Abstract
This study investigated the relationship between dyslipidemia prior to conception and the risk of preeclampsia (PE) in women pregnant by in vitro fertilization and embryo transfer (IVF-ET). The retrospective cohort study consisted of 2994 women who conceived by IVF-ET and delivered live neonates. The study population was divided into two components: a training set for the prediction model development (2288 women) and a test set for validation (706 women). Multivariable logistic regression was used for the development and validation of predictive model for the risk of PE. Among the 2288 women in the training set, 266 women (11.6%) developed PE. Multiple logistic regression analysis identified independent predictors for PE: triglyceride (TG) [adjusted odds ratio (aOR) 1.284; 95% confidence interval (CI) 1.113–1.489, P < 0.001]; pre-pregnancy BMI; pre- chronic hypertension; twin pregnancy; embryo transfer protocol. These independent predictors for PE were used to form a risk prediction model, and the area under the receiver-operator characteristic (ROC) curve (AUC) in the training and the test set was 0.77 (95% CI 0.73–0.80)and 0.71 AUC (95% CI 0.65–0.77), respectively. In conclusion, higher TG levels before pregnancy were independently associated with the risk for PE in women pregnant by IVF-ET.
Similar content being viewed by others
Preeclampsia (PE) affects 2–8% of pregnancies and is the leading cause of maternal and fetal mortality and morbidity globally1,2,3. It also increases women’s long-term risk of cardiovascular diseases4,5. Studies have shown that PE and cardiovascular diseases have many common risk factors, such as obesity, history of hypertension, diabetes and dyslipidemia6,7,8. The relationship between dyslipidemia and PE has drawn much attention in recent years9,10,11,12.
In normal pregnancies, there is a physiological increase in serum lipid concentration, which ensures the development of the fetus13. However, an abnormal increase in serum lipid levels during pregnancy has been associated with the development of PE9,10,11,12. In contrast to dyslipidemia during gestation, the relationship between pre-gestational dyslipidemia and PE has not been studied extensively.
Nowadays, 8–12% childbearing age women are affected by infertility worldwide14, and more than 15% of couples suffer from infertility in China15.The use of in vitro fertilization and embryo transfer (IVF-ET) has risen steadily in the worldwide16,17. While IVF-ET benefits many couples, it has been established that IVF–ET is associated with adverse pregnancy outcomes, including PE18,19. Women pregnant by IVF-ET are older and more likely to have chronic disease (i.e. hypertension, diabetes and dyslipidemia) than women who have spontaneously conceived20,21. It has been reported that dyslipidemia was highly prevalent in women undergoing IVF-ET20,21. Although significant evidence links dyslipidemia and PE9,10,11,12, studies focusing on women undergoing IVF-ET are still lack. Thus, the purpose of this study was to investigate the relationship between dyslipidemia prior to conception and the risk of PE in women pregnant by IVF-ET. We also aim to establish and validate a prediction model for the risk of PE based on dyslipidemia, maternal characteristics and IVF-ET related variables in this specific group of women.
Method
Study population
This was a retrospective cohort study conducted at Peking University Third Hospital, a leading tertiary university hospital with an excellence reproductive medical center in China. Women conceived by IVF-ET and delivered live neonates between 1 January 2017 and 31 December 2022 in this hospital were included. Fasting lipid profile tests were routinely conducted before IVF-ET. Exclusion criteria were: histories of PE during previous pregnancy; no record of lipid measurements, the time duration between lipid measurement and conception exceeding 12 months, histories of taking lipid-lowering agents and histories of PE. For women with more than one birth during the study period, only data from the first pregnancy were analyzed.
The study population was divided into two components: a training set for the prediction model development (women delivered between 1 January 2017 and 31 December 2020) and a test set for validation (women delivered between 1 January 2021 and 31 December 2022).
This study was approved by the ethics review board of Peking University Third Hospital, and was performed in accordance with the Declaration of Helsinki. Due to the retrospective nature of the study, the ethics review board of Peking University Third Hospital waived the need of obtaining informed consent.
Date collection
Data were collected from the hospital’s electronic medical records, including age, pre-pregnancy body mass index (BMI), previous history of diabetes mellitus and autoimmune diseases, parity, number of fetus, gestational diabetes mellitus, time at delivery. Blood pressure (BP) was routinely measured at the first visit to the reproductive medical center in the seated position after resting for at least 5 min using an Omron automated sphygmomanometer. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were recorded. Autoimmune diseases included systemic lupus erythematosus (SLE), rheumatoid arthritis, Sjögren’s syndrome, antiphospholipid-antibody syndrome (APS), undifferentiated connective tissue diseases, etc.
