Abstract
As a kind of hazardous solid wastes, municipal solid waste incineration (MSWI) fly ash leads to serious environmental pollution. Converting MSWI fly ash into zeolites is an economically beneficial and environmentally sound way of disposal. In this paper, the effect of different additives (kaolin, milled glass powder, Na2SiO3) on the synthesis of zeolites by microwave-assisted hydrothermal process using MSWI fly ash was investigated through a series of single-factor experiments. The cation exchange capacity (CEC) was used to evaluate the synthesis effect and the adsorption property of zeolites. Na2SiO3 was found to be an ideal additive with the optimal dosage of 30 wt%, and the optimal time of magnetic stirring process was about 8 h. Na-P1 zeolite with high absorbability was synthesized in this condition, whose CEC was about 1.70 meq/g. In addition, the effect of Na2SiO3 dosage and various hydrothermal conditions on the CEC of zeolites were investigated through the orthogonal experiment and analysis of variance. It was found that the Na2SiO3 dosage had the strongest impact on the CEC of zeolites, followed by hydrothermal temperature, concentration of NaOH and hydrothermal time. The work of this paper provides a reference basis for synthesizing high-performance zeolites by MSWI fly ash.
Similar content being viewed by others
Introduction
A large amount of municipal solid waste (MSW) is generated in China every year. According to China Statistical Yearbook in 2021, the MSW disposal capacity in China was 249 million tons, of which 180 million tons (accounting for 72.29 wt%) were treated by incineration. Although the incineration disposal has the advantages of waste minimization, it generates a large quantity of secondary pollutants such as MSWI fly ash. Due to its richness in toxic heavy metals and dioxins, many scholars have been attracted on how to treat MSWI fly ash harmlessly.
Traditional treatments of MSWI fly ash include separation/extraction1,2, solidification/stabilization (S/S)3,4 and thermal treatment5. Nowadays, the conversion of MSWI fly ash into utilizable products has been widely concerned. Si and Al elements are considered to be the two main elements widely present in MSWI fly ash6, demonstrating that its utilization in the production of zeolites has a broad application prospect7,8. For instance, Qiu et al.9 modified MSWI fly ash to synthesize zeolites and the great potential in the field of adsorption was discovered. Currently, the synthesis of zeolites is mainly performed by the hydrothermal method. Its mechanism is to dissolve the ash (containing SiO2 and Al2O3) in an alkaline solution (usually in NaOH) to form aluminum silicate as the precursor of zeolites. And after a period of aging, the aluminum silicate solution is placed into an autoclave to carry out hydrothermal reaction at a specific temperature and pressure, which gradually results in the formation of aluminosilicate zeolites (e.g., P zeolite, FAU zeolite, Na-A zeolite)10,11. Heavy metals and dioxins can be effectively stabilized and degraded during the hydrothermal synthesis period12,13, and the zeolites synthesized can be commonly used as adsorbents14, catalyst carriers14 and ion exchangers16 due to their high specific surface aera and porosity.
Generally, the synthesis of zeolites is influenced by parameters such as Si/Al content, pH, activation reagents (or additives), and hydrothermal conditions17. Therefore, numerous studies have been carried out to investigate these parameters in the synthesis of zeolite. For example, Shi et al.18 added 30 wt% of a 1:1 mass ratio mixture of coal fly ash and diatomite as Si-Al modifier, as well as 3 wt% of tobermorite crystal seed, to the MSWI fly ash. After mixed with NaOH solution, the mixture was heated separately at 150 ℃ for different durations. The result showed that the diffraction peak of tobermorite appeared 3 h earlier than that of the product without crystal seed added. Insights from the above research, it is clear that suitable additives and hydrothermal conditions are essential for the synthesis of zeolites. Due to the limited Si and Al content in fly ash, and the fact that SiO2 usually exists in the form of insoluble crystalline phase (quartz), additives are often needed to adjust the Si/Al molar ratio. The hydrothermal conditions are mainly to promote the heterogeneous reaction and the dissolution of SiO2. Conventional hydrothermal method is time- and energy-consuming, which usually requires hours to weeks of heating time under high temperatures. Thus, the hydrothermal method needs to be improved, such as microwave-assisted technology, ultrasound-assisted technology. Microwave heating has the advantages of accelerating the dissolution and increasing the utilization rate of SiO2 from fly ash19. Ultrasonic heating can also accelerate the reaction between solid and liquid reactants, thus reducing the crystallization time and temperature20. But the temperature is usually not high enough (not higher than 100 ℃). Therefore, ultrasonic heating always needs to be combined with alkaline melting reaction process or hydrothermal treatment21,22.
