Abstract
To evaluate the efficacy and safety of ultrasound - guided transabdominal injection of Botulinum toxin A (BoNT - A) in the treatment of refractory overactive bladder (rOAB). This retrospective cohort study included 64 patients with rOAB admitted to the Department of Urology, the Third People’s Hospital of Hangzhou, from January 2021 to February 2025. They were divided into an observation group (ultrasound - guided transabdominal injection, 32 cases) and a control group (transurethral cystoscopic injection, 32 cases). Both groups received BoNT - A (100 U) intramural injection of the bladder. In the observation group, the injection was performed under real - time ultrasound guidance through the abdominal wall, while the control group underwent the standard cystoscopic injection method. The primary outcome measures included the parameters of the micturition diary (urgency episodes, daytime urination frequency, nocturia episodes) and bladder capacity (initial desire capacity, maximum bladder capacity) before treatment, 1 month, and 6 months post-treatment. The secondary outcomes covered the standardized scale scores of patients (ICIQ - OAB, OAB - Q), global impression of improvement (PGI - I), visual analog pain score (VAS), incidence of complications, retreatment rates, and immediate willingness to repeat the procedure. There were no statistically significant differences in baseline characteristics (age, sex, and pre - treatment symptom scores) between the two groups (all P > 0.05). After treatment, both groups of patients showed significant improvement in urgency episodes, daytime urination frequency, nocturia episodes, bladder capacity, and ICIQ - OAB and OAB - Q scores (all P < 0.05), but there was no significant difference in therapeutic effects between the groups (all P > 0.05).Six months after treatment, the observed indicators in the observation and control groups increased compared with 1 month after treatment, but were still significantly better than the baseline level (all P < 0.05). There was no significant difference in efficacy between the two groups (all P > 0.05). The incidence of complications in the observation group was significantly lower than that in the control group (P < 0.01), and the VAS pain score was lower (3.32 ± 1.25 vs. 4.82 ± 1.61, P = 0.006), with a higher immediate willingness to repeat the procedure (8.28 ± 1.54 vs. 6.86 ± 2.19, P = 0.004). There was no significant difference in PGI-I scores between the groups (2.27 ± 0.92 vs. 1.95 ± 0.43, P = 0.08). There was no significant difference in the repeat injection rate between the two groups at 6 months after treatment (28.13% vs. 18.75%, P = 0.375). Ultrasound - guided transabdominal injection of BoNT - A for rOAB is as effective as transurethral cystoscopic injection, but it can significantly reduce the risk of complications and improve patient acceptance of treatment, providing a safer and more compliant alternative option for clinical practice.
Similar content being viewed by others
Introduction
Overactive bladder (OAB) is a syndrome characterized by urinary urgency, often accompanied by urinary frequency and nocturia, with or without urgency incontinence1. At present, the pharmacological treatment for OAB in clinical practice mainly involves anticholinergic drugs, such as oxybutynin, and tolterodine2. However, for some patients, the effects of these traditional treatment drugs are not satisfactory. The American Urological Association defines patients with refractory OAB(rOAB) as those who do not experience significant symptom relief after prolonged behavioral training, or who fail to respond to 6–12 weeks of treatment with an anticholinergic drug (including inadequate symptom relief or intolerable adverse reactions)3.
For patients with rOAB, further invasive treatments may be required, such as intravesical injection of botulinum toxin or sacral nerve modulation4,5. Extensive research has demonstrated that intravesical injection of Botulinum toxin type A (BoNT-A) for the treatment of OAB is effective and has a high safety profile4,6. Since the US Food and Drug Administration (FDA) approved botulinum toxin for the treatment of OAB in 2013, it has become a highly utilized third - line therapeutic agent for OAB7.
However, the traditional transurethral intravesical injection of BoNT - A is an invasive procedure that requires experienced specialists to inject under cystoscopic guidance, which may lead to adverse reactions such as urinary tract infection, bladder bleeding, urethral injury, and urinary retention. Moreover, after intravesical injection of BoNT - A, its impact on OAB symptoms lasts for about 6–9 months. Therefore, to maintain the efficacy of this treatment, repeated injections are often needed8. These factors may affect patients’ long - term treatment compliance and also limit the promotion and popularity of this treatment method. Therefore, how to reduce the difficulty of BoNT - A treatment for rOAB and increase patients’ long - term treatment compliance is one of the key issues that need to be solved at present. In this study, we attempted to inject botulinum toxin into the bladder wall through the abdomen under ultrasound guidance, and compared the effectiveness, safety, and patient compliance of this new injection technique with the standard transurethral injection technique.
