Abstract
Transarterial chemoembolization (TACE) is considered unsuitable for hepatocellular carcinoma (HCC) that exceeds up-to-7 criteria. Balloon-occluded alternative infusion of cisplatin solution and gelatin particles of transarterial chemoembolization (BOAI-TACE) has shown promise in the treatment of HCC and preservation of liver function. This prospective, single-arm study enrolled patients with HCC beyond up-to-7 criteria from five hospitals. The primary endpoint was objective response ratio (ORR) for BOAI-TACE, according to response evaluation criteria in cancer of the Liver (RECICL), at 2 months after treatment. Eighteen patients were enrolled in this study. Fourteen patients achieved response, resulting in an ORR of 77.8% (95% confidence interval [CI] 54.3–91.5%) according to both RECICL and modified response evaluation criteria in solid tumor (mRECIST) guidelines, meeting the primary endpoint. Disease control rate was 88.9% (95% CI 66.0–98.1%). No worsening of either Child–Pugh or albumin–bilirubin (ALBI) scores was observed. No serious adverse events were recorded, indicating that BOAI-TACE retains utility even in severe HCC cases while preserving liver function.
Similar content being viewed by others
Introduction
Treatment of hepatocellular carcinoma (HCC) includes surgical resection, radiofrequency ablation, transarterial chemoembolization (TACE), chemotherapy, and radiation therapy1,2. TACE is a method of transcatheterally injecting anticancer drugs and embolic substances into the tumor feeding artery to induce ischemia and necrosis of HCC. It is widely used, especially for intermediate-stage HCC as categorized by the Barcelona Clinic Liver Cancer (BCLC) classification1,2. Recently, new drug therapies, such as molecularly targeted agents and immune checkpoint inhibitors, have been added to these existing therapies3,4, demonstrating superior results compared to existing chemotherapy and forcing a major paradigm shift in HCC treatment. There is a major trend to argue that molecularly targeted agents and immune checkpoint inhibitors should be used instead of TACE for intermediate-stage HCCs beyond the up-to-7 criteria (i.e., when the sum of the maximum diameter in centimeters of the tumor and the tumor number is 7 or greater), which was previously considered indicative for TACE in the past4. This is because conventional TACE is considered ineffective in treating HCC beyond the up-to-7 criteria and preservation of hepatic reserve is also difficult4. Because molecularly targeted drugs and immune checkpoint inhibitors are contraindicated once hepatic reserve is compromised, preservation of hepatic reserve is crucial for advancing HCC treatment.
Although TACE is the currently established treatment for HCC, its methodology remains variable. Anthracycline agents (doxorubicin and epirubicin) or platinum-based agents are typical5, while ethiodized oil, gelatin sponges, and drug-eluting beads are used as embolic materials. Treatment may also employ balloon occlusion6. A previous report on balloon-occluded alternative infusion transarterial chemoembolization (BOAI-TACE) found an ORR of 73% and fewer cases of liver function deterioration versus conventional techniques7. The treatment efficacy of BOAI-TACE for HCC beyond up-to-7 criteria was also better than previous TACE. However, these results were from a secondary analysis of a prospective trial. In the present study, evaluation of BOAI-TACE treatment efficacy for HCC beyond up-to-7 criteria and liver function were prospectively examined.
Materials and methods
Ethics
This prospective, single-arm study was performed at five hospitals from March 2021 to December 2023. This study was approved by the University of Tsukuba Clinical Research Review Board (CRB3180028) and written, informed consent was obtained from individual participants. This study complied with the tenets of the Declaration of Helsinki. This study was registered in the Japan Registry of Clinical Trials. The clinical trial registration number is jRCTs031200334, and the date of first registration was 29/01/2021.
