Abstract
This study aimed to identify the risk factors associated with intraventricular hemorrhage (IVH) in extremely preterm infants (EPIs), focusing on early-stage prediction to improve clinical outcomes. A retrospective cohort study was conducted at Guangzhou Women and Children’s Medical Center, including 189 EPIs born between January 2019 and December 2023. Infants were categorized into IVH and non-IVH groups based on head ultrasound findings. Risk factors were assessed using univariate and multivariate analyses, and a predictive model for IVH was developed. Of the 189 EPIs, 80 (42.3%) developed IVH, with 26 (13.8%) experiencing severe IVH. Gestational age was identified as a significant protective factor (OR = 0.565, p = 0.023), while invasive mechanical ventilation (IMV) was a key risk factor (OR = 2.718, p = 0.012). The predictive model demonstrated good performance, with an AUC of 0.753 (95% CI: 0.681–0.825). Gestational age and IMV are critical factors in the development of IVH in EPIs. Early identification of high-risk infants based on these factors can aid in timely interventions to reduce IVH incidence and improve outcomes.
Similar content being viewed by others
Introduction
Advancements in medical science have significantly improved the survival rates of extremely preterm infants (EPIs), defined as those born at a gestational age of less than 28 weeks. However, these infants remain highly susceptible to severe complications, including intraventricular hemorrhage (IVH), due to their immature cerebrovascular systems and underdeveloped regulatory functions1. A nationwide epidemiological study in China highlighted a concerning increase in the incidence of severe IVH in EPIs, rising from 6.4 to 17.2% between 2010 and 20192. This trend underscores the urgent need for effective preventive and therapeutic strategies for these vulnerable infants.
IVH, particularly in its severe form, is closely associated with long-term neurological impairments, including cerebral palsy, cognitive delays, and sensory deficits such as hearing loss and visual impairments3,4,5,6,7. The pathophysiology of IVH is multifactorial, and while the exact mechanisms remain incompletely understood, early interventions have been shown to mitigate the incidence and severity of the condition, thereby improving outcomes for affected infants. IVH generally occurs within the first week of life, with the majority of cases developing within the first 3 days8,9. Despite extensive research into the risk factors for IVH in preterm infants10,11,12,13, there remains a lack of studies focused specifically on the early-stage prediction and risk assessment of IVH in EPIs. This knowledge gap presents significant challenges in the prevention and treatment of this devastating condition.
The present study aims to address this gap by retrospectively analyzing a cohort of EPIs admitted to our medical center. Our primary objective is to identify the risk factors associated with the development of IVH in this population. By identifying these factors, we hope to provide evidence-based recommendations for the timely implementation of interventions that can enhance survival rates and improve the quality of life for these critically ill infants.
Methods
Study design and patients
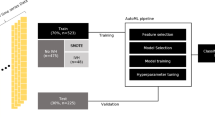
A retrospective cohort study was conducted at the Women and Children’s Medical Center of Guangzhou Medical University, focusing on extremely preterm infants (EPIs) admitted to the neonatal intensive care unit (NICU) between January 2019 and December 2023. The inclusion criteria for the study were: (1) admission to the NICU within 24 h of birth, (2) gestational age of less than 28 weeks, and (3) performance of head ultrasound (HUS) within the first 3 days (days 1–3) and again between days 5–7 after delivery. Infants with structural organ abnormalities, congenital metabolic diseases, chromosomal abnormalities, or those who died or were discharged within the first 7 days of life were excluded.
Following the HUS examinations, the study participants were divided into two groups: the intraventricular hemorrhage (IVH) group and the non-IVH group (Fig. 1). The study protocol and data collection procedures were approved by the Ethics Committee of the Women and Children’s Medical Center of Guangzhou Medical University (Approval No. 350B00), in compliance with ethical guidelines and standards. Given the retrospective nature of the analysis, informed consent was not required, as approved by the Ethics Committee.