Materials and methods
Patient selection and data collection
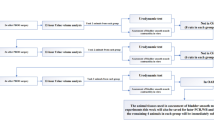
This retrospective cohort study screened all patients diagnosed with rOAB in our hospital between January 2021 and February 2025 through the electronic medical record system. Inclusion and exclusion criteria were applied to identify eligible cases. A total of 214 patients were initially identified, of whom 64 met the predefined criteria and were included in the final analysis. According to the different injection methods used by the patients, they were divided into observation and control groups, with 32 people in each group, as shown in Fig. 1. This study employed a consecutive enrollment design, in which all patients fulfilling the predefined inclusion criteria during the study period were enrolled, with no eligible participants excluded for non-protocol reasons.
Inclusion criteria: ①Age over 18 years and meeting the diagnosis of rOAB3; ②Disease duration of 6 months or longer; ③No effect or partial effect after treatment with traditional drugs such as tolterodine, or oxybutynin, and those who could not tolerate the side effects; ④Received BoNT-A injection and signed treatment informed consent. Exclusion criteria: ①Micturition diary showing an average single - void volume > 200 mL, or < 8 micturition episodes; ②Bladder outlet obstruction; ③With urinary tract stones, infection, tuberculosis, or tumor; ④Those who had received botulinum toxin injections in other parts recently, or were allergic to botulinum toxin; ⑤Those who were taking anticoagulants and had bleeding disorders; ⑥With neurogenic bladder; ⑦With neurological diseases such as lumbar - sacral vertebral dysraphism. This study was approved by the Ethics Committee of Hangzhou Third People’s Hospital (Y-KL2025025) and conducted in accordance with the Declaration of Helsinki and relevant guidelines. Written informed consent was obtained from all participants. No identifying information or images without specific permission are included.
Treatment implementation
Control group: transurethral cystoscopic injection
Patients were placed in the lithotomy position. After routine disinfection and draping, local anesthesia was administered. A cystoscope was inserted through the urethra. Under direct cystoscopic guidance, a specialized 6 F long needle was used to inject Botulinum toxin type A (trade name: Hengli, Lanzhou Bio - products Research Institute Co., Ltd., specification: 100 units/bottle) into the detrusor muscle of the bladder in an intermittent manner. The drug was dissolved in 10 mL of saline, and the standard 20-point injection method was used, with 0.5 mL injected at each point. The injection sites were distributed in the bladder base, lateral walls, and dome, as evenly as possible. The injection sites should avoid the bilateral ureteral orifices, bladder trigone, and bladder neck. The injection depth reached the submucosal muscle layer about 2 mm, taking care to avoid puncturing the bladder wall. After the injection, the bladder was emptied, the cystoscope was withdrawn, and a catheter was left in place. In the absence of special circumstances, the catheter was removed 2–3 days after the injection.
Observation group: Ultrasound - Guided transabdominal injection into the bladder wall
Patients were in the supine position. 100 U of Botulinum toxin type A was dissolved in 2 mL of 0.9% sodium chloride injection and drawn into a 2-mL syringe for standby use. Under real-time ultrasound guidance, a standardized four-point injection protocol was implemented. Two injection sites were selected in each of two orthogonal imaging planes: (1) on the anterior lateral walls within the maximum transverse section, positioned at the farthest possible distance along the bladder’s long axis (left-to-right orientation), and (2) on the anterior walls within the maximum sagittal section, placed maximally apart along the longitudinal axis (cephalad-to-caudad orientation), as shown in Figs. 2 and 3. This spatial distribution—totaling four injection points (two per plane)—was designed to optimize BoNT-A diffusion within the detrusor muscle while minimizing procedural complexity. Patients were instructed to maintain moderate bladder filling (approximately 200 mL of urine) and remain in the supine position. A portable ultrasound machine (S - II type, American - made SonoSite) was used to scan the lower abdominal bladder surface projection area of the patient and determine the target point location. After disinfection and draping, 2% lidocaine (Hunan Kelen Pharmaceutical Co., Ltd., specification 5 mL: 0.1 g) was injected subcutaneously at the puncture site for local anesthesia. Using the in-plane needle insertion technique, a 21G, 10 cm puncture needle (Shanghai Jumo Medical Device Co., Ltd.) was inserted into the subcutaneous tissue 2 cm lateral to the ultrasound probe on the skin surface. After entering the skin, the direction of the puncture needle was adjusted to minimize the angle between the needle and the bladder wall. When the tip of the needle passed through the bladder serosal layer into the muscle layer, it continued to advance in that layer (taking care to keep the tip of the needle parallel to the bladder wall at that point) for 3–5 mm. After confirming no blood or urine aspiration, 0.5 mL of the botulinum toxin solution was injected. The needle was retained, and the syringe was replaced with a saline-filled one to inject 0.5 mL saline for flushing any residual toxin. The needle was left in place for 10 s before withdrawal. The remaining target points were injected in sequence using the same method, with 0.5 mL of the botulinum toxin solution injected at each point. After each injection, the abdominal puncture site was pressed until there was no obvious bleeding. The patient was observed for half an hour, and if there were no obvious abnormalities, they could leave. All patients received only one injection of botulinum toxin. After the treatment, the VAS was used to score the patients’ pain during the treatment and their immediate willingness to repeat the procedure.
