized into five distinct types: (1) NIH, (2) neoatherosclerosis, (3) uncovered lesions, (4) underexpanded stents, or (5) protruding calcified nodule (CN) (Fig. 2). Neoatherosclerosis is described in previous studies as pathological modifications at the MLA site inside the stent, including calcified NIH, attenuated NIH, and ruptured NIH19.In specific instances where a CN was identified within neoatherosclerotic calcified NIH, it was classified as a neoatherosclerotic calcified nodule. Conversely, a protruding CN without neoatherosclerosis has been described as a non-neoatherosclerotic protruding CN, characterized by a convex and irregular calcium formation within the old stent, absent of NIH20. Uncovered plaque was defined as persistent plaque burden extending beyond the stent edge without full strut coverage and based on IVUS assessment at the time of SER diagnosis. Stent underexpansion was identified when the MSA was < 4 mm2 or the stent expansion rate was < 50%18. In the instances where there were several possible contributing factors, the main cause of SER has been identified based on its greatest impact on stenosis. Figure 3 illustrates the evaluation of underlying mechanical and biological mechanisms and diagnostic flowchart of each primary cause.
Representative cases for each pattern of SER. In each example, the coronary angiogram at the time of SER (A) is shown accompanied by a white dotted line indicating the old stents. B-E in the coronary angiograms correspond to the IVUS image (B-E). B′-E′ are the same images with annotation compared with B-E. Blue dotted lines in the IVUS images indicate old stent struts. (A) Excessive neointimal hyperplasia with good stent expansion; the blue asterisks indicate excessive neointimal hyperplasia. (B) Neoatherosclerosis with good stent expansion; the blue asterisks indicate neointimal attenuated plaque (signal attenuation without calcium) representing lipidic plaque. These represent neoatherosclerosis within the old stent. (C) Uncovered lesion; the stent struts (blue dotted lines) were observed only in D and E with no struts in B and C indicating the lack of stent coverage associated with a new stenosis. (D) Underexpanded stent. A severely underexpanded stent (minimum stent area = 4.5mm2 ) at C compared to the distal segment (stent area of 10.9 mm2 ) in E. (E) Protruding calcified nodule within the stent. A calcified nodule (blue asterisks) was observed within the old stent struts (blue dotted lines) without adjacent neointimal hyperplasia. SER stent edge restenosis, IVUS intravascular ultrasound.
Diagnostic flowchart of each primary cause. For SERs with multiple patterns, the primary pattern was hierarchally diagnosed based on the main morphology at the stenosis. The prevalence of biological causes (neointimal hyperplasia, neoatherosclerosis, or uncovered ostium) was 89.7% and 10.3% for mechanical causes. PCI percutaneous coronary intervention, SER stent edge restenosis, IVUS intravascular ultrasound.
Clinical follow-up and outcomes
The study endpoints, collectively termed as device-oriented clinical endpoints (DoCE), encompassed cardiac mortality, myocardial infarction (MI) associated with the target vessel or stent thrombosis, as well as target lesion revascularization (TLR). The definitions of these clinical outcomes adhered to the guidelines set by the Academic Research Consortium21. The primary endpoint was to determine the frequency of DoCE occurrence, while the secondary endpoint was to evaluate the incidence of each individual component of DoCE. Periodic clinical follow-up was conducted at six-month intervals either by a clinical visit or a telephonic interview. The follow-up duration extended up to two years, with a minimum of one year completed for all research participants.
Statistical analysis
For continuous variables, descriptive statistics were employed via either mean ± standard deviation or median (first to third quartile), while categorical variables were presented as number (percentage). Comparisons of continuous outcome data were performed via the Student’s t-test/Mann–Whitney U test, whereas categorical outcome data were analyzed via the Fisher’s exact test or chi-square test. Kaplan-Meier survival assessment was employed to estimate event rates, and Cox regression assessment was used to generate hazard ratios (HR) with 95% confidence intervals (95% CI). As patients may have experienced multiple DoCE, each participant was evaluated until the occurrence of their first event and only once during the assessment. Statistical significance was set at P < 0.05 for all analyses, which were conducted via SPSS 24.0 (SPSS Inc., Chicago, IL, US).
Results
A total of 126 SER lesions meeting the inclusion criteria were evaluated via IVUS between January 2010 and December 2021 (Fig. 1).The clinical characteristics of the patients are presented in Table 1. The mean age of the patients was 71.5 ± 8.7 years, with women comprising 72.2% of the research population. The median duration between the previous stent implantation and the current procedure was 1.3 years, with the first quartile (Q1) at 0.7 years and the third quartile (Q3) at 3.2 years. Data from the index PCI procedural information revealed that 84.9% of the previously implanted stents were second-generation DES, 14.3% were first-generation DES, and 0.8% were bare metal stent (BMS). Angiographic and procedural findings are summarized in Table 2. SER was performed on the left anterior descending coronary artery in 54.8% of cases. All lesions were successfully treated, with 60.3% of patients receiving a new stent and 39.7% undergoing DCB deployment (Table 2).
