Abstract
Distinguishing benign and malignant endometrial lesions on the basis of endometrial thickness (ET) may lead to a missed diagnosis of endometrial carcinoma (EC) in women with postmenopausal bleeding (PMB) or increased invasive examination and pain in women with benign endometrial lesions. Our research aims to establish an ultrasonic prediction model for differentiating between benign endometrial lesions and EC in women with PMB. PMB women with ET ≥ 5 mm (n = 412) or ET < 5 mm who presented with recurrent vaginal bleeding (n = 57) were enrolled in this prospective observational study. According to the pathological examination results of the endometrium, women with PMB were divided into endometrial atrophy (EA) (n = 231), endometrial polyp (EP) (n = 98), endometrial hyperplasia (EH) (n = 58) and EC (n = 82) groups. Ultrasonic parameters were compared among the four groups. The predictive value of different parameters for differentiation between benign endometrial lesions and EC in women with PMB was determined via receiver operating characteristic (ROC) curves. The best cut-off of ultrasonic parameters analyzed by ROC curves was used to establish prediction model. Women with EC had significantly thicker endometrium and higher endometrial volume (EV), vascularization index (VI), flow index (FI) and vascularization-flow index (VFI) than women with other pathological types of endometrium (P < 0.05). The endometrial VI, FI and VFI of women with EH were significantly higher compared with those in women with EA and EP (P < 0.05). For patients with ET ≥ 5 mm, the best parameter for distinguishing between benign lesions and EC was the FI, with an area under the curve (AUC) of 0.86, a sensitivity of 86.7% and a specificity of 81.4%. In addition, for patients with ET < 5 mm, the best parameter for distinguishing between benign lesions and EC was the VI, with an AUC of 0.92, a sensitivity of 92.1% and a specificity of 72.9%. The ultrasonic prediction model based on the FI and VI had better predictive value for EC in both patients with ET ≥ 5 mm and patients with ET < 5 mm. The ultrasonic parameters differed among the different pathological types of the endometrium in women with PMB. The ultrasonic prediction model based on the endometrial FI and VI was clinically useful for differentiating between benign endometrial lesions and EC, especially in postmenopausal patients with recurrent vaginal bleeding presenting with ET of less than 5 mm.
Similar content being viewed by others
Introduction
Endometrial cancer (EC) is the most common gynecologic malignancy in developed countries1, as well as in some developed cities in China2. Postmenopausal bleeding (PMB) is the initial presenting symptom in 80–90% of women with EC3. Therefore, distinguishing between benign endometrial lesions and EC in women with PMB is very important to provide accurate and timely diagnoses for surgical treatment4.
At present, the diagnosis of EC is mainly based on clinical symptoms, measurement of endometrial thickness (ET) by transvaginal ultrasound scanning (TVS), endometrial sampling devices, dilatation and curettage (D&C) and hysteroscopy5. Although different types of endometrial sampling devices have been used in the screening and diagnosis of EC in recent years, TVS has been recommended as an initial tool because it is less invasive compared with other tests, and TVS is not affected by the atrophic cervix due to menopause6. In most women, the measurement of ET is used to assess the risk of endometrial lesions7. However, studies reported that some patients who subsequently developed EC with ET less than 5 mm were missed or had a delayed diagnosis. A cohort research showed that approximately 6% of women with PMB and with a confirmed diagnosis of EC had thinner ET(≤ 4 mm)8. In addition, other studies have analyzed the pathological results of women with PMB whose ET greater 5 mm measured via TVS, approximately 70% of women are diagnosed with endometrial benign lesions9. Therefore, distinguishing benign and malignant endometrial lesions on the basis of ET may lead to missed diagnoses of EC in women with ET less than 5 mm, or increased invasive examination and pain in women with benign endometrial lesions.