Evaluation indices
Primary indices
The micturition diary was recorded continuously for 3 days before treatment, 1 month, and 6 months post-treatment, and the average values of various parameters were calculated, including the number of urinary urgency episodes, daytime urination frequency, and nocturia episodes. The bladder capacity at the initial desire to void and the maximum bladder capacity of the patients were measured before and after treatment.
Secondary indices
The Overactive Bladder Questionnaire (OAB - Q) is divided into the symptom distress score and the quality - of - life score, with each score ranging from 1 to 6. The higher the score, the greater the symptom distress and the poorer the quality of life. The International Consultation on Incontinence Questionnaire - overactive bladder (ICIQ - OAB) scores range from 0 to 16, with higher scores indicating more severe symptoms. These two surveys are recommended by the International Continence Society as the highest - level evidence for OAB research9. The Patient Generated Index (PGI - I) is a single - item survey that assesses patients’ perception of symptom improvement after treatment, with 7 levels (1–7 points). The lower the score, the better the effect. This survey provides a subjective perception of symptom severity and treatment effect, and the score is related to objective outcomes such as the number of micturitions and the number of incontinence episodes10. The pelvic pain score uses the 11 - point Visual Analogue Scale (VAS), with a range of 0–10 points (0 = no pain, 10 = severe pain). Patients’ immediate willingness to repeat the procedure was assessed within 30 min post-injection using the same VAS scale (0–10), reflecting patients’ immediate tolerance to the procedure, with 0 points indicating complete unwillingness and 10 points indicating complete willingness to repeat the treatment.
Safety indices
The main safety indices included subcutaneous bleeding, hematuria, urinary tract infection, and urinary retention. One month after treatment, after the completion of the rating scale survey, urine routine and bladder residual urine examination were performed. If urinary tract infection was present, antibiotic treatment was given. If the residual urine volume was greater than 200 mL and the bladder was not completely emptied, or greater than 400 mL, regardless of symptoms, patients were instructed to perform clean intermittent catheterization.
Statistical analysis
The statistical analysis was performed using SPSS 19.0 software. Continuous variables were tested for normality with the Kolmogorov-Smirnov test and presented as Mean ± SD or median (range) accordingly. Group comparisons used the t-test for normally distributed data and the Mann-Whitney U test for non-normally distributed data. A post-hoc power analysis was conducted using G*Power 3.1 to evaluate the statistical power of the observed differences in PGI-I scores between groups. Categorical variables were compared using the chi-square test. P-value < 0.05 was considered statistically significant.
Results
General data
A total of 64 patients were included in this study according to the inclusion and exclusion criteria, with 32 in the control group (10 males and 22 females, aged 48.72 ± 6.24 years) and 32 in the observation group (12 males and 20 females, aged 45.91 ± 5.36 years). All 64 patients received botulinum toxin type A injection. Before treatment and 1 month after treatment, all patients completed the micturition diary, bladder capacity at the initial desire to void, maximum bladder capacity examination, and ICIQ - OAB and OAB - Q score survey. Immediately after the treatment, all patients completed the VAS scoring and the survey of immediate willingness to repeat the procedure; 1 month after treatment, all patients completed the PGI - I score survey. There were no significant differences in gender, age, and comorbidities between the two groups (P > 0.05), which were comparable, as shown in Table 1.