Therefore, the MH technology was used in this study to explore the influence of different additives on the synthesis of zeolite types. Besides, considering further improving the efficiency of zeolite synthesis, an innovative magnetic stirring process has been introduced to strengthening the dissolution of silicon aluminum substances. In addition, with the use of the optimal additive, the effect of different additive dosages and hydrothermal conditions on the zeolite adsorption performance (analyzed in terms of CEC) were investigated, providing a referable experimental basis for the synthesis of high-performance zeolites.
Materials and methods
Raw materials
The MSWI fly ash utilized in this research was gathered from the bag filter of a circulating fluidized bed waste incinerator at a facility in Hangzhou, Zhejiang Province. Analytical-grade NaOH, CH3COONH4, CH3COONa, C3H8O (isopropanol), Na2SiO3 (particle size 75–180 μm) were purchased from Sinopharm Chemical Reagent Co. In addition, kaolin (particle size ≤ 106 μm) and milled glass powder (particle size 150 μm) were used as additives in the synthesis of zeolites.
Single-factor experiment
The aim of this series of experiments is to study the effects of additive species (kaolin, milled glass powder, Na2SiO3), additive dosage (5–30 wt%) and magnetic stirring time (4–12 h) on the zeolite types and the CEC of the zeolites synthesized. The experimental parameter settings are presented in Table 1. The MSWI fly ash was first placed in an oven (DGG-9070, China) and dried at 105 °C for 6 h. Then, the MSWI fly ash was mixed with NaOH solution and additives through magnetic stirring, and finally placed in a special container (AEX00050, Italy) and heated in a microwave dissolver (ETHOS UP, Italy).
Orthogonal experiment
The effects of different additive doses and hydrothermal conditions on the CEC of zeolites were studied through orthogonal experiments. The optimal additive was determined by single-factor experiment. Hence, the orthogonal parameters were designed as in Table 2.
Detection procedure of CEC
CEC can be used as the criteria for judging the adsorption ability of zeolite synthesis. It is generally believed that the higher the CEC value, the better the synthesis effect of zeolite23. CEC is measured according to Method 9081 developed by the US Environmental Protection Agency. The CEC value of the raw fly ash is about 0.02 meq/g.
Characteristic analysis
Element composition, morphology and microstructures, crystal phases of the fly ash and zeolite samples was determined using X-ray Fluorescence Spectrometer (XRF, PANalytical Epsilonl, Dutch), field emission scanning electron microscope (SEM, SU-70, Japan) and X-ray diffractometer (XRD, D/max-3B, Japan), respectively.
Results and discussion
Characterization of MSWI fly ash
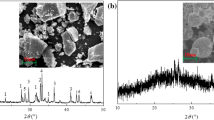
The primary chemical constituents of samples are presented in Table 3. Silicon and aluminum make up 10.8 wt% and 10.3 wt%, respectively, indicating a Si/Al molar ratio of 1.01, which is theoretically conducive to the synthesis of Na-P1 zeolite (Na6Al6Si10O32·12H2O)23. The fly ash contains a significant amount of CaO, rendering the fly ash alkaline and facilitating the preparation of zeolite in alkaline environments. The Ca element is the most abundant in fly ash, reaching up to 31.8 wt%, which restricts the synthesis of calcium-free zeolites like P zeolite and analcime. During the hydrothermal synthesis of zeolites, the content of Si and Al elements, as well as the ratio between Ca, Si, and Al, are crucial influencing factors. The relatively low content of Cl (4.96 wt%) is brought by soluble salts formed during the incineration. The XRD pattern is shown in Fig. 1a, which indicates the main components including quartz, anhydrite, calcium carbonate, calcium hydroxychloride and hematite. The morphology and microstructures are illustrated in Fig. 1b,c, which shows a porous structure consisting of stacks of lamellar units. The leaching toxicity of heavy metals in the raw fly ash and the leaching toxicity after disposal were studied in detail in our other study24.