IVF-ET related information were collected from the electronic medical records of the reproductive medical center, including basal follicle-stimulating hormone (FSH), Luteinizing Hormone (LH), estradiol (E2), etiology of infertility, embryo transfer protocol, type of embryo transfer and stage of transferred embryo. The etiology of infertility was determined as the most important cause of infertility. Embryo transfer protocol included natural cycle thawed embryo transfer (abbreviated as ‘natural cycle’), hormone replacement therapy cycle frozen-thawed embryo transfer (abbreviated as HRT cycle’), ovulation induction cycle frozen-thawed embryo transfer (abbreviated as ‘OI cycle’) and fresh stimulated embryo transfer (abbreviated as ‘Fresh cycle’) .
Biochemical analyses were routinely performed in a fasting state at the first visit to the reproductive medical center using an automatic biochemical analyzer (BECKMAN COULTER CHEMISTRY ANALYZER AU5800 Serie, USA). The total cholesterol (TC), triglyceride (TG), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C) and glucose levels were collected. TG was test by Glycerol-3-Phosphate Oxidase-Peroxidase Method.
In our hospital, the criteria for diagnosing PE adhere to the guidelines by the American College of Obstetricians and Gynecologists (ACOG)22: a systolic BP of 140 mmHg or more or a diastolic BP of 90 mmHg or more, on two occasions at least 4 h apart after 20 weeks’ gestation with proteinuria, or with severe features: thrombocytopenia, renal insufficiency, impaired liver function, pulmonary edema, new-onset headache unresponsive to medication, or visual symptoms. The diagnosis of proteinuria, thrombocytopenia, renal insufficiency, and impaired liver function followed the criteria in ACOG guidelines22.
Statistical analyses
The Shapiro-Wilk test was utilized to evaluate the normality of data distribution. Continuous variables of normal distribution were expressed as mean ± standard deviation (SD), and the comparison between two groups was conducted using the independent sample t test. Categorical data were reported as counts (percentages) and the comparison between two groups was conducted using Chi-square test. Independent predictors for PE were identified by multiple logistic regression analysis (backward stepwise), including maternal age and variables with a value of P < 0.10 by univariate analysis. A prediction model of PE was developed using the multivariable logistic regression. Regression coefficients were used to generate a nomogram.
The prediction model was assessed by examining discrimination and calibration in the development cohorts (2017–2020 population) and the validation cohorts (2021–2022 population). The discrimination was assessed by the area under the receiver-operator characteristic (ROC) curve (AUC) and its 95% CI. The calibration was constructed to examine the agreement between the predicted probabilities with the observed outcome, which was assessed by the Hosmer-Lemeshow goodness-of-fit test and calibration plots. The calibration plot was calculated by the 1000 repetitions Bootstrap resampling. Development and reporting of the prediction model followed the Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD) Statement.
Statistical tests were done with R software (version 4.3.2) and SPSS (version 25.0). Statistical significance was set at two-sided P values less than 0.05.
Results
Population characteristics
There were 118,279 women undergoing IVF-ET between 1 January 2017 and 31 December 2022 in our hospital. Among them, 2994 women were included in this study (Fig. 1). The study population was divided into a training set and a test set. The training set included 2288 women delivered between 1 January 2017 and 31 December 2020, and the test set included 706 women delivered between 1 January 2021 and 31 December 2022.
Screening for independent predictors of preeclampsia in women pregnant by IVF-ET
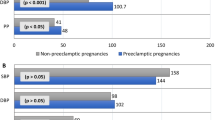
Among the 2288 women in the training set, 266 women (11.6%) developed PE. Women with PE had higher pre-pregnancy BMI, pre-pregnancy systolic BP, pre-pregnancy diastolic BP compared to women without PE. Twin pregnancy and previous history of chronic hypertension and diabetes mellitus were more common in the PE group. Women with PE delivered at earlier gestational weeks. More women in the PE group underwent IVF-ET due to anovulation and by hormone replacement therapy (HRT) cycles than those in the non- PE group. The pre-pregnancy TC, TG and LDL-C levels were significantly higher and the HDL-C levels were significantly lower in the PE group than in the non- PE group. There were no significant differences between the two groups in terms of maternal age, histories of parturition, histories of autoimmune diseases, glucose level, basal FSH, LH, and E2, type of embryo transfer and stage of transferred embryo (Table 1).