The occurrence of EC involves cancerous changes in endometrial epithelial cells under the action of multiple factors, which are accompanied by abnormal proliferation of blood vessels. Therefore, the assessment of EC occurrence based solely on ET has certain limitations, and the accuracy is higher if blood flow parameters are combined. Three-dimensional (3D) power Doppler ultrasound is generally superior to color Doppler imaging for detecting low-velocity flows and visualizing small vessels. Studies have reported that the endometrial volume (EV), vascularization index (VI), flow index (FI) and vascularization-flow index (VFI) are significantly higher in EC than in endometrial hyperplasia (EH)10. Therefore, 3D power Doppler parameters are more useful than ET for differentiating between EH and EC, and blood flow in tumors could predict the spread of EC11. Gynecological doctors want to identify women at high risk for EC when they present with PMB. Therefore, ultrasonic prediction model based on the 3D power Doppler may help to assist doctors in triaging patients for further investigations and reduce missed diagnoses, especially for women with PMB whose endometria are less than 5 mm. Our research aims to establish the ultrasonic prediction model for EC in patients with PMB.
Materials and methods
Study design
We conducted a prospective observational study on patients with PMB. All methods were performed in accordance with the relevant guidelines and regulations. This study was approved by the ethics committee of The First Affiliated Hospital of Xi’an Jiaotong University (XJTU1AF2016LSL-022). All the participants gave written informed consent. This study has been registered on Clinical Trials.gov (NCT 03289468). The study complied with the STROBE guidelines for observational studies.
Participants
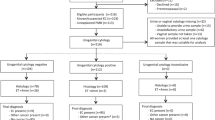
Figure 1 presents the flow chart of study participation. Five hundred and ninety-two patients with PMB at the First Affiliated Hospital of Xi’an Jiaotong University from November 2015 to February 2020 were initially enrolled in this prospective observational study. Patients had a median age of 57.8 years, with an age range of 45–79 years. The inclusion criteria were as follows: ① women with natural menopause, and menopause was defined as at least 1 year of menstrual cessation after the age of 40, ② definitive endometrial histological diagnosis was obtained in our hospital. The exclusion criteria were as follows: ① menstruation of women stopped for less than 1 year, ② women who received tamoxifen or menopausal hormone therapy (MHT), ③ women who presented with PMB because of coagulation related diseases, iatrogenic factors, cervical disease or reproductive tract infection. The recurrent vaginal bleeding (RVB) was defined as two or more separate bleeding events within the last 12 months12. Data were collected including body mass index (BMI), age at menopause, duration of PMB, and history of disease (diabetes, hypertension). The definition of normal endometrial thickness after menopause is controversial, and most academic institutions recommend a low risk of EC when the endometrium is less than 5 mm. However, a small number of women with postmenopausal bleeding are eventually diagnosed with EC when the endometrial thickness is less than 5 mm, especially women with RVB or risk factors for endometrial cancer. The standard recommended by the Society of Gynecologic Oncology and Society of Obstetricians and Gynecologists of Canada was less than 5 mm13. The American College of Obstetricians and Gynecologists (ACOG) committee recommends ≤ 4 mm14. Therefore, in order to reduce unnecessary testing and medical costs, patients with ET ≥ 5 mm (n = 412) or ET < 5 mm who presented with RVB (n = 57) received D&C or hysteroscopy to obtained histopathological diagnosis in this study. The histopathology of the endometrium was assessed standardized by senior attending physicians, and then reviewed by senior pathologists at our hospital. The patients were divided into four groups according to the pathological examination results of the endometrium: endometrial atrophy (EA) (n = 231), endometrial polyp (EP) (n = 98), EH (n = 58) and EC (n = 82) groups.
Outcome measures
Ultrasonic examinations were performed using Voluson E8 (GE Medical Systems, USA) ultrasound system with vaginal probe frequency of 4–9 MHz. All the participants were examined by TVS. The maximum thickness of the endometrium on both sides of the midline was measured in a longitudinal plane from the echogenic interface at the junction of the endometrium and myometrium.