Participant population
The inclusion criteria for participants in this study were as follows: (i) HCC diagnosed by contrast-enhanced CT or MRI according to the guidelines of the European Association for the Study of Liver Disease8, (ii) HCC classified as beyond up-to-7 criteria, (iii) indication for TACE in more than one subsegment, (iv) age of 20 years or more, (v) Eastern Cooperative Oncology Group performance status of 0 or 1, (vi) obtaining of consent, (vii) and expected to have a survival prognosis of at least 3 months after TACE. The exclusion criteria for participants in this study were: (i) Child–Pugh score greater than or equal to seven, (ii) renal function with an estimated glomerular filtration rate (eGFR) of less than 40 ml/min/1.73 m2, (iii) tumor invasion to the portal or hepatic vein, (iv) the largest tumor was greater than or equal to 15 cm in diameter, (v) presence of sarcomatous changes (tumor size increases 2 times or more over the course of 2–3 months and lack of arterial enhancement), (vi) lymph node metastasis or distant metastasis, (vii) previous surgical or endoscopic biliary reconstruction, (viii) dilatation of bile ducts larger than the accompanying portal vein, (ix) severe arterial-portal or arterial-venous shunts that could interfere with treatment, (x) severe mental disorders, (xi) severe allergy to iodine contrast media or other drugs, (xii) pregnancy or lactation, (xiii) a history of radiotherapy for HCC, (xiv) and/or those deemed unsuitable by the principal investigator or a sub-investigator of the study. An additional restriction was that each eligible patient could only be enrolled once. Patients who received treatment for HCC during the period from the time of BOAI-TACE to the time of post-treatment image evaluation were excluded from the study.
BOAI-TACE
BOAI-TACE was performed according to a previous method7. A detailed methodology is available in the Supplemental Methods section.
Participant characteristics and laboratory data
Participant data obtained included age, sex, performance status, cause of hepatitis, comorbidities, medical history, Child–Pugh score, warfarin history, and previous treatment for HCC.
Serum white blood cell count, hemoglobin, platelet count, albumin, total bilirubin, aspartate aminotransferase (AST), alanine transaminase (ALT), γ-glutamyl transferase (GTP), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), serum creatinine, eGFR, amylase, and prothrombin time international normalized ratio (PT/INR) were measured within 2 weeks before treatment and then on the day after treatment, 7 days after treatment, 1 month after treatment, and 2 months after treatment. Alpha fetoprotein (AFP) and protein induced by vitamin K absence (PIVKA)-II were measured within 2 weeks before and 2 months after treatment.
All data were stored in an electronic data capture system; all entry dates and alterations were recorded electronically.
Primary and secondary endpoints
The primary endpoint in this study was objective response ratio (ORR) (complete response plus partial response) for BOAI-TACE for HCC beyond up-to-7 criteria 2 months after treatment according to response evaluation criteria in cancer of the liver (RECICL)9. The secondary endpoint was ORR 2 months after treatment by modified response evaluation criteria in solid tumor (mRECIST)10. Another secondary endpoint was evaluation of Child–Pugh and albumin–bilirubin (ALBI) scores 2 months after treatment11. As a secondary analysis of a prospective trial, the ORR of BOAI-TACE for HCC beyond up-to-11 criteria (i.e., when the sum of the maximum diameter in centimeters of the tumor and the tumor number is 11 or greater) was evaluated.
Imaging assessment
Dynamic contrast-enhanced CT or MRI was used for imaging evaluation of HCC; both CT and MRI slices were less than 2.5 mm thick. Anonymized images were evaluated central-review style by two board-certified radiologists (29 and 25 years of experience) who did not conduct BOAI-TACE during the study period. Both size and number of tumors were quantified. Pre- and post-treatment images were evaluated by RECICL and mRECIST. A medical image analysis workstation (AZE Virtual Space, Canon Medical Systems Corporation, Tochigi, Japan) was used for imaging assessment.
Adverse events
Adverse events were evaluated according to the Society of Interventional Radiology guidelines12. Post-TACE laboratory data were assessed using Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. Adverse events were assessed up to 3 months after treatment.