Multiple logistic regression analysis included maternal age and 12 variables with a value of P < 0.10 by univariate analysis: maternal age, pre-pregnancy BMI, pre-pregnancy systolic BP, pre-pregnancy diastolic BP, twin pregnancy, previous history of chronic hypertension and diabetes mellitus, TC, TG, LDL-C, HDL-C, etiology of infertility, and embryo transfer protocol. Independent predictors for PE identified by multiple logistic regression analysis were: TG [adjusted odds ratio (aOR) 1.284; 95% confidence interval (CI) 1.113–1.489, P < 0.001]; pre-pregnancy BMI (aOR 1.108; 95% CI 1.059–1.159, P < 0.001); pre-chronic hypertension (aOR 6.015; 95% CI 3.542–10.188, P < 0.001); twin pregnancy (aOR 4.289; 95% CI 3.211–5.755, P < 0.001); embryo transfer protocol (aOR for HRT cycles 2.158; 95% CI 1.435–3.277, P < 0.001)(Table 2).
Establishment and internal validation of the prediction model
The independent predictors for PE identified by multiple logistic regression analysis were used to establish the logistic regression equation and form a risk prediction model. The final model included triglycerides, BMI, chronic hypertension, twin pregnancy, and embryo transfer protocol. The logistic regression equation for estimating the probability of developing PE was as follows:
In this model, P is the predicted probability of PE. “Natural cycle” was the reference group for the embryo transfer protocol. “No” was the reference group for the chronic hypertension and twin pregancy.
The prediction probability of this model was plotted on the ROC (AUC = 0.77, 95%CI 0.73–0.80, Fig. 2). We then drew a nomogram to identify the risk of developing PE in women pregnant by IVF-ET (Fig. 3). The characteristics of women with and without PE in the test set were shown in Supplementary Table S1 online. The validation of the prediction model showed that it had good discriminative ability and calibration (AUC = 0.71, 95%CI 0.65–0.77, Fig. 2). The calibration curve shows a good consistency between the observed probabilities and the predicted probabilities (Fig. 4).
Discussion
This cohort study examined the association between pre-conception dyslipidemia and the risk for PE in women pregnant by IVF-ET and delivered live neonates. The result showed that the increase in TG levels measured before pregnancy was independently associated with the risk for PE. Furthermore, we developed a model to predict the development of PE in women undergoing IVF-ET. The predictors in this model that had significant effect on the risk of PE were: TG, BMI, chronic hypertension, twin pregnancy and HRT cycles.
During normal pregnancy, there is a physiological change in lipid metabolism because of the effects of estrogen, progesterone and lactogen13. From the 12th week of pregnancy, serum levels of TC, TG, HDL-C and LDL-C gradually increase, especially in the second and third trimesters13. By late pregnancy, the four lipid components increase by 45%, 150%, 35% and 35% respectively23. Previous studies indicated a relationship between TG and PE7,9,10,11,12,23,24. A meta-analysis by Spracklen et al.23 showed that PE was associated with elevated TG levels during all trimesters of pregnancy, and the differences in TG levels between women with and without PE were substantially more remarkable during the third trimester than in the first/second trimesters. The Amsterdam Born Children and Their Development (ABCD) cohort study observed 4008 women and showed that maternal TG concentrations in early pregnancy (12–14 gestational weeks) were linearly associated with the risk of pregnancy-induced hypertension, PE, preterm birth and large for gestational age (LGA)10. Several studies also investigated the relationship between preconception TG and risk of PE7,24. A community-based Cohort study included 13,217 singleton pregnancies without preexisting hypertension, and indicated that elevated TG levels (≥ 1.7 mmol/L) could predict the risk for PE ( OR 2.4, 95%CI 1.71–3.30)7. A study by Baumfeld et al.24 had similar results, and reported that high TG was independently associated with the composite outcome of gestational diabetes mellitus / or PE with OR of 1.61 (95%CI 1.29–2.01). The relationship between dyslipidemia and the risk of PE in women undergoing IVF-ET has not been reported yet. In the present study, we included women who conceived by IVF-ET and delivered live neonates, and found that TG measured before conception was an independent predictor for the risk of PE. The specific role of TG in the pathogenesis of the PE is still not well established. The possible mechanism is that accumulation of TG in endothelial cells could trigger a decreased production of prostaglandins and nitric oxide and consequently cause endothelial dysfunction13.