To further detect the EV and index of blood flow, the ultrasound machine was switched to the 3D mode with power Doppler. The patient was asked to remain as still as possible. All the ultrasonic scans were performed by one operator to avoid interobserver variation. The ultrasonic parameters were measured three times, and the average value was used for the final statistical analysis. The manual mode of the VOCAL contour editor was used to cover the entire 3D volume of the endometrium with a 15° rotation step. A total of 12 endometrial slices were obtained outlining the endometrium at the myoendometrial junction from the fundus to the internal os. A histogram automatically showed the vascularization indices. The 3D volume was formed using basic units called voxels. The voxel contains all information about the grey scale and colour, according to an intensity scale that ranges from 0 to 100. The vascularization indices are as follows: VI refers to the colour voxel-tototal voxel ratio, FI refers to the weighted colour voxel divided by the total colour voxel ratio and provides an amplitude value for the colour signal, and VFI refers to the weighted colour voxel-to-total voxel ratio. The EV, VI, FI and VFI were calculated automatically by the VOCAL software (GE Medical Systems, USA). VI manifests the number of vessels in the endometrium. FI estimates the average intensity of flow. VFI manifests a combination of vascularity and blood flow15.
Statistical analysis
Statistical analyses were performed using SPSS 20.0 software (IBM, USA) (http://www.onlinedown.net/soft/1229603.htm). The Kolmogorov–Smirnov test was used to check the normal distribution prior to statistical tests. The continuous data variables were given as mean ± standard deviation, and statistical comparison was performed by using the analysis of variance or Student’s t-test. The enumeration data were given as number and percentage (%), which were compared by chi-square test. The predictive value of different parameters for differentiation between benign endometrial lesions and EC in women with PMB was performed by ROC curves, and the cut-off value of each parameter was calculated by these curves. The sensitivity, specificity, negative predictive value (NPV), positive predictive value (PPV) and accuracy of the 3D power Doppler ultrasound with surgico-pathologic findings as the reference standard. P < 0.05 was considered statistically significant.
Results
Among the 469 women with PMB, pathological examination results revealed 231 that (49.3%) women with EA, 98 (20.9%) with EP, 58 (12.4%) with EH, and 82 (17.5%) with EC including 8 women with ET < 5 mm presented RVB. Table 1 shows the clinical data of patients according to their histopathological diagnosis. There was significant difference in the duration of PMB among the four groups (P = 0.045), women with EC had the longest duration of PMB, followed by women with EH. However, no significant differences were found when comparing other characteristics among the four groups (P = 0.126–702).
The ET, EV, VI, FI and VFI of women with EC were significantly higher compared with those of women with other pathological types of endometrium (P = 0.006–0.020). The ET and EV of women with EH were significantly higher than in women with EA (P = 0.032, 0.028). In addition, the endometrial VI, FI and VFI of women with EH were significantly higher than those of women with EA and EP (P = 0.017–0.033). However, no significant difference was found in the ultrasonic parameters between women with EA and those with EP (P = 0.189–0.560) (Fig. 2).
For patients with ET ≥ 5 mm, the data in our study suggested that the FI was the best predictor for EC, with AUC of 0.86. However, ET had a poorer predictive value for EC compared with other parameters, with AUC of 0.74 (Table 2) (Fig. 3A). In addition, for patients with ET < 5 mm, the data in our study suggested that the VI was the best predictor for EC, with AUC of 0.92 (Table 2) (Fig. 3B). Because the FI and VI had better predictive values in both patients with ET ≥ 5 mm and those with ET < 5 mm, so they were used to establish ultrasonic prediction model. Table 3 shows the assignment score of FI and VI according to the cut-off analyzed by ROC curves, which were used to establish prediction model. The risk of EC increased in women with PMB as the score of the model increased. For patients with ET ≥ 5 mm, the AUC for ultrasonic prediction model was 0.90 (95% CI: 0.81–0.98) (P = 0.006). The cut-off of the ultrasonic prediction model was 3 points, with sensitivity and specificity were 92.2% and 83.6%, respectively (Fig. 3C). For patients with ET < 5 mm, the AUC for the ultrasonic prediction model was 0.92 (95% CI: 0.84–0.99) (P = 0.005). The cut-off of the ultrasonic prediction model was 3 points, with sensitivity and specificity of 95.2% and 84.7%, respectively (Fig. 3D).