Statistical analysis
The required sample size was estimated based on a threshold ORR of 33.3%3 and an expected ORR of 82.6%7, with 90% power, and an alpha value of 0.1 (one-side) using the binomial test. Given a 10% ineligible participant rate, the target sample size was determined to be at least 19 participants.
Worsening of Child–Pugh and/or ALBI scores before and 2 months after treatment was evaluated by Wilcoxon signed-rank test. Tumor markers were also analyzed by the Wilcoxon signed-rank test. Statistical analyses were performed using SPSS for Windows version 26 (IBM SPSS Inc., Chicago, IL). The significance level was set at 0.05.
Results
Participant characteristics
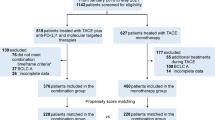
Eighteen participants were enrolled during the study period (Fig. 1). Although this was one case less than the planned 19, the study was completed as planned because there were no dropouts. As 10% dropout was expected, the registration of 18 cases was within the acceptable range of the research plan and the research period was not extended. Informed consent was obtained in all 18 cases and there were no cases of ineligibility or withdrawal of consent. Safety and efficacy evaluations were performed on all enrolled patients.
Characteristics of the participants enrolled in this study are summarized in Table 1. Mean participant age was 77.6 years (standard deviation: 7.2, range: 66–94, Male:Female = 14:4). The mean maximum tumor diameter was 39.2 mm (range: 12–143 mm) while the mean number of tumors was 9.4 (range: 3–20). Pretreatment Child–Pugh scores were 5 in 14 participants and 6 in 4 participants. The mean pretreatment ALBI score was− 2.55. Thirteen of the 18 enrollees in the study were beyond up-to-11 criteria.
BOAI-TACE and treatment efficacy outcomes
The procedure was completed in all participants with a mean cisplatin dosage used in BOAI-TACE per participant of 80.4 mg (range: 50–100 mg). The mean number of treated segmental arteries of the liver was 5.9 (range: 3–8). In 11 of 18 participants, treatment from extrahepatic parasitic arteries was added. Eight participants were treated from the right inferior phrenic artery, one from the bilateral inferior phrenic arteries, one from the right inferior phrenic and cystic arteries, and one from the cystic artery.
Overall RECICL and mRECIST evaluation between two readers matched for all participants, although there were disagreements on tumor numbers in three cases which were resolved after discussion of images. Of the two reader measurements, the smaller pre-treatment tumor diameter was adopted while the larger diameter was used for the post-treatment measurement. In other words, the most unfavorable reading for the overall evaluation was adopted. ORR and DCR (disease control rates) of BOAI-TACE for HCCs beyond up-to-7 criteria in this study were 77.8% (95% confidence intervals [CI] 54.3–91.5%) and 88.9% (95% CI 66.0–98.1%) according to both RECICL and mRECIST (Table 2). Some representative cases are shown in Figs. 2, 3, 4 and 5. Pre- and post-treatment median values for serum AFP were 13.8 ng/ml (range: 2.5–35,000) and 11.2 ng/ml (range: 2.2–1690). We did not find evidence of differences between pre- and post-treatment AFP (p = 0.156). Pre- and post-treatment median values for serum PIVKA-II were 123 mg/ml (range: 26–22,900) and 27 mg/ml (range: 16–399). Post-treatment PIVKA-II was significantly decreased (p = 0.002).
Arterial phase of contrast-enhanced CT before (a, b, c) and after (d, e, f) BOAI-TACE in a 70-year-old man. Three hepatocellular carcinomas (HCCs) were found in the liver, including a 45 mm HCC in S6 (a, arrow). The contrast effect of a 45 mm HCC in S6 disappeared after treatment and complete response (CR) was achieved (d). (b, e) A 14 mm HCC in S6 was also achieved CR (arrowhead). (c, f) An HCC in S2 (curved arrow) did not achieve CR and the overall evaluation was partial response.