Primary indices
One month after treatment, the number of urinary urgency episodes, nocturia episodes, and daytime urination frequency decreased in both groups compared with before treatment, and the differences were statistically significant (all P < 0.05). However, there were no significant differences between the two groups (all P > 0.05). The bladder capacity at the initial desire to void and the maximum bladder capacity after treatment were significantly higher than those before treatment in both groups (all P < 0.05), but there were no differences between the two groups (all P > 0.05). At the 6-month follow-up, both groups exhibited a mild resurgence in urinary urgency episodes and daytime voiding frequency compared to the 1-month post-treatment assessment. Notably, all parameters remained significantly improved relative to baseline levels(all P < 0.05). In contrast, nocturia episodes demonstrated limited sustained efficacy, with post-treatment values approaching pre-treatment levels(P > 0.05). Additionally, initial bladder capacity and maximum bladder capacity decreased. However, compared to pre-treatment values, all indicators remained significantly improved (all P < 0.05), as shown in Table 2.
Secondary indices
There were no significant differences in the baseline scores of each rating scale before treatment. One month after treatment, the OAB - Q score and ICIQ - OAB score improved compared with the baseline levels before treatment (P < 0.05). Six months after treatment, Both indicators have rebounded, but are still below pre-treatment levels(all P < 0.05). At each follow-up time point, there were no significant differences between the two groups after treatment (all P > 0.05), as shown in Table 2. After treatment, the VAS score and the score of immediate willingness to repeat the procedure were higher in the observation group than in the control group (P = 0.006, 0.004),while 1 month after treatment, the PGI - I score in the observation group was similar to that in the control group (P = 0.08). Within 6 months after treatment, 9 patients (28.13%) in the observation group and 6 patients (18.75%) in the control group received repeat injections due to symptom recurrence (P = 0.376). All patients who underwent retreatment chose the same injection method as their original group, with no crossover treatments observed, as shown in Table 3.
Safety indices
All patients successfully completed the surgical treatment. In the observation group, 5 cases had subcutaneous ecchymosis, 2 cases had hematuria of varying degrees, and 2 cases had urinary retention, with no urinary tract infections(UTI). In the control group, 15 cases had hematuria, 6 cases had UTI, and 3 cases had urinary retention, with no subcutaneous ecchymosis. The differences were statistically significant, as shown in Table 3.
Discussion
The results of this study indicate that ultrasound - guided transabdominal injection of botulinum toxin into the bladder wall can effectively alleviate urinary symptoms in patients with refractory overactive bladder, contributing to an improved quality of life and facilitating their early return to family and society. Numerous domestic and international studies have demonstrated that injecting botulinum toxin into the detrusor muscle of the bladder is highly effective, safe, and minimally invasive. It has been widely used in the rehabilitation of patients with refractory neurogenic detrusor overactivity, enhancing their quality - of - life indices and urodynamic parameters, and thus serves as an effective therapeutic approach for patients with detrusor overactivity4,11,12.
Currently, the primary route of administration for botulinum toxin injection in clinical practice is through cystoscope - guided intravesical injection into the bladder wall. Cystoscopy provides direct visualization of the submucosal layer, which theoretically enhances precision in targeting the detrusor muscle. However, this method requires specialized settings such as operating rooms or cystoscopy suites. Despite its anatomical precision, the inability to accurately determine the intramural injection depth under the cystoscope, coupled with the continuous flushing of saline, can lead to significant discrepancies between the single - point injection dose and the target dose. Even with adjustments to the needle angle and delayed needle withdrawal, these issues cannot be entirely avoided. Additionally, this injection technique is prone to inducing bladder bleeding and infections, which to some extent affects the therapeutic outcome and the widespread adoption of this method.
In this study, ultrasound guidance was utilized to insert the puncture needle percutaneously into the lower abdomen to reach the bladder, dynamically displaying the position of the needle tip in the bladder wall muscle layer throughout the entire process, with flexible and controllable injection depth and angle. There is currently no unified standard for the dosage and number of injection sites for intramural bladder injections of botulinum toxin. A study reported that injecting different doses of botulinum toxin into the detrusor muscle of children with refractory OAB led to significant improvements in urodynamic parameters and quality - of - life(QOL) scores13. Some researchers compared the therapeutic effects of injecting botulinum toxin into 20 sites versus 1–3 or 5 sites in the bladder wall of patients with detrusor overactivity and found similar improvements in urodynamic aspects14,15. Ultrasound - guided percutaneous injection of botulinum toxin has been widely used to treat conditions such as limb spasticity after stroke and Piriformis Muscle Syndrome, and has achieved certain effects16,17.