Delivery room management for premature infants
Prior to delivery, a multidisciplinary team consisting of neonatologists, obstetricians, and anesthesiologists developed individualized treatment plans for each preterm infant. These plans were discussed with the family to ensure they were fully informed of potential risks and complications, and informed consent was obtained accordingly. At the time of delivery, the neonatal team was on standby, ready to provide immediate care. All necessary resuscitation equipment was prepared in advance, and the resuscitation process in the delivery room followed a well-coordinated approach, including initial stabilization, airway management, and support for respiration and circulation. This process was guided by the American Neonatal Resuscitation Program (NRP) guidelines. Once the infant was stabilized, they were promptly transferred to the neonatal intensive care unit (NICU) for ongoing monitoring and care. If necessary, pulmonary surfactant (PS) was administered via minimally invasive surfactant therapy (MIST) to support lung function. For infants who did not require intubation, non-invasive respiratory support was continued to maintain respiratory stability. The primary goal of this approach was to optimize the infant’s chances of survival and recovery during the critical first hours and days after birth.
Definitions of clinical conditions
In this study, Respiratory Distress Syndrome (RDS) was defined as infants who met the diagnostic criteria for RDS, including clinical signs such as tachypnea, grunting, nasal flaring, and chest retractions, along with radiological evidence of lung underexpansion or reticulogranular pattern on chest X-ray, and received pulmonary surfactant (PS) therapy. Hypotension was defined as a mean arterial pressure (MAP) below the gestational age-specific norm (for infants < 28 weeks, MAP < 30 mmHg), accompanied by the need for vasopressor treatment. Anemia was defined as hemoglobin (Hb) or hematocrit (HCT) levels falling below the normal physiological reference range, necessitating a blood transfusion. We adopted a restrictive transfusion strategy, with transfusion thresholds primarily based on HCT/Hb levels, postnatal age, and the clinical status of the infant. Sepsis included blood culture-positive sepsis and blood culture-negative clinical sepsis. Severe acidosis was defined as a pH ≤ 7.0, refers to a condition in which the blood pH drops significantly below the normal range, which is typically between 7.35 and 7.45. Thrombopenia was defined as a platelet count of less than 150,000 platelets/µL. PDA referred to all infants with PDA detected by echocardiography within the first week of life, including both hemodynamically significant and medically treated PDA. Invasive mechanical ventilation (IMV) referred to infants who failed to wean from IMV within the first week of life, typically due to conditions such as severe RDS, apnea, respiratory failure, shock, or hemodynamic instability. Failure to withdraw IMV was defined as the inability to maintain spontaneous breathing, requiring reconnection to invasive support within 24 h of attempted weaning.
Ultrasound screening and IVH classification
Head ultrasound screenings were conducted using the Philips Epiq5 color Doppler ultrasound system on days 1–3 and 5–7 after birth to evaluate the presence and severity of intraventricular hemorrhage (IVH). The severity of IVH was classified according to the modified Papile grading system: Grade I – subependymal hemorrhage; Grade II – intraventricular hemorrhage involving less than 50% of the ventricle; Grade III – intraventricular hemorrhage with associated ventricular enlargement; Grade IV – intraventricular hemorrhage extending into the brain tissue. Grades I and II were categorized as mild, while Grades III and IV were classified as severe.
Data collection
Clinical data from extremely preterm infants admitted to the NICU between January 2019 and December 2023 were retrospectively collected. A total of 189 infants were included, 80 of whom were diagnosed with IVH (IVH group) and 109 without IVH (non-IVH group). The following variables were assessed:
-
Demographic data: Sex (male/female), gestational age, and birth weight (presented as median and interquartile range).
-
Delivery factors: Mode of delivery (vaginal or cesarean) and delivery site (in-born or out-born).
-
Prenatal factors: Multiple gestation, in vitro fertilization (IVF), fetal distress, intrauterine infection, threatened preterm labor, stained amniotic fluid, premature rupture of membranes, antenatal steroid use, magnesium sulfate use, gestational diabetes mellitus, and gestational hypertension.