ROC curves for the prediction of EC. (A) Different ultrasonic parameters for patients with ET ≥ 5 mm. (B) Different ultrasonic parameters for patients with ET < 5 mm. (C) Ultrasonic prediction model based on assignment scores of FI and VI for patients with ET ≥ 5 mm. (D) Ultrasonic prediction model based on assignment scores of FI and VI for patients with ET < 5 mm.
Discussion
In our study, 17.5% of women with PMB were diagnosed with EC, similar to previous studies and the meta-analyses by Gupta et al. showed that the prevalence of EC among all PMB patients was 10–24%16,17. However, the incidence of EC in some studies was less than 10%, such as 3.8% reported among 4383 women with PMB in Hong Kong18 and 4.9% reported in the United Kingdom12. This difference may be explained by differences in the health-care system, use of screening methods, sample size, habits of the participants and place of residence.
Most studies have confirmed that ET can be used to distinguish benign and malignant endometrial lesions19,20. Makled et al. reported that the cut-off value of the ET was 11.5 mm21. Similarly, in this study, we found that the cut-off of the ET was 9.4 mm. With pathological examination of the endometrium as the gold standard diagnostic tool for detecting EC, the diagnostic performance of ET for EC was as follows: sensitivity 75.2%, specificity 65.4%, PPV 68.2%, NPV 72.2% and AUC 0.74. There are different ET cut-off values recommended by various professional groups. Although ET has been used to assess the risk of EC in women with PMB, approximately 4% of EC are still missed, and this parameter has a false-positive rate as high as 50%22. Therefore, there is a limitation in predicting EC based on ET alone, so prediction models based either on clinical risk factors or a combination of clinical risk factors plus ET have been reported to differentiate between benign endometrial lesions and EC in women with PMB15,23.
Changes from EH to EC are accompanied with angiogenesis and neovascularization, which can be tested through power Doppler ultrasound. The 3D power Doppler technique has high sensitivity for detecting blood flow with very low velocities24. Therefore, very small vessels and low velocity blood flow can be detected via power Doppler25. With these features, the blood flow indexes of 3D power Doppler have good predictive value for EC, especially in patients with borderline ET or women presenting with PMB whose ET is less than 5 mm. Some scholars have been confirmed that 3D power Doppler can be used to predict endometrial lesions, including EP, EH and EC19. The data in this study revealed that the ET, EV, VI, FI and VFI of women with EC were significantly increased than those of women with other pathological types of endometria. The ET and EV of women with EH were significantly higher than those of women with EA. In addition, the VI, FI and VFI of women with EH were significantly higher than in women with EA and EP. Therefore, as endometrial lesions progress, the endometrial thickness and blood flow increase.
Many studies have been conducted to evaluate the accuracy of indexes measured by 3D power Doppler in differentiating benign and malignant lesions of the endometrium in women with PMB. Larger EV and higher Doppler indices were correlated with malignant lesions. Merce et al. reported that ET and EV were significantly higher in women with EC than in those with EH26. In 2016, indices measured by 3D power Doppler were used to differentiate between EH and EC, which improved the sensitivity and specificity. The cut-off values for ET in their study were EV (7 cm3), VI (0.7%), FI (0.22) and VFI (25), respectively27, which were similar to the results of our study. However, other researchers have revealed different cut-off measurements of 3D power Doppler for EC in women with PMB. Epstein et al. demonstrated that the cut-off values of the EV, VI, FI and VFI were 2.7 cm3, 4.3%, 1.15 and 30.1, respectively19. In 2018, Pandey et al. indicated that the variables with good discriminatory potential between benign and malignant statuses were the VI and VFI28. The differences in results could be explained by differences in the use of screening methods, sample sizes, places of residence and habits of the subjects, EC standards of endometrial pathology results obtained (≥ 4 mm or ≥ 5 mm), ultrasonic equipment, etc.