Primary mechanism of stent edge restenosis
The primary causes that mediated SER were evaluated to be NIH in 42.9% (n = 54) of the lesions, neoatherosclerosis in 32.5% (n = 41), uncovered lesions in 14.3% (n = 18), stent underexpansion in 7.9% (n = 10), and protruding CN in 2.4% (n = 3). Biological causes, which included NIH, neoatherosclerosis, and uncovered lesions, accounted for 89.7% of SER cases, while mechanical causes (stent underexpansion as well as protruding CN) were observed in 10.3% of cases (Fig. 3). When secondary causes of SER were considered, 12.7% (n = 16) of lesions exhibited more than one potential mechanism. The results from IVUS analysis are provided in Table 3. The smallest lumen area recorded was 2.3 mm2 (range: 1.7–2.9 mm2), while the MSA measured 6.4 mm2 (range: 5.6–7.8 mm2). The highest NIH area observed was 65.7% (range: 50.4–72.5%). The peak calcium arc inside the stent reached 85° (range: 0–147°), whereas the maximum calcium arc posterior to the stent was 165° (range: 86–282°). Following the procedure, the MSA was 7.0 mm2 (range: 6.2–9.0 mm2), with a minimum stent expansion rate of 71.8% (range: 61.3–80.6%). It should be noted that although lumen area increased, the actual plaque volume was not reduced; rather, the calculated plaque burden ratio decreased due to vessel expansion. The rate of restenting was significantly higher in biologically caused SERs (62.8%, 71/113) compared to mechanically caused SERs (38.5%, 5/13; p < 0.001) (Fig. 4A).
(A) The prevalence of each primary cause with new DES implantation or with DCB. (B) Kaplan-Meier curves for DoCE due to biologically cause with DCB vs. others. SER stent edge restenosis, IVUS intravascular unltrasound, PCI percutaneous coronary intervention, DCB drug-coated balloon, DES drug-eluting stents, NIH neointimal hyperplasia, CN calcified nodule, DoCE device-oriented clinical endpoints, 95% CI 95% confidence intervals, HR hazard ratio.
2-year clinical outcomes
Over a 2-year observational period, the occurrence of DoCE was 7.1% (n = 9), encompassing one cardiac-related mortality, two instances of target vessel-associated MI, a single case of target vessel-related stent thrombosis, and six TLR events. The SERs were categorized into four distinct groups: 71 SERs attributed to biological causes treated with a new stent, 42 SERs attributed to biological causes treated with DCB, 5 SERs attributed to mechanical causes treated with new stent placement, and 8 SERs attributed to mechanical causes handled with DCB. The cohort of biologically-induced SERs treated with DCB exhibited a DoCE rate (9.5%) that was comparable to the other three groups combined (6.0%, HR 2.78, 95% CI: 0.91–9.21; p = 0.161) (Table 4; Fig. 4B).
Discussion
This study identified the following principal outcomes: (1) Biological factors, including NIH, neoatherosclerosis, and uncovered lesions, accounted for 89.7% of SER cases; (2) The 2-year clinical event rates were similar between the biologically and mechanically induced SER groups, regardless of whether a new stent was implanted.
Biologically caused SERs
SER was found to be a serious clinical concern during the period of brachytherapy2. Even though SER has not been notable during the BMS period, various reports indicated that it can occur following both BMS and DES implantation2. The development, severity, and patterns of SER are influenced by multiple factors, including biological, mechanical, and operator-related technical aspects2. In this study, NIH or neoatherosclerosis, defined as tissue proliferation within the stent, was detected in 75.4% of SERs. The prevalence of NIH (42.9%) was higher than that reported in previous IVUS and OCT studies17,22. DESs avoid excessive neointimal formation by releasing drugs that suppress stenotic wall proliferation following a specific release schedule, thereby reducing positive remodeling, preventing medial smooth muscle cell growth and migration, and reducing inflammatory reactions23. However, hypersensitivity to the polymer and drug, local inflammation, and delayed healing are considered to be the major causes of neointima formation in DES-related ISR18. SER often exhibits a focal pattern, characterized by proteoglycan-rich tissue with reduced cellularity24. Furthermore, dense signal pattern is significantly more common in patients with SER if tumor tissue layered echogenicity is used25.
Neoatherosclerosis, which is also known as in-stent new atherosclerosis, is described by the infiltration of lipid-laden foamy macrophages, which may also have a necrotic core and/or calcium in the newly formed intima after stent implantation26. Since the use of DES has been growing in the recent past, neoatherosclerosis is a likely cause for stent failure27. Although neoatherosclerosis appears to progress more quickly than native vessel atherosclerosis, it consequently may result in equally severe pathologies28.