The relationship of preconception TC and HDL-C levels with the risk of PE are still controversial. The meta-analysis by Spracklen et al.23 showed that women with PE had higher levels of TC during all trimesters of pregnancy and lower levels of HDL-C only in the third trimester. In the study by Wiznitzer et al.9, high TC but not low HDL-C during pregnancy was independently associ ated with the development of PE. However, Baumfeld et al.24 reported that low HDL-C (≤ 50 mg/dL) before conception was independently associated with the composite outcome of gestational diabetes mellitus / or PE ( OR 1.33, 95%CI 1.09–1.63). A population-based study from China investigated the relationship of TC, TG, HDL-C and LDL-C during pregnancy with pregnancy complications, and indicated that only TG was independently associated with increased risk of pregnancy complications11. Previous studies have consistently shown no significant correlation between LDL-C and the occurrence of PE9,11,23,24. The present study showed that although women with PE had significant higher TC, higher LDL-C and lower HDL-C levels before pregnancy, none of the three lipid components were independently associated with the development of PE in multiple logistic regression analysis.
It has been established that IVF-ET is associated with adverse pregnancy outcomes18,19. In this study, we established and validated a prediction model for the risk of PE in women pregnant by IVF-ET. We found that the model constructed based on the TG, BMI, chronic hypertension, twin pregnancy and embryo transfer protocol had good predictive power and clinical utility.
Clinical Relevance
There are currently no recommendations by guidelines to screen for dyslipidemia before ART. Dyslipidemia is common in women undergoing ART. Cirillo et al.21 Investigated 1003 women (median age 40 years) undergoing ART, and found that nearly 60% of them suffered from dyslipidemia. Lipids screening seems to be necessary before beginning infertility treatment. Recognizing dyslipidemia may allow for appropriate intervention (i.e. life style changes and weight management) which could modify lipid profiles and might contribute to improve maternal outcomes25. These interventions should be undertaken both before and during the gestation. Furthermore, increased vigilance for early signs of PE might be considered in women with dyslipidemia, especially those with high TG levels before ART.
This study had shown that the risks of PE was higher in patients conceiving after HRT cycles than in those conceiving after natural cycles. This was consistent with the results of previous studies26,27. Thus, we should consider obstetrical risks when we decide on the endometrium preparation method. Natural cycles and fresh cycles might be better in women with high risk of PE.
This study has certain limitations as it did not analyze chronic kidney disease, SLE, and APS as independent risk factors. However, given the relatively low prevalence of these diseases among women of childbearing age in preconception status, future research should incorporate larger sample sizes and more detailed analyses. Secondly, we did not included lipoproteins because this was a retrospective study and lipoproteins were not routinely measured before IVF-ET, although previous studies indicated lipoprotein(a) might also be as a predictor of PE28. Moreover, this study was conducted at a single center, and although internal validation showed good discrimination ability and calibration, external validation was not performed due to the lack of an independent dataset. Future studies with multi-center cohorts or prospective validation are needed to confirm the robustness and generalizability of our findings.
Conclusions
Higher TG levels before pregnancy were independently associated with the risk of PE in women pregnant by IVF-ET. The model encompassing TG, BMI, chronic hypertension, twin pregnancy and embryo transfer protocol could predict PE.
Data availability
The datasets used in the current study are available from the corresponding author on reasonable request.
References
American College of Obstetricians and Gynecolocists. Gestational hypertension and preeclampsia: ACOG practice bulletin, number 222. Obstet. Gynecol. 135, e237–e260. https://doi.org/10.1097/AOG.0000000000003892 (2020).
Jiang, L. et al. A global view of hypertensive disorders and diabetes mellitus during pregnancy. Nat. Rev. Endocrinol. 18 (12), 760–775. https://doi.org/10.1038/s41574-022-00734-y (2022). Epub 2022 Sep 15. PMID: 36109676; PMCID: PMC9483536.
Li, F., Qin, J., Zhang, S. & Chen, L. Prevalence of hypertensive disorders in pregnancy in China: A systematic review and meta-analysis. Pregnancy Hypertens. 24, 13–21. https://doi.org/10.1016/j.preghy.2021.02.001 (2021). Epub 2021 Feb 14. PMID: 33626437.