-
Intrapartum factors: Apgar scores at 1 and 5 min, as well as resuscitation methods (100% oxygen resuscitation, endotracheal intubation, chest compressions, fluid resuscitation).
-
Postnatal factors: RDS, sepsis, patent ductus arteriosus (PDA), hypotension, anemia, severe acidosis (pH ≤ 7.0), thrombocytopenia, and IMV.
All data were extracted from medical records to ensure completeness and accuracy. All postpartum factors were collected prior to the occurrence of IVH. For instance, if IVH occurred within the first 3 days after birth, postpartum factors for these infants were collected within the first 3 days. If IVH occurred by day 7 but not within the first 3 days, postpartum factors for these infants were collected within the first 7 days. In this study, there were indeed some missing data, but the missing rate for all variables is below 2%. Given that the proportion of missing data is very small and it did not have a significant impact on the sample size or the robustness of the results, we decided to use complete case analysis to handle the missing data.
Statistical analysis
Statistical analyses were performed using SPSS software version 25.0 (IBM Corp., Armonk, NY). Data were presented as means with standard deviation or medians with interquartile ranges (IQR), depending on the distribution. Categorical variables were expressed as numbers (n) and percentages (%). Group differences for normally distributed variables were analyzed using the independent sample t-test, and non-normally distributed variables were compared using the Mann-Whitney U test. The chi-square test was employed for categorical variables. Logistic regression analysis was performed to assess risk factors for IVH. Variance inflation factor (VIF) was used to assess the degree of multicollinearity among the variables before multivariate analysis. Model performance was evaluated using the receiver operating characteristic (ROC) curve, with an area under the curve (AUC) greater than 0.75 indicating good discrimination. The model calibration was assessed using the Hosmer-Lemeshow test. A p-value of < 0.05 was considered statistically significant.
In the multivariate model, the selection of confounders was based on a comprehensive consideration of existing literature and univariate analysis results. First, we referred to variables known to be associated with neonatal outcomes in the literature, and selected those potentially related to IVH through univariate analysis, further evaluating their impact in the multivariate model. Additionally, for factors strongly supported in the literature, such as antenatal corticosteroids, we included them in the model even if they did not show statistical significance in the univariate analysis, to ensure the model’s clinical relevance and comprehensiveness.
Results
Demographic characteristics
Among the 189 EPIs, 80 (42.3%) developed IVH. Of these, 26 (13.8%) experienced severe IVH, while 54 (28.6%) had mild IVH. Regarding the timing of onset, 53 cases (66.3%) occurred within the first 3 days after birth, and 27 cases (33.8%) developed between days 5 and 7(Fig. 2).
Distribution of IVH and severe IVH by gestational age and birth weight
Gestational age: The incidence of IVH showed a strong correlation with gestational age. In the 27 to 27+ 6 weeks group, 40 out of 113 infants (35.4%) developed IVH, with 9 (8.0%) experiencing severe IVH. Similarly, in the 26 to 26+ 6 weeks group, 18 out of 45 infants (40.0%) developed IVH, with 4 (8.9%) having severe IVH. Among the 25 to 25+ 6 weeks group, 13 out of 21 (61.9%) developed IVH, with 8 (38.1%) experiencing severe IVH. The highest incidence was observed in the 24 to 24+ 6 weeks group, where 9 out of 10 infants (90.0%) developed IVH, and 5 (50.0%) had severe IVH. Statistical analysis revealed significant differences in the proportions of IVH across gestational age groups (χ2 =14.929, p = 0.002), with a similar trend for severe IVH (χ 2= 25.651, p < 0.001)(Figure 3).