Results of single-factor experiments
Effect of kaolin
The elemental composition of kaolin is shown in Table 4. Si and Al are the main elements, in addition to small amounts of Ca, Ti, Fe and other elements. As the XRD results shown in Fig. 2a, the kaolin is mainly composed of mullite (Al2.272Si0.728O4.864, PDF#83-1881), with minor amounts of quartz and alumina (Al2O3, PDF#51-0769). For its richness of Si and Al, the kaolin can be used as an additive in the synthesis of zeolites.
The effect of different magnetic stirring time on the synthesis of zeolites was first investigated (Table 1). According to the XRD (shown in Fig. 2b), tobermorite (Ca5Si6O24(O,OH,F)18.5H2O, PDF#45-1480) and katoite (Ca2.93Al1.97Si0.64O2.56(OH)9.44, PDF#84-0917) were observed in E1–E3, demonstrating that MSWI fly ash was conversed with the addition of kaolin. Furthermore, it could be seen that the intensity of the diffraction peaks of katolite was first enhanced and then weakened with the magnetic stirring, indicating that the magnetic stirring promotes the dissolution of quartz in the fly ash and mullite in the kaolin, which provides Si and Al. However, with the increase of stirring time to 12 h, the Si dissolved increased while the Al dissolved was relatively stable, which makes the Si/Al molar ratio increase and not conducive to the synthesis of katoite25. It should be emphasized that the product diffraction peaks in the XRD patterns are all weak in intensity, and meanwhile the addition of kaolin does not significantly promote zeolites synthesis.
The CEC results align with the aforementioned trends. The CEC value of tobermorite is about 0.03 meq/g, and that of the raw fly ash is about 0.02 meq/g. Thus, it can be seen that the direct impact of tobermorite on the CEC value of the hydrothermal products is not significant. As depicted in Fig. 2c, the CEC of E1–E3 initially increased and then decreased with magnetic stirring, mirroring the trend in the intensity of the kaolinite diffraction peak. The CEC of the MH products with magnetic stirring for 4 h, 8 h, and 12 h were 0.54 meq/g, 0.67 meq/g, and 0.44 meq/g, respectively. However, the CEC of the MH product with magnetic stirring for 12 h was smaller than that of the MH product with stirring for 4 h. This suggests that changes in magnetic stirring time affects the Si/Al molar ratio dissolved into the liquid phase, and further affects the CEC value. Although zeolites were not directly detected in the products with the addition of kaolinite, the CEC of the products has been significantly improved. This may be because under hydrothermal conditions, reactions occur between kaolinite and fly ash to generate some undetected amorphous or microcrystalline substances with potential CEC. For example, some amorphous phases containing aluminosilicates may be formed, and there are exchangeable cation sites in their structures. In addition, the gel-like substances rich in aluminum or silicon formed in the early stage of the hydrothermal reaction may gradually transform into crystalline or amorphous phases with a certain CEC as the reaction proceeds and the conditions change.
Then, the effect of the dosage of kaolin was investigated. The XRD patterns of MH products obtained by adding different dosages of kaolin are shown in Fig. 3a. Diffraction peaks caused by tobermorite and katoite were observed in E2, E4–E6, but the intensity of the diffraction peaks did not show a regular change with the increase of kaolin addition. As shown in the XRD patterns, the intensity of the diffraction peaks of tobermorite and katoite was relatively high when 5 wt% and 20 wt% kaolin were added, while the intensity was weak when 10 wt% and 30 wt% kaolin were added. It indicates that the dosage of kaolin is not the more the better, since the Si and Al dissolved and effectively utilized in kaolin are limited. And the changing trend of CEC results (Fig. 3b) is also consistent with the XRD patterns.
In summary, the incorporation of kaolin facilitates the transformation of the morphological structure of MSWI fly ash, including the formation of tobermorite and katoite. Additionally, the optimal conditions for maximizing the CEC value of the product involve adding kaolin accounting for 20% by weight and conducting magnetic stirring for 8 h.