Arterial phase of contrast-enhanced MRI before (a, b, c) and after (d, e, f) BOAI-TACE in a 75-year-old man. Sixteen hepatocellular carcinomas (HCCs) were found in the liver, including a 32 mm HCC in S6 (a, arrow). The contrast effect of a 32 mm HCC in S6 disappeared after treatment and complete response (CR) was achieved (d). (b, e) A 22 mm HCC in S6 was also achieved CR (arrowhead). (c, f) An HCC in S5 (curved arrow) was also achieved CR. CR was obtained for the target lesions; however, there was a residual tumor in the non-target lesions and the overall evaluation was partial response.
Arterial phase of contrast-enhanced CT before (a, b) and after (c, d) BOAI-TACE in a 70-year-old woman. Five hepatocellular carcinomas (HCCs) were found in the liver, including a 143 mm HCC in S8/4 (a, arrow). The largest lesion was markedly reduced in size (c). (b, d) Complete response was obtained for the other HCCs (arrowhead). The overall evaluation was partial response.
Arterial phase of contrast-enhanced CT before (a, b, c, d) and after (e, f, g, h) BOAI-TACE in a 78-year-old man. Seven HCCs were found in the liver, including a 14 mm hepatocellular carcinoma (HCC) in S3 (arrow), a 13 mm HCC in S4/8 (white arrowhead), a 11 mm HCC in S8/5 (curved arrow), and a 6 mm HCC in S5 (black arrowhead). After treatment, the contrast effect of all lesions disappeared and complete response was obtained.
Secondary analysis of this prospective trial revealed that treatment efficacies for HCC beyond up-to-11 criteria were an ORR of 69.2% (95% Cl 42.0–87.6%) and a DCR of 84.6% (56.5–96.9%) (Table 2).
Liver function outcomes
Mean Child–Pugh and ALBI scores 2 months after BOAI-TACE were 5.16 and − 2.54. We did not find evidence of differences between Child–Pugh and ALBI scores before or after BOAI-TACE (p = 0.56 and p = 0.65, respectively). The Child–Pugh scores before and after BOAI-TACE are presented in Table 3 and the ALBI scores in Table 4.
Adverse events
Of the 18 participants, one participant developed grade 2 renal dysfunction and right cerebral infarction the day after BOAI-TACE. Since the infarction was unilateral and there was stenosis of the internal carotid artery, hemodynamic factors were regarded as the cause. The decrease in renal function was also thought to be largely owed to dehydration. A direct causal relationship between these adverse events and BOAI-TACE was considered unlikely. After 2 months, renal function trended towards improvement. Evaluation after 6 months showed that paralysis had almost disappeared and did not interfere with daily living.
No abnormal leukocytosis or leukopenia was observed. Grade 1 anemia was observed in eight participants and grade 2 anemia in one participant but both were present before treatment and were not treatment related. Nine of the 18 participants had grade 1 thrombocytopenia and five had grade 2 thrombocytopenia, while platelet counts decreased in four cases compared to pre-treatment levels. Elevated AST was seen in all participants (grade 2 in 10 and grade 3 in 4) but all were transient. ALT was elevated in 14 of the 18 participants: 4 were grade 2 and 1 was grade 3, but all were transient. Grade 1 increases in LDH were observed in 14 of 18 participants, but ALP increases were absent. Serum creatinine was elevated to grade 1 in three participants and grade 2 in one, but all had improved renal function after 2 months. One participant had grade 2 serum amylase elevation, but it was transient and without symptoms. Prolonged PT/INR grade 1 was observed in 6 participants and grade 2 in one participant, but values recovered to pre-treatment levels after 2 months.