In the present study, uncovered lesions were identified as the primary mechanism in 14.3% of SERs. Uncovered stent-edge lesions are well-established as a predictor of future adverse clinical outcomes5. Previous studies have demonstrated that increased plaque burden (> 54.5%) at the stent edge is a predictor of ISR and future TLR4,5,29. IVUS enables the evaluation of plaque morphology at stent landing zones with its high penetration depth, making it the ideal modality for quantifying plaque burden23. Therefore, in cases of SER with an uncovered lesion longer than 3 mm or with a circumferential extent greater than 60°, the implantation of an additional stent to cover the plaque should be considered30.
Mechanically caused SERs
Stent underexpansion is a prevalent factor contributing to ISR, including SER31, This can be attributed to two primary reasons. First, the resulting smaller lumen area makes even minimal neointimal proliferation clinically significant. Second, substrate malapposition in particular is caused by suboptimal stent expansion; this results in an unfavorable hemodynamic environment characterized by high wall shear stress (WSS) at the underexpanded segment and low WSS in the regions proximal and distal to the stenosis. This environment promotes neointimal proliferation and the formation of neoatherosclerotic lesions in DES32. Computational fluid dynamics (CFD) models and in vivo studies have demonstrated that these hemodynamic disturbances play a crucial role in ISR progression. Low WSS regions (< 0.4 Pa) are associated with endothelial dysfunction, increased platelet adhesion, and neointimal hyperplasia, whereas high WSS zones (> 10 Pa) can lead to excessive endothelial shear stress and plaque rupture20,32. Several strategies to optimize stent expansion and mitigate hemodynamic disturbances, including IVUS-guided PCI, lesion preparation with RA or IVL, and high-pressure post-dilatation with NC balloons. These techniques help to minimize abnormal WSS regions and reduce ISR or SER risk. While our study did not directly employ CFD modeling, the findings support the importance of maximizing stent expansion and optimizing lesion preparation to minimize the hemodynamic disturbances that drive ISR/SER. Future studies should consider integrating CFD-based WSS analysis with IVUS-guided PCI to develop patient-specific strategies for stent optimization and restenosis prevention. Although stent underexpansion is typically a greater concern within the main stented segment, IVUS assessment in this study identified 7.9% of SER cases with underexpansion at the stent edge. Several factors may contribute to this phenomenon, including vessel compliance mismatch, unrecognized calcification at the stent landing zone, balloon-to-vessel size mismatch, and the influence of hinge motion. These anatomical and procedural variables highlight the complexity of achieving optimal stent expansion at the edges, even with IVUS guidance. For severely calcified restenotic lesions, RA or orbital atherectomy (OA) can be employed to improve stent expansion. However, in cases of extensive stent malapposition, traditional RA/OA presents challenges such as burr entrapment, wire passage difficulty, and increased risk of stent strut fracture18,20. To mitigate these risks, pre-procedural IVUS imaging is essential for evaluating the extent of malapposition and guiding lesion preparation30. Procedural modifications include adjusting RA/OA parameters, such as using smaller burr sizes (≤ 1.5 mm) and controlled rotational speeds (140,000–180,000 rpm) to minimize structural damage18. Additionally, enhanced guidewire and device support techniques, including low-profile guidewires with microcatheter support and guide extension catheters, can improve device delivery and stability20. When feasible, pre-dilatation with NC balloons can help reduce malapposition before performing RA/OA18. Post-procedural IVUS assessment is crucial to ensure optimal stent expansion and evaluate debris burden30. Furthermore, novel approaches, such as RA/OA combined with IVL or CFD-guided rotational paths, warrant further investigation to enhance safety and improve outcomes in complex lesions33. Scoring balloons or cutting balloons were utilized in non-circumferential calcified lesions to improve plaque modification before stent deployment. High-pressure post-dilatation with NC balloons further ensures adequate stent expansion and minimizes the risk of underexpansion-related SER. IVL has demonstrated significant potential in treating calcified lesions; however, its efficacy remains limited in highly stenotic (> 80%) or circumferentially calcified cases (> 270° calcium arc), where device delivery and energy transfer present challenges34. Recent advancements aim to overcome these limitations through higher-energy shockwave systems, which enhance calcium penetration, and smaller-diameter IVL balloons, improving device deliverability in severe restenotic lesions35. Additionally, combination therapy approaches, such as RA followed by IVL to increase calcium fracture depth and IVL combined with DCB to optimize vascular remodeling, have emerged as promising strategies36. Moreover, next-generation IVL systems are being developed with targeted energy delivery mechanisms that minimize trauma to healthy tissue while improving calcified plaque fragmentation37. Excimer laser appears to be satisfactory in most circumstances but is used less frequently in catheterization laboratories. By intravascular imaging, it is possible to assess procedural outcomes, make changes in the management plan in case of inadequate lesion preparedness, and implant another DES with further aggressive postdilatation when the process reaches the optimal stage38.