Vitale et al. demonstrated a prediction model based on the presence and duration of abnormal uterine bleeding, the ultrasound vascular pattern and echogenicity, and the hysteroscopic appearance of the endometrium. The sensitivity and specificity were 79.17% and 95.19%, respectively, with an area under the ROC curve of 0.96529. Additionally, previous studies reported two prediction models for the development of endometrial cancer in the general population (risk models) and one extension. The risk models included epidemiological variables related to the reproductive history of women, hormone use, BMI, and smoking history. The diagnostic models also included clinical predictors, such as endometrial thickness and recurrent bleeding. The data showed that the concordance statistic, which was used to assess discriminative ability, varied from 0.68 to 0.77 in the risk models and from 0.73 to 0.96 in the diagnostic models30. The data in our study suggested that the FI and VI had better predictive value in patients with both ET ≥ 5 mm and ET < 5 mm. Hence, to predict EC in women with PMB more conveniently and accurately, especially for women with ET less than 5 mm, the FI and VI were used to establish ultrasonic prediction model. According to the results of this study, for patients with ET ≥ 5 mm, the AUC for the ultrasonic prediction model was 0.90, and for patients with ET < 5 mm, the AUC for the ultrasonic prediction model was 0.92. Therefore, the ultrasonic prediction model based on 3D power Doppler scanning had better predictive value for EC in both patients with ET ≥ 5 mm and patients with ET < 5 mm.
Our study has several limitations. First, the sample size of this study was not large enough, and it was a single-center study. Second, although the effect of MHT on the endometrium was excluded in this study, the BMI and metabolic syndrome, which are considered risk factors for EC, were not analyzed in depth. Third, patients with endometrial thickness less than 5 mm and no history of RVB did not undergo pathological examinations, which may include EC. In addition, specific equipment is required to measure endometrial blood flow parameters, such ultrasound instruments need to be equipped with 3D ultrasonic probes, and sonographers need to be trained to ensure the accuracy and repeatability of the measurements. Therefore, the validity and reproducibility of the ultrasonic prediction model in this study remain to be studied in the future.
Conclusion
The ultrasonic parameters differed among the different pathological types of the endometrium in women with PMB. The ultrasonic prediction model based on the endometrial FI and VI was clinically useful for differentiating between benign endometrial lesions and EC, especially in postmenopausal patients with recurrent vaginal bleeding presenting with ET of less than 5 mm.
Data availability
All datasets generated for this study are included in the article. Further inquiries can be directed to the corresponding author (Xiaofeng Yang).
References
Siegel, R. L. et al. Cancer statistics, 2025. CA Cancer J. Clin. 5, 10–45. https://doi.org/10.3322/caac.21871 (2025).
Jiang, D. Y. et al. Endometrial sampling devices for early diagnosis of endometrial lesions. J. Cancer Res. Clin. Oncol. 142, 2515–2522. https://doi.org/10.1007/s00432-016-2215-3 (2016).
Clarke, M. A. et al. Association of endometrial cancer risk with postmenopausal bleeding in women: a systematic review and meta-analysis. Jama Intern. Med. 178, 1210–1222. https://doi.org/10.1001/jamainternmed.2018.2820 (2018).
Kim, S. et al. Long-term diabetes risk among endometrial cancer survivors in a population-based cohort study. Gynecol. Oncol. 156, 185–193. https://doi.org/10.1016/j.ygyno.2019.10.015 (2020).
Van Hanegem, N. et al. The accuracy of endometrial sampling in women with postmenopausal bleeding: a systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 197, 147–155. https://doi.org/10.1016/j.ejogrb.2015.12.008 (2016).