Birth weight: The incidence of IVH also increased with decreasing birth weight. In the ≥ 1000 g group, 25 out of 68 infants (36.8%) developed IVH, with 3 (4.4%) experiencing severe IVH. In the 750–999 g group, 33 out of 86 infants (38.4%) developed IVH, with 11 (12.8%) experiencing severe IVH. Among the 501–749 g group, 19 out of 30 infants (63.3%) developed IVH, and 11 (36.7%) had severe IVH. In the < 500 g group, 3 out of 5 infants (60.0%) developed IVH, with 1 (20.0%) experiencing severe IVH. Statistical analysis confirmed significant differences in the proportions of IVH across birth weight groups (χ2 = 7.475, p = 0.058), with severe IVH showing a similar trend (χ 2 =18.509, p < 0.001) (Figure 4).
Risk factors for IVH
Univariate analysis: Univariate analysis was performed to identify potential risk factors for IVH. Significant factors included younger gestational age (p < 0.001), lower birth weight (p = 0.011), 1-minute Apgar score (p < 0.001), 5-minute Apgar score (p = 0.007), 100% oxygen resuscitation (p = 0.006), endotracheal intubation (p < 0.001), and postnatal complications such as sepsis (p = 0.011), IMV (p < 0.001), hypotension (p = 0.001), and anemia (p < 0.001). All details are shown in Table 1.
Multivariable logistic regression analysis: A multivariable logistic regression analysis was conducted to identify independent risk factors for IVH, controlling for confounding variables. No multicollinearity was present among the selected variables (including gestational age and birth weight). The analysis revealed that gestational age was a significant protective factor (OR = 0.565, 95% CI: 0.346–0.923, p = 0.023), while IMV was identified as an independent risk factor (OR = 2.718, 95% CI: 1.245–5.932, p = 0.012). Other variables, including birth weight, antenatal steroids, Apgar scores, and sepsis, were not statistically significant (p > 0.05). These findings emphasize the critical role of gestational age as a protective factor and IMV as a key risk factor for IVH (Fig. 5).
Model performance
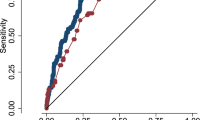
The predictive model for IVH demonstrated good performance. The ROC curve yielded an AUC of 0.753 (95% CI: 0.681–0.825), with a maximum Youden index of 0.452. The sensitivity and specificity were 70.9% and 74.3%, respectively (Fig. 6). The omnibus test confirmed that the model significantly outperformed the null model (χ2 = 39.330, p < 0.001), and the Hosmer-Lemeshow test indicated a good fit (χ2 = 5.683, p = 0.683).
Discussion
This study aimed to investigate the risk factors for intraventricular hemorrhage (IVH) in extremely preterm infants (EPIs). Through a retrospective analysis of 189 EPIs, we found that gestational age is a significant protective factor for IVH, while the use of invasive mechanical ventilation (IMV) is an independent risk factor for IVH.
First, gestational age plays a critical role in the occurrence of IVH. Our findings indicate that as gestational age increases, the incidence of both IVH and severe IVH (severe IVH) significantly decreases. Specifically, the incidence of IVH and severe IVH was highest in the 24 to 24+ 6-week group (90.0% and 50.0%, respectively), while in the 27 to 27+ 6-week group, the incidence dropped significantly to 35.4% and 8.0%, respectively (Fig. 3). These results are consistent with multiple studies14,15,16, further confirming gestational age as an important predictor of IVH. Extremely preterm infants with lower gestational age have underdeveloped cerebral vasculature, making them more vulnerable to hemodynamic fluctuations, insufficient oxygen supply, and other factors that increase the risk of IVH. More mature EPIs (e.g., 27 to 27+ 6 weeks) exhibit a significantly lower risk of IVH compared to those born at earlier gestational ages (e.g., 24 to 24+ 6 weeks), supporting gestational age as a protective factor for IVH (OR = 0.565, P = 0.023). While gestational age is indeed a well-established predictor of IVH, our study uniquely focuses on early prediction in EPIs born at < 28 weeks, a subgroup with distinct vulnerabilities due to immaturity of cerebral autoregulation and hemodynamic instability. Prior studies often combine EPIs with infants ≥ 28 weeks, which may dilute insights specific to this critically high-risk population. By narrowing the cohort to EPIs and validating gestational age as a protective factor within this group, our findings reinforce its clinical utility for risk stratification in the most vulnerable neonates, an aspect that is underexplored in existing literature.