The final interesting finding of the present study is that CN were visualized in 3 SER lesions. In recent literature, there has been a clear relation between coronary hinge motion, severely calcified lesions, and the development of CN2. The mechanisms through which CN leads to SER involve both mechanical and hemodynamic disturbances. Protruding CN can result in stent underexpansion and malapposition, creating low WSS zones that promote neointimal hyperplasia and neoatherosclerosis, thereby accelerating restenosis39. Additionally, in segments exposed to hinge motion, particularly in the right coronary artery, repetitive mechanical stress can induce CN fragmentation and rupture, further exacerbating endothelial dysfunction and inflammation, which are known to contribute to ISR and SER33. To mitigate CN-induced SER and address hinge motion as a risk factor, IVUS imaging aids in classifying CN and guiding lesion preparation. RA and IVL optimize stent expansion, while scoring/cutting balloons enhance plaque modification. Ultra-thin strut stents reduce mechanical stress, and bioresorbable scaffolds improve long-term vessel adaptability. High-pressure NC balloon post-dilatation ensures full expansion, with final IVUS confirmation (MSA ≥ 5.5 mm2) to eliminate malapposition and minimize SER risk.
Clinical implications
The findings of this study indicate that the clinical outcomes of DCB therapy to treat SER are comparable to those achieved with DES. This is consistent with the results of a previous investigation that demonstrated similar efficacy and safety profiles between DCB and DES for the management of SER40. DCB angioplasty is regarded as a suitable option for SER due to its reported ability to inhibit NIH as well as negative vessel remodeling12. It suggests that the transfer of antiproliferative agents from the DCB inhibits the modules involved in the regulation of neointimal formation and may reduce SER. Furthermore, additional advantages have been noted with DCB therapy, such as regression of the plaque, healing responses, and positive vessel remodeling41. As to the improved outcomes of SER discovered in this study, several aspects may have influenced the favorable results of DCB therapy. One possibility is the utilization of IVUS during the interventional procedure. We used this imaging to assess lesions before and after DES implantation or before DCB angioplasty. This approach enables the identification and appropriate management of restenosis mechanisms that could potentially render DCB treatment ineffective, thereby increasing the probability of successful PCI40. Furthermore, calcified lesions are known to be a risk factor for TLR following DCB therapy42; it is recognized that full coverage of the plaque with DES is crucial, necessitating the positioning of the stent edge segment in a vessel region with minimal plaque and calcification43. These strategies are important in the prevention of ISR as well as thrombosis after implantation of DES. Nonetheless, SER is still possible with the stent, underexpansion, or calcification of the artery. Under these circumstances, high-pressure NC balloons are superior to DCB since the latter cannot perform high-pressure ballooning. If stent underexpansion in SER is detected by imaging modalities, a high-pressure, noncompliant balloon will be used to predilate before deploying DES or performing DCB angioplasty. Therefore, appropriate predilatation was performed for lesions with stent underexpansion or CN lesions.
DCB therapy is a viable alternative to DES, particularly in ISR and small-vessel disease. However, its efficacy may be limited in severely calcified plaques, high-recoil fibrotic segments, long lesions (> 30 mm), chronic total occlusions, and acute coronary syndrome due to impaired drug penetration, lack of mechanical support, and lower long-term patency in certain cases44. The injection of scoring balloons prior to DCB treatment may also extend the positive outcomes of the approach. Kufner et al. described that the prior application of scoring balloons reduced restenosis after DCB treatment45. The scoring balloons may give a sufficient increase in lumen and reduce malignant dissection. Further, it might be expected that the plaque and vessel cracks generated by the scoring balloon might promote increased transfer of drugs46. In the present study, scoring balloons were often employed for lesion modification. Therefore, based on the mentioned factors above, they might have contributed positively to the enhanced result of the DCB treatment for SER that is comparable with the new generation of DES.
Intravascular imaging plays a critical role in optimizing PCI, with OCT and near-infrared spectroscopy (NIRS) providing superior lesion characterization and procedural guidance47. OCT enables high-resolution plaque morphology assessment, facilitating early detection of vulnerable plaques and guiding lesion preparation, while NIRS identifies lipid-core plaques, helping to assess high-risk lesions47. During PCI, OCT improves stent expansion and strut apposition, whereas NIRS refines treatment approaches, particularly in acute coronary syndrome (ACS) patients48. Post-procedural assessment with OCT helps detect stent malapposition and neoatherosclerosis, while NIRS-based lipid core burden index (LCBI) predicts future cardiac events49.