Effect of milled glass powder
The elemental composition of milled glass powder is shown in Table 5, and Si, Al and Ca are the main elements. The content of Si is 45.39 wt% and the content of Al is 13.03 wt%, which can provide abundant Si source for the synthesis of zeolite. No other elements are more than 1.0 wt%. From the XRD pattern (Fig. 4a) of the milled glass powder, it can be seen that it consists mainly of amorphous material with no diffraction peaks for specific crystals.
The XRD patterns of MH products with different magnetic stirring time is shown in Fig. 4b. In E7–E9, the diffraction peaks caused by tobermorite, katoite and sodlite (hydrated) (Na7.6Al6Si6O24(CO3)0.93.2.93H2O, PDF#89-9099) were all observed, indicating that the MWSI fly ash was converted to zeolites with the addition of milled glass powder. It could be also seen that the intensity of the diffraction peaks of sodlite increased and then decreased with the magnetic stirring, the diffraction peaks of tobermorite increased from none to some, and the diffraction peaks of katoite did not change obviously. In addition, the intensity of SiO2 diffraction peaks of E7–E9 showed a gradual weakening trend, indicating that the dissolution of SiO2 in the MSWI fly ash into the liquid phase was promoted by the magnetic stirring25. Namely the sodlite and katoite were synthesized firstly, and with the prolongation of stirring time, the Si in the milled glass powder and the MSWI fly ash was further dissolved, which promoted the synthesis of the tobermorite. However, the diffraction peaks of sodlite were not further enhanced when the stirring time was increased to 12 h. This was attributed to that the milled glass powder, which contained abundant Si source, continued to dissolve, and the Si/Al molar ratio continued to increase. It was not conducive to the synthesis of sodlite with Si/Al molar ratio of 1.0.
The changing trend of CEC of MH products is shown in Fig. 4c. It could be seen that the CEC of the MH products increased and then decreased with the magnetic stirring, which was also consistent with the changing trend of the intensity of the zeolite diffraction peaks. The CEC of the MH products with magnetic stirring for 4 h, 8 h, and 12 h were 0.56 meq/g, 0.67 meq/g, and 0.44 meq/g, respectively. Among them, the product with 8 h magnetic stirring had the highest CEC.
With respect to the effect of the dosage of milled glass powder, the XRD patterns of MH products obtained by adding different dosages of milled glass powder are shown in Fig. 5a. In E8, E10–E12, the diffraction peaks caused by tobermorite, katoite and sodlite were observed, and the intensity of the sodlite diffraction peaks gradually increased as the amount of ground glass powder added increased. However, there was no significant difference in diffraction peak intensity between the zeolite synthesized with 20 wt% milled glass powder and that synthesized with 30 wt% milled glass powder. Although the addition increased, the Si source that could be dissolved into the liquid phase was limited, and the amount of zeolites synthesized did not increase linearly all the time with the increase of the addition of milled glass powder.
The CEC of MH products with adding different dosages of milled glass powder are shown in Fig. 5b. It could be seen that the CEC increased as the dosage increased to 20 wt%. When the dosage increased to 30 wt%, the CEC decreased compared to the dosage of 20 wt%. Evidently, the CEC of MH products did not always increase with the increase of the addition of milled glass powder, and even showed a decreasing trend. The reason is that the Si/Al molar ratio of the synthesis system with the addition of 20 wt% milled glass powder is 1.49, which is close to that of sodlite (1.0) and is conducive to the synthesis of sodlite; the Ca/Si molar ratio is 1.39, which is close to that of tobermorite (1.50) used in the synthesis of tobermorite in other studies26, and is conducive to the synthesis of tobermorite.
In conclusion, the involvement of milled glass powder facilitated the conversion of MSWI fly ash to zeolites like sodlite (hydrated). Furthermore, the most conducive conditions to the synthesis of zeolites were the addition of 20 wt% milled glass powder and the magnetic stirring time for 8 h.