In this study, we conducted stratified analyses based on birth weight and found that infants with a birth weight under 750 g had a higher risk of IVH and severe IVH (Fig. 4). Low birth weight is often associated with a higher incidence of intraventricular hemorrhage17. Infants with low birth weight typically also have lower gestational age and face higher physiological stress, making them more susceptible to cerebral vascular instability, hypoxia, and hemodynamic fluctuations, which contribute to IVH. However, despite the higher incidence of IVH in low birth weight infants, our multivariate analysis did not find birth weight itself to be an independent predictor of IVH. This may be due to the broad range of birth weights and the interaction of multiple risk factors. Future studies could explore how birth weight interacts with other clinical factors to form more precise risk assessment models, providing deeper insights into the role of birth weight in the occurrence of IVH.
Regarding IMV use, our study found that IMV is an independent risk factor for IVH. Infants requiring mechanical ventilation exhibited a significantly increased risk of IVH. This finding aligns with several other studies, which suggest that mechanical ventilation, especially invasive mechanical ventilation, can lead to hemodynamic fluctuations and intracranial pressure changes, thereby increasing the risk of periventricular hemorrhage18,19,20. Specifically, we observed that infants who were unable to be weaned from IMV within the first week of life had a 2.718-fold increased risk of IVH compared to those managed with non-invasive ventilation. However, due to the overlap in the timing of IVH onset and the duration of IMV use, it is difficult to definitively determine whether IMV is a cause of IVH or a consequence of IVH leading to continued ventilation support. To clarify, we performed a thorough analysis of the 57 IVH cases requiring prolonged IMV (≥ 7 days). Key findings revealed that the inability to wean from IMV was predominantly driven by severe pulmonary disease (e.g., refractory RDS, pulmonary infection) or systemic conditions (e.g., sepsis-induced hemodynamic instability), rather than directly resulting from IVH itself. This suggests that prolonged IMV use likely reflects baseline respiratory compromise in high-risk infants, which may predispose them to IVH through mechanisms such as hemodynamic fluctuations or ventilator-induced inflammation. While the temporal overlap between IVH onset (days 1–7) and IMV duration complicates causal inference, our analysis supports IMV as a marker of critical illness severity rather than solely a consequence of IVH. Therefore, future research should focus on identifying risk factors at earlier time points (e.g., within the first three days of life) to better understand the etiology of IVH.
Although we did not find a significant association between antenatal steroid treatment and IVH in our multivariate analysis, this result differs from findings in some other studies21,22. Antenatal steroids are commonly thought to promote fetal lung maturity, reduce the incidence of neonatal respiratory distress syndrome (RDS), and indirectly lower the risk of IVH23. The lack of association between antenatal corticosteroid use and IVH in our study may reflect variations in clinical practice, such as differences in the timing and dosing of steroid administration. In the current study, data on antenatal corticosteroids included both partial and complete courses, which might have differentially influenced outcomes. We acknowledge this limitation and plan to rigorously evaluate the effects of steroid timing (e.g., < 24 h vs. ≥24 h before delivery) and dosing regimens (single vs. multiple courses) on IVH risk in a forthcoming prospective study.
Some important perinatal confounders, for example, twin-to-twin transfusion syndrome(TTTS) and small for gestational age (SGA), are known to influence neonatal outcomes.However, due to the small sample size of TTTS in our study (only 2 cases) and SGA (only 10 cases), we did not include it as a separate variable in the model. We will consider them in future studies if the data allows.
Another strength of this study is its relatively high prediction model accuracy (AUC). By integrating clinical indicators such as gestational age, birth weight, and Apgar scores, this study provides a valuable tool for predicting the risk of IVH in extremely preterm infants. Nonetheless, further optimization and validation of this model are needed, potentially incorporating additional clinical variables such as blood gas parameters and imaging data, to improve prediction accuracy and clinical applicability.