Currently, no direct studies have been found the specific impact of pharmacotherapeutic regimens (e.g., statins, antiplatelets) on SER patterns. However, pharmacotherapy may be crucial in modulating SER by influencing neointimal proliferation, inflammation, and thrombosis. High-intensity statins reduce inflammation and plaque progression, particularly in lipid-rich lesions, while potent P2Y12 inhibitors (ticagrelor, prasugrel) better prevent inflammation-driven SER50. In calcified lesions, statins may have limited impact, making lesion preparation with RA/IVL essential. Optimized dual antiplatelet therapy duration can help mitigate SER in high-risk lesions, but bleeding risk must be considered.
Although this study did not incorporate computational fluid dynamics (CFD) modeling, the observed wall shear stress (WSS) disturbances associated with stent underexpansion and malapposition may be further elucidated in future studies through CFD-based simulations. Integration of CFD with intravascular imaging data could provide patient-specific hemodynamic insights and improve mechanistic classification and treatment planning in SER.
Limitations
This research has several limitations that should be taken into account. Firstly, the research was a non-randomized observational design with a limited sample size, potentially introducing selection and information biases. Secondly, the stents initially deployed at the SER target lesions could be either BMS, first-generation DES, or second-generation DES, which may influence plaque development to a certain degree. While SER mechanisms differ between BMS and DES, the number of BMS cases in our study was relatively small. Given this limited sample size, their impact on the overall findings is expected to be minimal. However, including BMS cases allows for a more comprehensive real-world analysis of edge ISR across different stent types, providing broader clinical applicability. Excluding BMS cases entirely might introduce selection bias and reduce the generalizability of our findings. Consequently, the conclusions of this study may not be universally applicable. Thirdly, we were unable to ascertain whether previous pharmacotherapy had an impact on the prevalence and morphological features of SER. Notably, the variable use of statins and antiplatelet medications might contribute to the variations in the pattern of SER stenotic tissue structure between the two patient cohorts. Fourthly, due to the presence of multiple contributing elements, the primary etiology categorization could be misinterpreted. Fifthly, IVUS possesses inherent restrictions in the qualitative assessment of restenotic tissues. Its imaging characteristics do not perfectly align with histological findings, thereby presenting a risk of misclassifying pathological attributes. Hence, additional investigations integrating OCT, NIRS, or alternative methodologies are warranted. Sixthly, although IVUS was applied in all cases, ideal landing zones with < 60% plaque burden could not always be selected in complex lesions, such as those with diffuse disease or severe calcification. In these cases, lesion preparation and stent expansion were prioritized. This reflects a practical limitation in applying guideline-based IVUS criteria to real-world anatomy. Lastly, these are problems that remain even if the exact underlying morphology is known, and hinge motion is one of those that can only be modified minimally.
Conclusions
This study comprehensively examines the biological and mechanical mechanisms underlying SER and evaluates long-term treatment outcomes. Our findings show that biological factors (NIH, neoatherosclerosis, and uncovered lesions) account for the majority of SER cases (89.7%), while mechanical factors (stent underexpansion, protruding CN) contribute to 10.3%. Treatment outcomes were comparable between DCB angioplasty and DES implantation, with a low DoCE incidence (7.1%) over two years, demonstrating the effectiveness of current interventions. Notably, DCB therapy was non-inferior to DES, making it a viable alternative, especially for biologically driven SER. IVUS analysis revealed a higher frequency of SER-related restenosis in the LAD and a greater tendency for repeat stenting in biological SER cases. Additionally, stent underexpansion (8%) highlights the need for optimal lesion preparation and post-dilatation to minimize restenosis risk. Our findings emphasize the importance of personalized treatment strategies based on SER mechanisms. While DCB therapy reduces NIH and metal burden, DES implantation remains necessary in cases with residual plaque or large-caliber lesions. Future research should explore advanced imaging techniques (OCT, near infrared reflectance spectroscopy (NIRS)) and refine interventional strategies to improve long-term outcomes. This study provides critical insights into SER pathophysiology and treatment efficacy, supporting a mechanism-based approach to intervention. Further research is warranted to optimize treatment strategies, minimize restenosis, and enhance long-term clinical durability in PCI.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Piccolo, R. et al. Drug-eluting or bare-metal stents for percutaneous coronary intervention: a systematic review and individual patient data meta-analysis of randomised clinical trials. Lancet. 393, 2503–2510. https://doi.org/10.1016/s0140-6736(19)30474-x (2019).
Otake, H. Stent edge Restenosis- an inevitable drawback of stenting?? Circ. J. Off. J. Jpn. Circ. Soc. 85, 1969–1971. https://doi.org/10.1253/circj.CJ-21-0581 (2021).