Based on the aforementioned reports and our own experience, this study chose a total of 4 injection sites on the anterior and lateral bladder walls, with 25 U injected at each point. It was found that after treatment, patients’ bladder capacity, number of micturitions, number of urinary urgency episodes, and scores on the ICIQ - OAB and OAB - Q were all significantly better than before treatment. Although the 6-month therapeutic effects of this study are better than the 6–8 month cycle reported in some literature18,19, but the efficacy does show an obvious attenuation. Future studies should extend follow-up (≥ 12 months) and optimize injection strategies (e.g., dose adjustment), considering patient subgroups, predominantly idiopathic OAB. No statistically significant difference was observed in the PGI-I scores between the observation group and the control group(P > 0.05). However, post-hoc power analysis for PGI-I revealed that the current sample size (n = 32 per group) yielded a statistical power of 58%, which may be insufficient to detect subtle between-group differences in patient-reported outcomes. Future studies with larger sample sizes may be needed. The patients’ pain scores and immediate willingness to repeat the procedure scores were higher than those in the control group (P < 0.05). It is important to note that the assessment of immediate willingness to repeat the procedure was conducted shortly after the injection, which primarily reflects patients’ tolerance to procedural discomfort (e.g., pain or anxiety during needle insertion) rather than their long-term satisfaction with therapeutic outcomes.
The comparable therapeutic outcomes observed with varying numbers of injection sites (4 vs. 20 sites) may be attributed to the intrinsic migration capacity of BoNT-A within the bladder wall. Preclinical evidence suggests that BoNT-A exhibits both passive molecular dispersion and active axonal retrograde transport, allowing its effects to extend beyond the immediate injection site20. This property likely compensates for reduced injection density by distributing the toxin to adjacent nerve terminals, thereby achieving broad neuromuscular blockade even with sparse injection patterns.
In terms of safety, apart from some patients experiencing subcutaneous bruising at the puncture site, the incidence of complications such as hematuria and urinary tract infection in the observation group was significantly lower than that in the control group (P < 0.05), while there was no significant difference in the incidence of urinary retention between the two groups (P > 0.05). This suggests that transabdominal injection is likely a safe and effective alternative technique. However, operators need to be proficient in the operation of B - ultrasound equipment and practice more to avoid damaging blood vessels around the bladder and adjacent organs during puncture.
Conclusions
In conclusion, ultrasound - guided transabdominal injection of botulinum toxin into the bladder wall has been demonstrated to effectively ameliorate urinary symptoms and enhance the quality of life in patients with refractory overactive bladder. Moreover, this therapeutic approach is characterized by a high degree of safety and good patient compliance. However, the long-term subjective outcomes and comparative efficacy in patient-reported improvement require further validation through adequately powered, prospective trials.
It should be noted, however, that the present study has several limitations. The sample size is relatively small and the sources are singular, which may have limited the statistical power for secondary endpoints such as PGI-I scores.There is a lack of stratified discussion on the bladder detrusor compliance of patients. Long - term therapeutic follow - up has not been conducted. The selection of injection targets and the dosage of botulinum toxin injection still need to be optimized. Additionally, this technique has relatively high requirements for equipment and personnel skills. Future research will further address these shortcomings, providing valuable data for the clinical improvement of treatment methods for overactive bladder patients.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Malde, S., Kelly, S., Saad, S. & Sahai, A. Case-finding tools for the diagnosis of OAB in women: A narrative review. Neurourol. Urodyn. 39, 13–24 (2020).
El-Zawahry, A. Combination pharmacotherapy for treatment of overactive bladder (OAB). Curr. Urol. Rep. 20, 33 (2019).
De Wachter, S. et al. Six-month results of selective bladder denervation in women with refractory overactive bladder. J. Urol. 201, 573–580 (2019).
Harris, S. & Rizzolo, D. Botulinum toxin as a treatment for refractory overactive bladder. JAAPA 29, 1–4 (2016).
Kraus, S. R. et al. Treatment patterns and costs among patients with OAB treated with combination oral therapy, sacral nerve stimulation, percutaneous tibial nerve stimulation, or OnabotulinumtoxinA in the united States. Neurourol. Urodyn. 39, 2206–2222 (2020).
Jiang, Y. H., Jhang, J. F. & Kuo, H. C. The clinical application of intravesical botulinum toxin A injection in patients with overactive bladder and interstitial cystitis. Tzu Chi Med. J. 35, 31–37 (2023).