Discussion
In the present study, a multicenter, prospective trial of 18 participants was conducted using BOAI-TACE, limiting eligibility to participants with HCC beyond up-to-7 criteria. The ORR and DCR were 77.8% (95%CI 54.3–91.5%) and 88.9% (95%CI 66.0–98.1%) according to both RECICL and mRECIST. As the previously reported ORR for TACE for HCC beyond up-to-7 criteria in a prospective study was 33.3%, the lower 95% CI limit seen in this study exceeds the ORR of previous reports3. Thus, the pre-specified primary endpoint of efficacy was met. The ORR obtained in this study is almost equivalent to a previously reported ORR of 86.2% (95%CI 62.3–93.6%), indicating stable outcomes from BOAI-TACE7. Since BOAI-TACE does not require stringent vessel selection, the technique is easier than conventional TACE, which may lead to more stable results. Additionally, treatment with BOAI-TACE significantly reduced PIVKA-II and this result may reflect a positive therapeutic effect. Although AFP did not decrease significantly, this may be due to the fact that there were more cases in the normal range before treatment compared to PIVKA-II.
Albeit as an ad hoc analysis, we found the ORR for HCC beyond the up-to-11 criteria was 69.2% (95% CI 42.0–87.6%) in this study. Although reports of TACE efficacy for HCC beyond the up-to-11 criteria are scarce, a systematic review of conventional TACE for HCC reported an ORR of 52.5% (95% CI 43.6–61.5)13. Even in patients beyond the up-to-11 criteria, which is considered difficult to control with TACE, our results are tentatively comparable to conventional TACE for even advanced HCC.
The prevailing paradigm is that therapeutic effect from TACE is a trade-off with hepatic reserve14,15. Notably, previous reports have shown percentages of worsening Child–Pugh classification after TACE ranging from 9 to 41.2%16,17,18, highlighting TACE as a risk factor for worsening liver function, especially in HCC beyond the up-to-7 criteria19. Progressive liver deterioration itself not only worsens quality of life and prognosis, but also interferes with systemic therapy featuring molecularly targeted drugs and immune checkpoint inhibitors4. It is thus considered critical to maintain hepatic reserve in HCC patients. In this study, there was no deterioration in either Child–Pugh or ALBI scores at 2 months after BOAI-TACE. Not a single case fell from A to B after 2 months in the Child–Pugh classification, even though all study participants had HCC beyond the up-to-7 criteria and 72% were beyond the up-to-11 criteria. This study demonstrates that even wide-range BOAI-TACE does not impair liver function, giving options for patients beyond the up-to-11 criteria that are otherwise considered to be at a severely elevated risk of progressive liver deterioration20. TACE for hepatocellular carcinoma beyond the up-to-7 or -11 criteria is not recommended due to poor therapeutic effect and worsened liver function from necessarily extensive embolization. BOAI-TACE, however, has good therapeutic value for these types of HCC and preserves liver function.
No major complications related to BOAI-TACE were observed in this study, similar to a previous report of 41 patients7. Long-term studies have yet to be accomplished, but Miksad and colleagues found in the LiverT study that most TACE-related complications happen in the acute phase (0–29 days after treatment) and persist through the chronic phase (30–90 days after treatment)21. Thus, our 90-day post-procedure follow-up is comparable to existing literature. When this study is combined with previous studies, BOAI-TACE was performed on 59 patients in prospective studies, and no safety issues were observed7. A major paradigm shift in the treatment of HCC is currently underway with the surfeit of recently developed immune checkpoint inhibitors and molecularly targeted drugs22,23. Because liver function must be preserved to safely use these drugs, the trend has been to avoid TACE, which impairs liver function4. On the other hand, BOAI-TACE does not cause a decrease in hepatic reserve and responsive patients can be easily transitioned to immune checkpoint inhibitors or molecularly targeted agents. Sequential treatment combining these agents with BOAI-TACE is definitely possible, as tumor shrinkage and cell death caused by BOAI-TACE may enhance the efficacy of immune checkpoint inhibitors for backward treatment effects. BOAI-TACE as a second-line treatment when immune checkpoint inhibitors or molecular-targeted drugs are refractory or intolerable may also be considered.