Dangas, G. D. et al. In-stent restenosis in the drug-eluting stent era. J. Am. Coll. Cardiol. 56, 1897–1907. https://doi.org/10.1016/j.jacc.2010.07.028 (2010).
Ino, Y. et al. Optical coherence tomography predictors for edge restenosis after Everolimus-Eluting stent implantation. Circ. Cardiovasc. Interv. 9 https://doi.org/10.1161/circinterventions.116.004231 (2016).
Kang, S. J. et al. Intravascular ultrasound predictors for edge restenosis after newer generation drug-eluting stent implantation. Am. J. Cardiol. 111, 1408–1414. https://doi.org/10.1016/j.amjcard.2013.01.288 (2013).
Kim, Y. G. et al. Mechanism of edge restenosis after drug-eluting stent implantation. Angulation at the edge and mechanical properties of the stent. Circ. J. Off. J. Jpn. Circ. Soc. 77, 2928–2935. https://doi.org/10.1253/circj.cj-12-1259 (2013).
Sakurai, R. et al. Predictors of edge stenosis following sirolimus-eluting stent deployment (a quantitative intravascular ultrasound analysis from the SIRIUS trial). Am. J. Cardiol. 96, 1251–1253. https://doi.org/10.1016/j.amjcard.2005.06.066 (2005).
Zbinden, R. et al. Ultrathin strut biodegradable polymer Sirolimus-Eluting stent versus Durable-Polymer Everolimus-Eluting stent for percutaneous coronary revascularization: 2-Year results of the BIOSCIENCE trial. J. Am. Heart Assoc.. 5, e003255. https://doi.org/10.1161/jaha.116.003255 (2016).
Tandjung, K. et al. Clinical outcome following stringent discontinuation of dual antiplatelet therapy after 12 months in real-world patients treated with second-generation zotarolimus-eluting resolute and everolimus-eluting Xience V stents: 2-year follow-up of the randomized TWENTE trial. J. Am. Coll. Cardiol. 61, 2406–2416. https://doi.org/10.1016/j.jacc.2013.04.005 (2013).
Kitahara, H. et al. Impact of stent size selection on acute and Long-Term outcomes after Drug-Eluting stent implantation in de Novo coronary lesions. Circ. Cardiovasc. Interv. 10. https://doi.org/10.1161/circinterventions.116.004795 (2017).
Jimba, T., Ikutomi, M., Tsukamoto, A., Matsushita, M. & Yamasaki, M. Effect of hinge motion on stent Edge-Related restenosis after right coronary artery treatment in the current Drug-Eluting stent era. Circ. J. Off. J. Jpn. Circ. Soc. 85, 1959–1968. https://doi.org/10.1253/circj.CJ-21-0196 (2021).
Jeger, R. V. et al. Drug-Coated balloons for coronary artery disease: third report of the international DCB consensus group. JACC Cardiovasc. Intervent. 13, 1391–1402. https://doi.org/10.1016/j.jcin.2020.02.043 (2020).
Ryan, T. J. et al. Guidelines for percutaneous transluminal coronary angioplasty. A report of the American college of cardiology/american heart association task force on assessment of diagnostic and therapeutic cardiovascular procedures (Subcommittee on percutaneous transluminal coronary Angioplasty). Circulation 78, 486–502. https://doi.org/10.1161/01.cir.78.2.486 (1988).
Ellis, S. G. et al. Coronary morphologic and clinical determinants of procedural outcome with angioplasty for multivessel coronary disease. Implications for patient selection. Multivessel angioplasty prognosis study group. Circulation 82, 1193–1202. https://doi.org/10.1161/01.cir.82.4.1193 (1990).
Mehran, R. et al. Angiographic patterns of in-stent restenosis: classification and implications for long-term outcome. Circulation 100, 1872–1878. https://doi.org/10.1161/01.cir.100.18.1872 (1999).
Nasu, K. et al. Efficacy of Biolimus A9-eluting stent for treatment of right coronary ostial lesion with intravascular ultrasound guidance: a multi-center registry. Cardiovasc. Intervention Ther. 33, 321–327. https://doi.org/10.1007/s12928-017-0487-4 (2018).
Song, L. et al. Characteristics of early versus late in-stent restenosis in second-generation drug-eluting stents: an optical coherence tomography study. EuroIntervention: J. EuroPCR Collab. Working Group. Interventional Cardiol. Eur. Soc. Cardiol. 13, 294–302. https://doi.org/10.4244/eij-d-16-00787 (2017).
Shlofmitz, E., Iantorno, M. & Waksman, R. Restenosis of Drug-Eluting stents: A new classification system based on disease mechanism to guide treatment and State-of-the-Art review. Circ. Cardiovasc. Interv. 12, e007023. https://doi.org/10.1161/circinterventions.118.007023 (2019).