Garovic, V. D. et al. Incidence and Long-Term outcomes of hypertensive disorders of pregnancy. J. Am. Coll. Cardiol. 75 (18), 2323–2334 (2020). PMID: 32381164; PMCID: PMC7213062.
Turbeville, H. R. & Sasser, J. M. Preeclampsia beyond pregnancy: long-term consequences for mother and child. Am. J. Physiol. Ren. Physiol. 318 (6), F1315–F1326. https://doi.org/10.1152/ajprenal.00071.2020 (2020). Epub 2020 Apr 6. PMID: 32249616; PMCID: PMC7311709.
US Preventive Services Task Force et al. Screening for Hypertensive Disorders of Pregnancy: US Preventive Services Task Force Final Recommendation Statement. JAMA. ;330(11):1074–1082. (2023). https://doi.org/10.1001/jama.2023.16991. PMID: 37721605.
Egeland, G. M. et al. Preconception cardiovascular risk factor differences between gestational hypertension and preeclampsia: cohort Norway study. Hypertension 67 (6), 1173–1180. https://doi.org/10.1161/HYPERTENSIONAHA.116.07099 (2016). Epub 2016 Apr 25. PMID: 27113053; PMCID: PMC4861703.
Retnakaran, R. & Shah, B. R. The adverse cardiovascular risk factor profile of women with pre-eclampsia develops over time in the years before pregnancy. BJOG 129 (9), 1512–1520. https://doi.org/10.1111/1471-0528.17084 (2022). Epub 2022 Jan 13. PMID: 34954865.
Wiznitzer, A. et al. Association of lipid levels during gestation with preeclampsia and gestational diabetes mellitus: a population-based study. Am J Obstet Gynecol. ;201(5):482.e1-8. (2009). https://doi.org/10.1016/j.ajog.2009.05.032. Epub 2009 Jul 24. PMID: 19631920; PMCID: PMC5483324.
Vrijkotte, T. G. et al. Maternal lipid profile during early pregnancy and pregnancy complications and outcomes: the ABCD study. J. Clin. Endocrinol. Metab. 97 (11), 3917–3925. https://doi.org/10.1210/jc.2012-1295 (2012). Epub 2012 Aug 29. PMID: 22933545.
Jin, W. Y. et al. Associations between maternal lipid profile and pregnancy complications and perinatal outcomes: a population-based study from China. BMC Pregnancy Childbirth. 16, 60. https://doi.org/10.1186/s12884-016-0852-9 (2016). PMID: 27000102; PMCID: PMC4802610.
Serrano, N. C. et al. Lipid profile, plasma apolipoproteins, and pre-eclampsia risk in the GenPE case-control study. Atherosclerosis. ;276:189–194. doi: 10.1016/j.atherosclerosis.2018.05.051. Epub 2018 Jun 4. PMID: 29914672. (2018).
Poornima, I. G., Indaram, M., Ross, J. D., Agarwala, A. & Wild, R. A. Hyperlipidemia and risk for preclampsia. J. Clin. Lipidol. 2022 May-Jun ;16(3):253–260. https://doi.org/10.1016/j.jacl.2022.02.005. Epub 2022 Feb 20. PMID: 35260347; PMCID: PMC10320742.
Vander Borght, M. & Wyns, C. Fertility and infertility: Definition and epidemiology. Clin Biochem. ;62:2–10. (2018). https://doi.org/10.1016/j.clinbiochem.2018.03.012. Epub 2018 Mar 16. PMID: 29555319.
Zhou, Z. et al. Epidemiology of infertility in China: a population-based study. BJOG 125 (4), 432–441. https://doi.org/10.1111/1471-0528.14966 (2018). Epub 2017 Dec 28. PMID: 29030908.
Inhorn, M. C. & Patrizio, P. Infertility around the Globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum. Reprod. Update. 21 (4), 411–426. https://doi.org/10.1093/humupd/dmv016 (2015 Jul-Aug). Epub 2015 Mar 22. PMID: 25801630.
De Geyter, C. et al. European IVF-monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). ART in Europe, 2015: results generated from European registries by ESHRE. Hum Reprod Open. ;2020(1):hoz038. (2020). https://doi.org/10.1093/hropen/hoz038. Erratum in: Hum Reprod Open. 2020;2020(3):hoaa038. PMID: 32123753; PMCID: PMC7038942.