This study must acknowledge several limitations. Firstly, the observation period was short, as the primary and secondary endpoints of this study were the evaluation of treatment response and liver function at 2 months after BOAI-TACE. Long-term treatment effects are unknown and require further investigation in addition to evaluation of hepatic reserve after repeated BOAI-TACE. Secondly, this study was performed at five institutions, with a relatively small number of clinicians performing the procedures. In the future, it is necessary to examine whether BOAI-TACE can be performed at more facilities to reproduce the same therapeutic efficacy and safety profiles of past reports. Because the subgroup analysis of 13 patients beyond the up-to-11 criteria was based on an ad-hoc analysis and the number of cases is small, it is less reliable than the results of the analysis of patients beyond the up-to-7 criteria. However, our results, taken together, indicate the utility and safety of BOAI-TACE as a versatile option for HCC and a target for large, diverse future studies.
In conclusion, BOAI-TACE is effective for HCCs beyond the up-to-7 criteria and preserves liver function. BOAI-TACE could replace conventional TACE, since objective response and liver preservation are both crucial to prolong overall survival and maintain quality of life in HCC patients.
Data availability
The data that support the findings of this study are not publicly available due to their containing information that could compromise the privacy of research participants but are available from the corresponding author (S.H., hoshiai@md.tsukuba.ac.jp) upon reasonable request.
Abbreviations
- ALBI:
-
Albumin–bilirubin
- BOAI-TACE:
-
Balloon-occluded alternative infusion of cisplatin solution and gelatin particles of transarterial chemoembolization
- DCR:
-
Disease control rate
- HCC:
-
Hepatocellular carcinoma
- mRECIST:
-
Modified response evaluation criteria in solid tumor
- ORR:
-
Objective response ratio
- TACE:
-
Transarterial chemoembolization
- RECICL:
-
Response evaluation criteria in cancer of the liver
References
Bruix, J., Sherman, M. & American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: An update. Hepatology 53, 1020–1022 (2011).
Reig, M. et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 76, 681–693 (2022).
Kudo, M. et al. Lenvatinib as an initial treatment in patients with intermediate-stage hepatocellular carcinoma beyond up-to-seven criteria and Child–Pugh a liver function: A proof-of-concept study. Cancers 11, 1084 (2019).
Kudo, M. et al. A changing paradigm for the treatment of intermediate-stage hepatocellular carcinoma: Asia–Pacific primary liver cancer expert consensus statements. Liver Cancer 9, 245–260 (2020).
Horikawa, M., Miyayama, S., Irie, T., Kaji, T. & Arai, Y. Development of conventional transarterial chemoembolization for hepatocellular carcinomas in Japan: Historical, strategic, and technical review. AJR Am. J. Roentgenol. 205, 764–773 (2015).
Irie, T., Kuramochi, M. & Takahashi, N. Dense accumulation of lipiodol emulsion in hepatocellular carcinoma nodule during selective balloon-occluded transarterial chemoembolization: Measurement of balloon-occluded arterial stump pressure. Cardiovasc. Intervent. Radiol. 36, 706–713 (2013).
Hoshiai, S. et al. A Transarterial chemoembolization of balloon-occluded alternate infusions of cisplatin and gelatin particles for hepatocellular carcinoma: A phase I/II multicenter prospective study of safety and efficacy. J. Vasc. Interv. Radiol. 33, 169–176.e1 (2022).
European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu & European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 69, 182–236 (2018).
Kudo, M. et al. Response evaluation criteria in cancer of the liver version 6 (response evaluation criteria in cancer of the Liver 2021 revised version). Hepatol. Res. 52, 329–336 (2022).
Lencioni, R. & Llovet, J. M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 30, 52–60 (2010).
Johnson, P. J. et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J. Clin. Oncol. 33, 550–558 (2015).
Sacks, D., McClenny, T. E., Cardella, J. F. & Lewis, C. A. Society of interventional radiology clinical practice guidelines. J. Vasc. Interv. Radiol. 14, S199–202 (2003).
Lencioni, R., de Baere, T., Soulen, M. C., Rilling, W. S. & Geschwind, J.-F.H. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: A systematic review of efficacy and safety data. Hepatology 64, 106–116 (2016).