Lee, C. W. et al. Intravascular ultrasound findings in patients with very late stent thrombosis after either drug-eluting or bare-metal stent implantation. J. Am. Coll. Cardiol. 55, 1936–1942. https://doi.org/10.1016/j.jacc.2009.10.077 (2010).
Nakamura, N. et al. Formation of calcified nodule as a cause of early In-Stent restenosis in patients undergoing Dialysis. J. Am. Heart Assoc. 9, e016595. https://doi.org/10.1161/jaha.120.016595 (2020).
Cutlip, D. E. et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 115, 2344–2351. https://doi.org/10.1161/circulationaha.106.685313 (2007).
Yamamoto, K. et al. Mechanisms and treatment outcomes of ostial right coronary artery in-stent restenosis. EuroIntervent. J. EuroPCR Collab. Work. Group Intervent. Cardiol. Eur. Soc. Cardiol. 19, e383–e393. https://doi.org/10.4244/eij-d-23-00107 (2023).
Shafiabadi Hassani, N. et al. In-Stent restenosis overview: from intravascular imaging to optimal percutaneous coronary intervention management. Med. (Kaunas Lithuania). 60. https://doi.org/10.3390/medicina60040549 (2024).
Her, A. Y. & Shin, E. S. Current management of In-Stent restenosis. Korean Circ. J. 48, 337–349. https://doi.org/10.4070/kcj.2018.0103 (2018).
Nebeker, J. R. et al. Hypersensitivity cases associated with drug-eluting coronary stents: a review of available cases from the research on adverse drug events and reports (RADAR) project. J. Am. Coll. Cardiol. 47, 175–181. https://doi.org/10.1016/j.jacc.2005.07.071 (2006).
Subban, V. & Raffel, O. C. Optical coherence tomography: fundamentals and clinical utility. Cardiovasc. Diagnosis Ther. 10, 1389–1414. https://doi.org/10.21037/cdt-20-253 (2020).
Nusca, A. et al. Stent Neo-Atherosclerosis: pathophysiology, clinical implications, prevention, and therapeutic approaches. Life (Basel Switzerland). 12. https://doi.org/10.3390/life12030393 (2022).
Alfonso, F., Coughlan, J. J., Giacoppo, D., Kastrati, A. & Byrne, R. A. Management of in-stent restenosis. EuroIntervent. J. EuroPCR Collab. Work. Group. Intervent. Cardiol. Eur. Soc. Cardiol. 18, e103–e123. https://doi.org/10.4244/eij-d-21-01034 (2022).
Kobayashi, N. et al. Prevalence, features, and prognostic importance of edge dissection after Drug-Eluting stent implantation: an ADAPT-DES intravascular ultrasound substudy. Circ. Cardiovasc. Interv. 9, e003553. https://doi.org/10.1161/circinterventions.115.003553 (2016).
Ali, Z. A. et al. Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI): a randomised controlled trial. Lancet (London England). 388, 2618–2628. https://doi.org/10.1016/s0140-6736(16)31922-5 (2016).
Kang, S. J. et al. Comprehensive intravascular ultrasound assessment of stent area and its impact on restenosis and adverse cardiac events in 403 patients with unprotected left main disease. Circ. Cardiovasc. Interv. 4, 562–569. https://doi.org/10.1161/circinterventions.111.964643 (2011).
Torii, R. et al. Implications of the local hemodynamic forces on the formation and destabilization of neoatherosclerotic lesions. Int. J. Cardiol. 272, 7–12. https://doi.org/10.1016/j.ijcard.2018.06.065 (2018).
Sugane, H. et al. Cardiac outcomes in patients with acute coronary syndrome attributable to calcified nodule. Atherosclerosis 318, 70–75. https://doi.org/10.1016/j.atherosclerosis.2020.11.005 (2021).
Gupta, A. et al. Coronary intravascular lithotripsy in contemporary practice: challenges and opportunities in coronary intervention. Ther. Adv. Cardiovasc. Dis. 18, 17539447241263444. https://doi.org/10.1177/17539447241263444 (2024).
Armstrong, E. J. et al. Intravascular lithotripsy for peripheral artery calcification: 30-Day outcomes from the disrupt PAD III observational study. J. Endovasc. Ther.Off. J. Int. Soc. Endovasc. Spec. 15266028241283716 https://doi.org/10.1177/15266028241283716 (2024).
Golabkesh, M., Mundfortz, D. & Haude, M. First case report of a percutaneous coronary intervention with intracoronary lithotripsy in a heavily calcified and tortuous right coronary artery using the R-One(+) robotic system. Eur. Heart J. Case Rep. 8, ytae563. https://doi.org/10.1093/ehjcr/ytae563 (2024).