Timmermans, A. et al. Endometrial thickness measurement for detecting endometrial cancer in women with postmenopausal bleeding: a systematic review and meta-analysis. Obstet. Gynaecol. 8, 160–167. https://doi.org/10.1097/AOG.0b013e3181e3e7e8 (2010).
Verbakel, J. Y. et al. Validation of ultrasound strategies to assess tumor extension and to predict high-risk endometrial cancer in women from the prospective IETA (International endometrial tumour Analysis) 4 cohort. Ultrasound Obstet. Gynecol. 55, 115–124. https://doi.org/10.1002/uog.20374 (2020).
Wong, A. S. et al. Reappraisal of endometrial thickness for the detection of endometrial cancer in postmenopausal bleeding: a retrospective cohort study. BJOG 123, 439–446. https://doi.org/10.1111/1471-0528.13342 (2016).
Turnbull, H. L. et al. Investigating vaginal bleeding in postmenopausal women found to have an endometrial thickness of equal to or greater than 10 mm on ultrasonography. Arch. Gynecol. Obstet. 295, 445–450. https://doi.org/10.1007/s00404-016-4249-9 (2017).
Merce, L. T. et al. Clinical usefulness of 3-dimensional sonography and power doppler angiography for diagnosis of endometrial carcinoma. J. Ultrasound Med. 26, 1279–1287. https://doi.org/10.7863/jum.2007.26.10.1279 (2007).
Hanafi, S. et al. Value of three dimensional power doppler ultrasound in prediction of endometrial carcinoma in patients with postmenopausal bleeding. J. Turk. Ger. Gynecol. Assoc. 15, 78–81. https://doi.org/10.5152/jtgga.2014.07355 (2014).
Burbos, N. et al. Predicting the risk of endometrial cancer in postmenopausal women presenting with vaginal bleeding: the Norwich DEFAB risk assessment tool. Br. J. Cancer. 102, 1201–1206. https://doi.org/10.1038/sj.bjc.6605620 (2010).
Renaud, M. C., Le, T. & Bentley, J. Epidemiology and investigations for suspected endometrial cancer. J. Obstet. Gynaecol. Can. 35, 380–383. https://doi.org/10.1016/S1701-2163(15)30970-1 (2013).
The American College of Obstetricians and Gynecologists. Endometrial intraepithelial neoplasia. Obstet. Gynecol. 125, 1272–1278. https://doi.org/10.1097/01.AOG.0000465189.50026.20 (2015).
Yuan, T., Zhang, T. & Han, Z. Placental vascularization alterations in hypertensive disorders complicating pregnancy (HDCP) and small for gestational age with HDCP using three-dimensional power doppler in a prospective case control study. BMC Pregnancy Childbirth. 15, 240. https://doi.org/10.1186/s12884-015-0666-1 (2015).
Musonda, P. et al. Comparing the performance of two clinical models in estimating the risk of endometrial cancer in symptomatic postmenopausal women. Eur. J. Obstet. Gynecol. Reprod. Biol. 159, 433–438. https://doi.org/10.1016/j.ejogrb.2011.09.005 (2011).
Gupta, J. K. et al. Ultrasonographic endometrial thickness for diagnosing endometrial pathology in women with postmenopausal bleeding: a meta-analysis. Acta Obstet. Gynecol. Scand. 81, 799–816. https://doi.org/10.1034/j.1600-0412.2001.810902.x (2002).
Wong, A. S. et al. Development and validation of prediction models for endometrial cancer in postmenopausal bleeding. Eur. J. Obstet. Gynecol. Reprod. Biol. 203, 220–224. https://doi.org/10.1016/j.ejogrb.2016.05.004 (2016).
Epstein, E. et al. Ultrasound characteristics of endometrial cancer as defined by international endometrial tumor analysis (IETA) consensus nomenclature: prospective multicenter study. Ultrasound Obstet. Gynecol. 51, 818–828. https://doi.org/10.1002/uog.18909 (2018).