Effect of Na2SiO3
The XRD patterns of MH products with addition of Na2SiO3 at different magnetic stirring time is shown in Fig. 6a. In E13–E15, diffraction peaks caused by katoite and sodlite were observed, indicating that the zeolites were synthesized by the MH process after the addition of Na2SiO3. From the XRD patterns of E13–E15, it could be seen that the intensity of diffraction peaks of sodlite and katoite was not significantly different, while the intensity of diffraction peak of SiO2 was the weakest at 8 h of magnetic stirring and the strongest at 12 h of magnetic stirring. It indicated that magnetic stirring for 8 h could significantly promote the dissolution of the SiO2 in the fly ash.
The changing trend of CEC of MH products is shown in Fig. 6b. The CEC of the product initially increased and then decreased with magnetic stirring, which was consistent with the changing trend of the SiO2 peak intensity in the XRD. It verified that the magnetic stirring enhanced the dissolution of SiO2 in the fly ash, and boosted the quantity of synthesized zeolites. The CEC of the MH products with magnetic stirring for 4 h, 8 h, and 12 h were 0.75 meq/g, 0.96 meq/g, and 0.83 meq/g, respectively, with the highest CEC observed after magnetic stirring for 8 h.
Concerning the effect of different dosages of Na2SiO3 on the synthesis of zeolites, it can be seen by the XRD patterns of the MH products (Fig. 7a). According to the Fig. 7a, diffraction peaks caused by sodlite and Na-P1 zeolite (Na6Al6Si10O32⋅12H2O, PDF#71-0962) were observed in E14, E16–18. The zeolite phase in the MH products was sodlite when the addition of Na2SiO3 was 5 wt%, 10 wt%, and 20 wt%, whereas only with the addition of 30 wt% Na2SiO3 was the diffraction peaks of Na-P1 zeolite detected, and the intensity tended to be enhanced progressively with the increase of the Na2SiO3 dosage. The reason for this is that the Si/Al molar ratio of the Na-P1 zeolite is 1.66, and the Si/Al molar ratio of the synthesis system increases with the increase of Na2SiO3. With the addition of 30 wt% Na2SiO3, in the entire synthesis system stands at 1.44, aligning more closely with the Si/Al ratio of Na-P1 zeolite, thereby promoting the zeolite synthesis of Na-P1.
The changing trend of CEC of MH products with adding different dosages of Na2SiO3 is shown in Fig. 7b. It presented a gradual increase with the dosage of Na2SiO3 increasing. The CEC of the MH products were 0.80 meq/g, 0.93 meq/g, 0.96 meq/g, and 1.70 meq/g for the dosages of Na2SiO3 of 5, 10, 20, and 30 wt%, respectively. Furthermore, the CEC with the addition of 5–20 wt% Na2SiO3 were very close to each other for the reason that only sodlite and katoite, which have poorer cation exchange property, were synthesized. Whereas the maximum CEC (1.70 meq/g) with the addition of 30 wt% Na2SiO3 was due to the synthesis of Na-P1 zeolites resulted in a substantial increase in the CEC. The zeolite synthesized of MSWI fly ash with the addition of Na2SiO3 is more excellent compared to the addition of kaolin and milled glass powder since Na2SiO3 directly provides an ionic Si source for the synthesis of zeolites.
In conclusion, the involvement of Na2SiO3 facilitated the conversion of MSWI fly ash to zeolites like sodlite and Na-P1 zeolite24. Furthermore, the most conducive condition to the synthesis of zeolites was the addition of 30 wt% Na2SiO3 and the magnetic stirring time for 8 h.
The solubility of these three additives in NaOH solution is ranked in descending order as follows: Na2SiO3, glass powder, and kaolin. Among them, sodium silicate can dissolve quickly and completely in water, but its dissolution is somewhat inhibited in alkaline environments. On the other hand, kaolin is almost insoluble in alkaline solutions. The SiO2 in glass powder has an amorphous structure. Compared to crystalline SiO2, the amorphous structure exhibits higher activity and is more likely to react with OH⁻ in NaOH solution, but its dissolution rate is still much lower than that of Na2SiO3. Additionally, the hydrothermal heating process positively impacts the degree of dissolution. The solubility of these substances directly affects the content of Si element in the reaction solution, playing a crucial role in zeolite synthesis and enhancing CEC.