López Ramos, H., Torres Castellanos, L., Ponce Esparza, I., Jaramillo, A. & Rodríguez, A. Moreno Bencardino, C. Management of overactive bladder with OnabotulinumtoxinA: systematic review and Meta-analysis. Urology 100, 53–58 (2017).
Li, J., Liu, W., Tang, C., Pan, H. & Song, C. Clinical efficacy and safety analysis of type A botulinum toxin in the treatment of adolescents with refractory overactive bladder. Med. (Baltim). 103, e38803. (2024).
Abrams, P. et al. 6th international consultation on incontinence. Recommendations of the international scientific committee: EVALUATION AND TREATMENT OF URINARY INCONTINENCE, PELVIC ORGAN PROLAPSE AND FAECAL INCONTINENCE. Neurourol. Urodyn. 37, 2271–2272 (2018).
Bjelic-Radisic, V. et al. Psychometric properties and validation of two global impression questionnaires (PGI-S, PGI-I) for stress incontinence in a German-speaking female population. Neurourol. Urodyn. 37, 1365–1371 (2018).
Hu, J. C., Hsu, L. N., Lee, W. C., Chuang, Y. C. & Wang, H. J. Role of urological botulinum Toxin-A injection for overactive bladder and voiding dysfunction in patients with Parkinson’s disease or Post-Stroke. Toxins 15, 166 (2023).
De Sallmard, G. et al. Efficacy and safety of intradetrusor botulinum toxin injections for idiopathic overactive bladder syndrome in patients with an artificial urinary sphincter. World J. Urol. 40, 489–495 (2022).
Ingham, J., Angotti, R., Lewis, M. & Goyal, A. Onabotulinum toxin A in children with refractory idiopathic overactive bladder: medium-term outcomes. J. Pediatr. Urol. 15, 32e1–32e5 (2019).
DiCarlo-Meacham, A. M. et al. Reduced versus standard intradetrusor OnabotulinumtoxinA injections for treatment of overactive bladder. Neurourol. Urodyn. 42, 366–374 (2023).
Avallone, M. A., Sack, B. S., El-Arabi, A., Guralnick, M. L. & O’Connor, R. C. Less is more-A pilot study evaluating one to three intradetrusor sites for injection of OnabotulinumtoxinA for neurogenic and idiopathic detrusor overactivity. Neurourol. Urodyn. 36, 1104–1107 (2017).
Sun, L. C. et al. Efficacy and Safety of Botulinum Toxin Type A for Limb Spasticity after Stroke: A Meta-Analysis of Randomized Controlled Trials. BioMed Res. Int. 2019, 8329306. (2019).
Santamato, A. et al. Ultrasound-Guided injection of botulinum toxin type A for piriformis muscle syndrome: A case report and review of the literature. Toxins 7, 3045–3056 (2015).
Jiang, Y. H. et al. Real-Life Treatment Outcome of Botulinum Toxin A Injection on Overactive Bladder and Voiding Dysfunction in Patients with Central Nervous System LesionsToxins. Mar 01;16(3). (2024).
Barba, M. & Lazar, T. Learning curve of botulinum toxin bladder injection for the treatment of refractory overactive bladderInternational. J. Women’s Health. 14, 1–7 (2022).
Kajbafzadeh, A. M., Ahmadi, H. & Laleh Montaser-Kouhsari. Intravesical electromotive administration of botulinum toxin type A in improving the bladder and bowel functions: evidence for novel mechanism of action. J. Spinal Cord Med. 01 (1), 89–95 (2021).
Acknowledgements
The authors thank all the people who support investigators to complete this study.
Funding
There is no funding support for this study.
Author information
Authors and Affiliations
Contributions
C.S. and L.J. wrote the main manuscript text, T.C. and P.H. conducted statistical analysis, and All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical statement
This retrospective study was conducted in accordance with the ethical standards of the institutional and/or national research committee and with the Declaration of Helsinki.This study was conducted with approval from the Ethics Committee of Hangzhou Third People’s Hospital (Y-KL2025025). Written informed consent was obtained from all participants.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, J., Tang, C., Pan, H. et al. Ultrasound guided transabdominal botulinum toxin injection for refractory overactive bladder treatment. Sci Rep 15, 18162 (2025). https://doi.org/10.1038/s41598-025-03116-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-03116-2
Keywords
This article is cited by
-
Efficacy of ultrasound-guided transabdominal intradetrusor botulinum toxin injection for detrusor overactivity after spinal cord injury
European Journal of Medical Research (2025)