Doll, K. M. et al. Endometrial thickness as diagnostic triage for endometrial cancer among black individuals. JAMA Oncol. 11, 354. https://doi.org/10.1001/jamaoncol.2024.1891 (2025).
Makled, A. K. et al. Three-dimensional power doppler and endometrial volume as predictors of malignancy in patients with postmenopausal bleeding. J. Obstet. Gynaecol. Res. 39, 1045–1051. https://doi.org/10.1111/j.1447-0756.2012.02066.x (2013).
Tabor, A., Watt, H. C. & Wald, N. J. Endometrial thickness as a test for endometrial cancer in women with postmenopausal vaginal bleeding. Obstet. Gynecol. 99, 663–670. https://doi.org/10.1016/s0029-7844(01)01771-9 (2002).
Burbos, N. et al. Estimating the risk of endometrial cancer in symptomatic postmenopausal women-a novel clinical prediction model based on patients’ characteristics. Int. J. Gynecol. Cancer. 21, 500–506. https://doi.org/10.1097/IGC.0b013e31820c4cd6 (2011).
Nieuwenhuis, L. L. et al. Fibroid vascularisation assessed with three-dimensional power doppler ultrasound is a predictor for uterine fibroid growth: a prospective cohort study. BJOG 125, 577–584. https://doi.org/10.1111/1471-0528.14608 (2018).
Ni, J. et al. Three-dimensional 3D ultrasound combined with power doppler for the differential diagnosis of endometrial lesions among infertile women. Int. J. Gynaecol. Obstet. 145, 212–218. https://doi.org/10.1002/ijgo.12787 (2019).
Merce, L. T. et al. Endometrial volume and vascularity measurements by transvaginal threedimensional ultrasonography and power doppler angiography in stimulated and tumoral endometria: intraobserver reproducibility. Gynecol. Oncol. 100, 544–550. https://doi.org/10.1016/j.ygyno.2005.09.024 (2006).
Abd Elkhalek, Y. I., Mansour, M. G. & Farouk, O. Three dimensional transvaginal sonography and power doppler angiography in the differentiation between endometrial hyperplasia and endometrial carcinoma in postmenopausal women with abnormal uterine bleeding. Egypt. J. Radiol. Nucl. Med. 47, 1795–1801. https://doi.org/10.1016/j.ejrnm.2016.08.014 (2016).
Pandey, H. et al. Utility of three dimensional(3-D) ultrasound and power doppler in identification of high risk endometrial cancer at a tertiary care hospital in Southern India: a preliminary study. Taiwan. J. Obstet. Gynecol. 57, 522–527. https://doi.org/10.1016/j.tjog.2018.06.007 (2018).
Vitale, S. G. et al. Risk of endometrial malignancy in women treated for breast cancer: the BLUSH prediction model - evidence from a comprehensive multicentric retrospective cohort study. Climacteric 27 (5), 48248–48248. https://doi.org/10.1080/13697137.2024.2376189 (2024).
Alblas, M. et al. Prediction models for endometrial cancer for the general population or symptomatic women: a systematic review. Crit. Rev. Oncol. Hematol. 126, 92–99. https://doi.org/10.1016/j.critrevonc.2018.03.023 (2018).
Funding
This study was supported by the grant from the Health Research Project of Shaanxi Province (No. 2018D054), the Health Science and Technology Innovation Project of Shaanxi Province (No. 2024PT-11), and the China Maternal and Child Health Association Maternal and Child Health Research and Innovation Technology Project (No. ZGFYBJXH-KY2104).
Author information
Authors and Affiliations
Contributions
L.W. conceived the study, analyzed the data and wrote the manuscript. S.M.Q collated the data. X.F.Y. provided participants and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, L., Quan, S. & Yang, X. Ultrasonic prediction model using three-dimensional power doppler for endometrial cancer detection in women with postmenopausal bleeding. Sci Rep 15, 18302 (2025). https://doi.org/10.1038/s41598-025-02067-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-02067-y