Results of orthogonal experiment
After the single-factor experiment, it was found that Na2SiO3 was the best of the three additives. The analytical data for different Na2SiO3 dosages and hydrothermal conditions of the orthogonal experiment is listed in Table 6, and the relationship between each factor and the CEC is shown in Fig. 8. Combined with Table 6 and Fig. 8, the influence of different factors on the CEC of MH products can be derived. The greater the fluctuation of the corresponding line chart in Fig. 8, the greater the influence of this factor on the CEC of the products. Among them, the addition of Na2SiO3 had the greatest influence on the CEC of MH products, and the CEC of the products was the largest and most favorable for zeolite synthesis when 30 wt% was added. Followed by the MH temperature, obviously. When the temperature raised to 180 °C, the CEC value reach its maximum, while when the temperature raised from 160 to 180 °C, the increase of CEC is not significant. The concentration of NaOH had the third influence on the CEC of the products, after the concentration was higher than 1 mol/L, there was no significant change in the CEC. Finally, it is the MH time. Although the time was different, the CEC of MH products were very close, and the CEC of MH products was the smallest at the time of 1.5 h.
The ANOVA is supplemented and the results of the analysis are listed in Table 7. The greater the sum of squares in the table is, the greater the effect of the factor on the CEC is. The mean square is the sum of the squares divided by its own degrees of freedom, eliminating the effects of different levels of individual factors. The larger the mean square, the more important the factor. The column "F" is obtained by dividing the mean square by the mean square of the error, taking the degree of freedom of the error and the degree of freedom of the factor as parameters, respectively, and querying the table of F-distributions to obtain the significance of each factor. Results showed that Na2SiO3 addition was the most important influential factor, followed by MH temperature, concentration of NaOH and MH time.
Conclusions
In this work, the effect of different additives as well as different MH conditions on synthesis of zeolites was investigated. The main conclusions obtained are as follows:
-
1.
It was found that the CEC was maximum for 8 h of magnetic stirring regardless of which additive was used. Magnetic stirring time affects the Si/Al molar ratio dissolved into the liquid phase, and further affects the zeolites synthesis.
-
2.
When the addition of Na2SiO3 was increased to 30 wt%, Na-P1 zeolite was synthesized and the CEC of the MH product was as high as 1.70 meq/g.
-
3.
In orthogonal experiments, it was found that the addition of Na2SiO3 showed the greatest effect on the CEC of the MH products, followed by the MH temperature, then the concentration of NaOH, and finally the MH time.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Dongyang, H. E. et al. Carbothermal treatment of municipal solid waste incineration fly ash: Purification and valuable elements extraction. Sep. Purif. Technol. 331, 125713 (2024).
Dongyang, H. E. et al. Thermal separation of heavy metals from municipal solid waste incineration fly ash: A review. Chem. Eng. J. 467, 143344 (2023).
Yue, W. A. N. G., Ruiping, L. I. & Jiangang, Q. I. A. O. Solidification of heavy metals in municipal solid waste incineration washed fly ash by asphalt mixture. Chemosphere 343, 140281 (2023).
Xueying, Y. U. A. N. et al. Stabilization effect of chelating agents on heavy metals in two types of municipal solid waste incineration fly ash. Process Saf. Environ. Prot. 180, 169–180 (2023).
Zhijun, S. et al. An all-in-one strategy for municipal solid waste incineration fly ash full resource utilization by heat treatment with added kaolin. J. Environ. Manag. 329, 117074 (2023).
Ling, L. et al. Characteristics of fly ash from waste-to-energy plants adopting grate-type or circulating fluidized bed incinerators: A comparative study. Energy Sour. Part A Recovery Util. Environ. Effects 46, 1–17 (2020).
Xiaolu, G. U. O. & Meng, S. O. N. G. Micro-nanostructures of tobermorite hydrothermal-synthesized from fly ash and municipal solid waste incineration fly ash. Constr. Build. Mater. 191, 431–439 (2018).
Zhidong, Y. et al. Resource recovery of waste incineration fly ash: Synthesis of tobermorite as ion exchanger. J. Mater. Res. 14(44), 4437–4442 (1999).