Cassese, S. et al. Intracoronary stenting and additional results achieved by shockwave coronary lithotripsy: design and rationale of ISAR-WAVE trial. Am. Heart J. 282, 1–12. https://doi.org/10.1016/j.ahj.2024.12.008 (2025).
Chen, G., Zrenner, B. & Pyxaras, S. A. Combined rotational atherectomy and intravascular lithotripsy for the treatment of severely calcified in-Stent neoatherosclerosis: A Mini-Review. Cardiovasc. Revasc. Med. Mol. Intervent.. 20, 819–821. https://doi.org/10.1016/j.carrev.2018.10.007 (2019).
Lee, T. et al. Prevalence, predictors, and clinical presentation of a calcified nodule as assessed by optical coherence tomography. JACC Cardiovasc. Imaging. 10, 883–891. https://doi.org/10.1016/j.jcmg.2017.05.013 (2017).
Nagasaka, T. et al. Drug-coated balloons for the treatment of stent edge restenosis. Coron. Artery Dis. 34, 236–243. https://doi.org/10.1097/mca.0000000000001235 (2023).
Kleber, F. X. et al. Local Paclitaxel induces late lumen enlargement in coronary arteries after balloon angioplasty. Clin. Res. Cardiol. Off. J. German Cardiac Soc. 104, 217–225. https://doi.org/10.1007/s00392-014-0775-2 (2015).
Peng, N. et al. Drug-Coated Balloons versus Everolimus-Eluting Stents in Patients with In-Stent Restenosis: A Pair-Wise Meta-Analysis of Randomized Trials. Cardiovasc. Ther. 1042329. https://doi.org/10.1155/2020/1042329 (2020).
Cortese, B. et al. Treatment of coronary artery disease with a new-generation drug-coated balloon: final results of the Italian elutax SV rEgistry-DCB-RISE. J. Cardiovasc. Med. (Hagerstown Md). 19, 247–252. https://doi.org/10.2459/jcm.0000000000000632 (2018).
Camaj, A. et al. Drug-Coated balloons for the treatment of coronary artery disease: A review. JAMA Cardiol. 10, 189–198. https://doi.org/10.1001/jamacardio.2024.4244 (2025).
Costa, M. A. et al. Impact of stent deployment procedural factors on long-term effectiveness and safety of sirolimus-eluting stents (final results of the multicenter prospective STLLR trial). Am. J. Cardiol. 101, 1704–1711. https://doi.org/10.1016/j.amjcard.2008.02.053 (2008).
Kufner, S. et al. Neointimal modification with scoring balloon and efficacy of Drug-Coated balloon therapy in patients with restenosis in Drug-Eluting coronary stents: A randomized controlled trial. JACC Cardiovasc. Intervent. 10, 1332–1340. https://doi.org/10.1016/j.jcin.2017.04.024 (2017).
Mintz, G. S., Matsumura, M., Ali, Z. & Maehara, A. Clinical utility of intravascular imaging: past, present, and future. JACC Cardiovasc. Imaging. 15, 1799–1820. https://doi.org/10.1016/j.jcmg.2022.04.026 (2022).
Mitsis, A. et al. Innovations in intracoronary imaging: present clinical practices and future outlooks. J. Clin. Med. 13 https://doi.org/10.3390/jcm13144086 (2024).
Tufaro, V. et al. Emerging hybrid intracoronary imaging technologies and their applications in clinical practice and research. JACC Cardiovasc. Intervent. 17, 1963–1979. https://doi.org/10.1016/j.jcin.2024.07.007 (2024).
Xenogiannis, I. et al. Saphenous vein graft failure: from pathophysiology to prevention and treatment strategies. Circulation 144, 728–745. https://doi.org/10.1161/circulationaha.120.052163 (2021).
Funding
This work was supported by Natural Science Foundation of Hunan Province (No.2022JJ30575) and Health Research Project of Hunan Provincial Health Commission (No. 20233486).
Author information
Authors and Affiliations
Contributions
X.W. and L.W. had the idea for the paper, reviewed and edited it critically for important intellectual content. Z.L. and H.H. performed the literature search and analysis. X.W., M.X.W., L.W., H.B.H. and H.H. substantially contributed to the conception of the paper, drafted and critically revised the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The present research was carried out in accordance with the tenets mentioned in the Helsinki Declaration and was approved by the Ethical Board of Xiangtan Central Hospital (approval number:X201875231-1). Prior to the commencement of the research, our team obtained written informed consent from each patient.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wu, X., Liu, Z., Huang, H. et al. Intravascular ultrasound assessment of stent edge restenosis mechanisms and treatment outcomes following percutaneous coronary intervention. Sci Rep 15, 16298 (2025). https://doi.org/10.1038/s41598-025-01381-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-01381-9