This study provides valuable insights into the risk factors for IVH in extremely preterm infants, emphasizing gestational age as a protective factor and highlighting IMV as an independent risk factor for IVH. While factors such as birth weight, Apgar scores, and hypotension were associated with IVH in univariate analysis, they did not demonstrate independent predictive value in multivariate analysis. Future research should focus on identifying new biomarkers and imaging indicators to more accurately predict and intervene in IVH risk, thereby optimizing clinical management for extremely preterm infants.
There are some limitations to this study. First, the sample size was relatively small, particularly in subgroups with lower gestational ages and birth weights, which may not fully represent all clinical scenarios. Second, as a retrospective study, it did not control for all potential confounding factors. Furthermore, due to the lack of long-term follow-up data, this study did not assess the long-term neurodevelopmental outcomes of IVH in preterm infants. Future studies should address these issues to obtain more reliable and comprehensive conclusions.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request。.
References
Lai, G. Y. et al. Global incidence proportion of intraventricular haemorrhage of prematurity: A meta-analysis of studies published 2010–2020. Arch. Dis. Child-Fetal 107(5), 513–519 (2022) .
Zhang, W. W., Yu, Y. H., Dong, X. Y. & Reddy, S. Treatment status of extremely premature infants with gestational age < 28 weeks in a Chinese perinatal center from 2010 to 2019. World J. Pediatr. 18 (1), 67–74 (2022).
Kaempf, J. W., Guillen, U., Litt, J. S., Zupancic, J. A. F. & Kirpalani, H. Change in neurodevelopmental outcomes for extremely premature infants over time: a systematic review and meta-analysis. Arch. Dis. child-fetal. 108 (5), 458–463 (2023).
Pande, G. S. & Vagha, J. D. A review of the occurrence of intraventricular hemorrhage in preterm newborns and its future neurodevelopmental consequences. Cureus 15 (11), e48968 (2023).
Pascal, A. et al. The impact of intraventricular hemorrhage and periventricular leukomalacia on mortality and neurodevelopmental outcome in very preterm and very low birthweight infants: A prospective population-based cohort study. J. Pediatr.-US 262, 113600 (2023).
Rees, P. et al. Preterm brain injury and neurodevelopmental outcomes: A Meta-analysis. Pediatrics 150 (6), null (2022).
Sakaue, S. et al. Low-grade IVH in preterm infants causes cerebellar damage, motor, and cognitive impairment. Pediatr. Int. 63 (11), 1327–1333 (2021).
Kadri, H., Mawla, A. A. & Kazah, J. The incidence, timing, and predisposing factors of germinal matrix and intraventricular hemorrhage (GMH/IVH) in preterm neonates. Child. Nerv. Syst. 22 (9), 1086–1090 (2006).
Mitrea, G., Matasaru, M., Ilie, L., Diana-Andreea, C. & Filip, A. Intraventricular hemorrhage in premature infants: A review of risk factors, pathology, treatment, and prognosis. ARS Med. Tomitana. 29 (3), 191–196 (2023).
Haghshenas-Mojaveri, M. et al. The frequency of intraventricular hemorrhage and its risk factors. Curr. Pediatr. Rev., 20(4), 548–553 (2024).
Zhao, Y., Zhang, W. & Tian, X. Analysis of risk factors of early intraventricular hemorrhage in very-low-birth-weight premature infants: a single center retrospective study. BMC Pregnancy Childbirth. 22 (1), 890 (2022).
Xing, S., Sun, H. Q. & Li, M. C. [Clinical characteristics and risk factors of periventricular-intraventricular hemorrhage in extremely low birth weight infants]. Zhonghua Yi Xue Za Zhi. 102 (47), 3774–3778 (2022).