Qili, Q. et al. Adsorption of heavy metal ions using zeolite materials of municipal solid waste incineration fly ash modified by microwave-assisted hydrothermal treatment. Powder Technol. 335, 156–163 (2018).
Dezhi, S. et al. Silicon-aluminum additives assisted hydrothermal process for stabilization of heavy metals in fly ash from MSW incineration. Fuel Process. Technol. 165, 44–53 (2017).
Gordoncc, Y. & Tsung-yin, Y. Synthesis of zeolites from municipal incinerator fly ash. J. Hazard. Mater. 62(1), 75–89 (1998).
Yuyan, H. et al. Hydrothermal treatment of municipal solid waste incineration fly ash for dioxin decomposition. J. Hazard. Mater. 207–208, 79–85 (2012).
Qili, Q. et al. Adsorption of copper ions by fly ash modified through microwave-assisted hydrothermal process. J. Mater. Cycles Waste Manag. 21(3), 469–477 (2019).
de Lucianofernandes, M., Gilbertorodriguesda, S. & Antônioeduardoclark, P. Zeolite application in wastewater treatment. Adsorpt. Sci. Technol. 2022, 1–26 (2022).
Balakrishnan, M. et al. Waste materials—catalytic opportunities: An overview of the application of large scale waste materials as resources for catalytic applications. Green Chem. 13(1), 16–24 (2011).
Wei-heng, S. & Hsiao-lan, C. Conversion of fly ash into zeolites for ion-exchange applications. Mater. Lett. 28(4), 263–268 (1996).
Christian, V. & Muhammad, U. Municipal solid waste fly ash-derived zeolites as adsorbents for the recovery of nutrients and heavy metals—A review. Water 15(21), 3817–3864 (2023).
Dezhi, S. H. I. et al. Synergistic effect of silicon-aluminum addition and seed-induced on stabilization of heavy metals in MSW incineration fly ash during hydrothermal process. CIECS J. 69(8), 3651–3661 (2018).
Tijjani, A., Zawati, H. & Mohdhafizdzarfan, O. A review on sustainable synthesis of zeolite from kaolinite resources via hydrothermal process. Adv. Powder Technol. 28(8), 1827–1840 (2017).
Syedsalman, B., Sohrab, R. & Hossein, K. Effect of ultrasound energy on the zeolitization of chemical extracts from fused coal fly ash. Ultrason. Sonochem. 28, 47–53 (2016).
Pameli, P. et al. Synthesis of NaP zeolite at room temperature and short crystallization time by sonochemical method. Ultrason. Sonochem. 20(1), 314–321 (2013).
Sivamani, S. & Sujit, S. Valorization of coal fly ash into nanozeolite by sonication-assisted hydrothermal method. J. Environ. Manag. 235, 145–151 (2019).
Grégorio, C. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 97(9), 1061–1085 (2006).
Zhou, Q. et al. Synthesis of high-quality Na-P1 zeolite from municipal solid waste incineration fly ash by microwave-assisted hydrothermal method and its adsorption capacity. Sci. Total Environ. 855, 158741 (2023).
Hidekazu, T. & Atsushi, F. Effect of stirring on the dissolution of coal fly ash and synthesis of pure-form Na-A and -X zeolites by two-step process. Adv. Powder Technol. 20(5), 473–479 (2009).
Abdolhosseini Qomi, M. J. et al. Combinatorial molecular optimization of cement hydrates. Nat. Commun. 5(1), 4960–4969 (2014).
Funding
This work was supported by Jiangsu Provincial Natural Science Foundation of China [BK20201032] and the Research Program Foundation of Nanjing Institute of Technology [YKJ 201998].
Author information
Authors and Affiliations
Contributions
Qili Qiu and Yulong Hu wrote the main manuscript text. Qi Zhou prepared all figures and tables. Qili Qiu and Jun Zhang reviewed the maniscript. Dongping and Fan Zeng revised the manuscript. Dongping Zhang formatted and organized the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Qiu, Q., Zhang, J., Zhang, D. et al. Effect of magnetic stirring and different additives on the synthesis of zeolites from MSWI fly ash. Sci Rep 15, 18751 (2025). https://doi.org/10.1038/s41598-025-03368-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-03368-y