Cheng, K.-L., Cheng, Y.-M., Chan, C.-Y. & Wang, C.-C. Predictors of liver dysfunction after transhepatic arterial chemo-embolization in hepatocellular carcinoma patients. Dig. Dis. Sci. 68, 3467–3472 (2023).
Hsin, I.-F. et al. Liver failure after transarterial chemoembolization for patients with hepatocellular carcinoma and ascites: Incidence, risk factors, and prognostic prediction. J. Clin. Gastroenterol. 45, 556–562 (2011).
Kohla, M. A. S., Abu Zeid, M. I., Al-Warraky, M., Taha, H. & Gish, R. G. Predictors of hepatic decompensation after TACE for hepatocellular carcinoma. BMJ Open Gastroenterol. 2, e000032 (2015).
Park, K. H. et al. Risk factors for liver function deterioration after transarterial chemoembolization refractoriness in Child–Pugh class A hepatocellular carcinoma patients. Korean J. Gastroenterol. 75, 147–156 (2020).
Hiraoka, A. et al. Hepatic function during repeated TACE procedures and prognosis after introducing sorafenib in patients with unresectable hepatocellular carcinoma: Multicenter analysis. Dig. Dis. 35, 602–610 (2017).
Yasui, Y. et al. Up-to-seven criteria as a useful predictor for tumor downstaging to within Milan criteria and Child–Pugh grade deterioration after initial conventional transarterial chemoembolization. Hepatol. Res. 48, 442–450 (2018).
Chi, C.-T. et al. Effect of transarterial chemoembolization on ALBI grade in intermediate-stage hepatocellular carcinoma: Criteria for unsuitable cases selection. Cancers 13, 4325 (2021).
Miksad, R. A., Ogasawara, S., Xia, F., Fellous, M. & Piscaglia, F. Liver function changes after transarterial chemoembolization in US hepatocellular carcinoma patients: The LiverT study. BMC Cancer 19, 795 (2019).
Kudo, M. et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 391, 1163–1173 (2018).
Finn Richard, S. et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 382, 1894–1905 (2020).
Irie, T. Creation of small gelatin particles by pumping method for transarterial chemoembolization of hepatocellular carcinoma: Analysis of particle size and reproducibility. Jpn. J. Radiol. 33, 790–794 (2015).
Sugimoto, K., Saguchi, T., Saito, K., Imai, Y. & Moriyasu, F. Hemodynamic changes during balloon-occluded transarterial chemoembolization (B-TACE) of hepatocellular carcinoma observed by contrast-enhanced ultrasound. J. Med. Ultrason. 41, 209–215 (2014).
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number JP22K07684.
Funding
This work was supported by JSPS KAKENHI Grant Number JP22K07684.
Author information
Authors and Affiliations
Contributions
Study conception and data analysis: S.H., N.H., T.Y., and T.I. Acquisition of data: S.H., N.H., T.Y., N.T., K.M., K.M., K.F., D.T., and T.I. Interpretation of data: S.H., N.H., T.Y., N.T., K.M., B.M., T.N., and T.I. Drafting manuscript: all authors.
Corresponding author
Ethics declarations
Competing interests
Toshiyuki Irie receives royalties from Piolax Medical Devices, Inc. The remaining authors disclose no conflicts.
Ethical approval
This study protocol was reviewed and approved by the University of Tsukuba Clinical Research Review Board (CRB3180028), approval number TCRB20-013. Written informed consent was obtained from individual participants.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hoshiai, S., Hasegawa, N., Yamada, T. et al. Multicenter, prospective clinical trial for balloon-occluded alternative infusion of cisplatin solution and fragmented gelatin particles of transarterial chemoembolization for hepatocellular carcinoma beyond up-to-seven criteria. Sci Rep 15, 16502 (2025). https://doi.org/10.1038/s41598-025-01444-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-01444-x