Puerta-Martínez, A. G., López-Garrido, E., Guerrero-Nava, J. M., Vargas-Ruiz, R. & Martínez-Padrón, H. Y. Risk factors associated with intraventricular hemorrhage in very-low-birth-weight premature infants. Child Nerv. Syst. (2024).
Stoll, B. J. et al. Trends in care practices, morbidity, and mortality of extremely preterm Neonates, 1993–2012. JAMA J. Am. Med. Assoc. 314(10), 1039–1051 (2015).
Bolisetty, S. et al. Intraventricular hemorrhage and neurodevelopmental outcomes in extreme preterm infants. Pediatrics 133 (1), 55–62 (2014).
Incidence risk factors of severe intraventricular hemorrhage in very low and extremely low birth weight infants: A multi-center study. Zhonghua Er Ke Za Zhi. 57 (4), 258–264 (2019).
Zhang, J. et al. A multicenter epidemiological investigation of brain injury in hospitalized preterm infants in Anhui, China. Zhongguo Dang Dai Er Ke Za Zhi. 21 (2), 114–119 (2019). PMID: 30782271.
Piatek, M. et al. Severe intraventricular hemorrhage is associated with lung injury in preterm infants on mechanical ventilation. J. Pediatr. Perinatol. Child. Health. 7 (4), 229–234 (2023).
Brown, M. K. et al. Incidence of hypocapnia, hypercapnia, and acidosis and the associated risk of adverse events in preterm neonates. Resp. Care. 63 (8), 943–949 (2018).
Sauer, C. W. et al. Intubation attempts increase the risk for severe intraventricular hemorrhage in preterm Infants-A retrospective cohort study. J. Pediatr-US. 177 (null), 108–113 (2016).
Lee, R. et al. Influence of antenatal corticosteroids and sex on the mortality and morbidity of extremely prematurely born infants. J. Matern-Fetal Neo M. 35 (25), 8062–8065 (2021).
Fuma, K. et al. Impact of antenatal corticosteroids-to-delivery interval on very preterm neonatal outcomes: a retrospective study in two tertiary centers in Japan. BMC Pregnancy Childbirth. 24 (1), 607 (2024).
McGoldrick, E. et al. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst. Rev. 12, CD004454 (2020).
Acknowledgements
The authors would like to thank Zhang Huayan for her valuable assistance and guidance throughout this work.
Funding
This study was supported by the Guangzhou Health Science and Technology Project (Grant No. 20241A011025), the Wu Jieping Medical Foundation Clinical Research Special Funding (Grant No.320.6750.2025-9-15), the Guangdong Medical Science and Technology Research Foundation (Grant No. A2023164), and the Liuzhou Science and Technology Planning Project (Grant No. 2024SB0104A002).
Author information
Authors and Affiliations
Contributions
Cai-YJ designed the data collection tools, recruited participants, collected the data, performed data analysis, drafted the manuscript, and approved the final version. Zhou-Wei conceptualized and designed the study, supervised the data collection process, revised the manuscript, and approved the final version for submission. Li-XL, Zhao-XP, and Song-YY contributed to data collection and approved the final manuscript. All authors confirm that this manuscript is original, has not been published previously, and is not under consideration for publication elsewhere. The authors consent to the publication of this manuscript and agree to transfer copyright ownership to Scientific Reports upon acceptance.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Women and Children’s Medical Center affiliated with Guangzhou Medical University (Approval No. 350B00) and conducted in accordance with relevant ethical guidelines. As this was a retrospective study, the requirement for informed consent was waived by the Ethics Committee of Women and Children’s Medical Center affiliated with Guangzhou Medical University in accordance with the Regulations for Ethical Review of Biomedical Research Involving Humans issued in China (No. 11, 2016).
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cai, Y., Li, X., Zhao, X. et al. Risk assessment and early prediction of intraventricular hemorrhage in extremely preterm infants. Sci Rep 15, 17346 (2025). https://doi.org/10.1038/s41598-025-02061-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-02061-4