Wu, P. et al. In-Hospital complications in pregnancies conceived by assisted reproductive technology. J. Am. Heart Assoc. 11 (5), e022658. https://doi.org/10.1161/JAHA.121.022658 (2022). Epub 2022 Feb 22. PMID: 35191320; PMCID: PMC9075081.
Heshmatnia, F. et al. Is there a relationship between assisted reproductive technology and maternal outcomes? A systematic review of cohort studies. Int. J. Reprod. Biomed. 21 (11), 861–880. https://doi.org/10.18502/ijrm.v21i11.14651 (2023). PMID: 38292514; PMCID: PMC10823119.
Cirillo, M., Coccia, M. E., Dimmito, A. & Fatini, C. Preconception period in women and men undergoing assisted reproduction: A gender approach for reproductive health. Eur. J. Obstet. Gynecol. Reprod. Biol. 275, 1–8. https://doi.org/10.1016/j.ejogrb.2022.06.003 (2022). Epub 2022 Jun 8. PMID: 35691220.
Cirillo, M., Coccia, M. E. & Fatini, C. Lifestyle and comorbidities: do we take enough care of preconception health in assisted reproduction?? J. Family Reprod. Health. 14 (3), 150–157. https://doi.org/10.18502/jfrh.v14i3.4667 (2020). PMID: 33603806; PMCID: PMC7868650.
Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet Gynecol. ;135(6):e237-e260. (2020). https://doi.org/10.1097/AOG.0000000000003891. PMID: 32443079.
Spracklen, C. N., Smith, C. J., Saftlas, A. F., Robinson, J. G. & Ryckman, K. K. Maternal hyperlipidemia and the risk of preeclampsia: a meta-analysis. Am. J. Epidemiol. 180 (4), 346–358. https://doi.org/10.1093/aje/kwu145 (2014). Epub 2014 Jul 2. PMID: 24989239; PMCID: PMC4565654.
Baumfeld, Y. et al. Pre-Conception dyslipidemia is associated with development of preeclampsia and gestational diabetes mellitus. PLoS One. 10 (10), e0139164. https://doi.org/10.1371/journal.pone.0139164 (2015). Erratum in: PLoS One. 2015;10(11):e0142462. PMID: 26452270; PMCID: PMC4599807.
Raghuraman, N. & Tuuli, M. G. Preconception Care as an Opportunity to Optimize Pregnancy Outcomes. JAMA. ;326(1):79–80. (2021). https://doi.org/10.1001/jama.2020.27244. PMID: 34228078.
Saito, K. et al. Endometrial preparation methods for frozen-thawed embryo transfer are associated with altered risks of hypertensive disorders of pregnancy, placenta accreta, and gestational diabetes mellitus. Hum Reprod. ;34(8):1567–1575. (2019). https://doi.org/10.1093/humrep/dez079. PMID: 31299081.
Niu, Y. et al. Is artificial endometrial Preparation more associated with early-onset or late-onset preeclampsia after frozen embryo transfer? J. Assist. Reprod. Genet. 40 (5), 1045–1054. https://doi.org/10.1007/s10815-023-02785-0 (2023). Epub 2023 Mar 31. PMID: 37000343; PMCID: PMC10239427. 16117184.
Konrad, E. et al. Correlation of elevated levels of, high-density lipoprotein and low-density lipoprotein with severity of preeclampsia: a prospective longitudinal study. J. Obstet. Gynaecol. 40 (1), 53–58 (2020). Epub 2019 Jul 13. PMID: 31304822.
Author information
Authors and Affiliations
Contributions
Shaomin Chen and Yang Wang collected the data and wrote the manuscript; Liyuan Tao and Zhaoyu Wang were responsible for statistical analysis. Yongqing Wang and Yuan Wei helped perform the analysis with constructive discussions; Zhaoping Li contributed significantly to analysis and manuscript preparation; Rong Li designed the study. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, S., Wang, Y., Wang, Z. et al. Pre conception dyslipidemia and risk for preeclampsia in women undergoing IVF ET. Sci Rep 15, 18454 (2025). https://doi.org/10.1038/s41598-025-03513-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-03513-7
Keywords
This article is cited by
-
Metabolic dysfunction-associated steatotic liver disease and adverse pregnancy outcomes: a nationwide cohort study
Hepatology International (2025)