Abstract
The increasing antimicrobial resistance and recurrence is a major public health concern leading to high rates of morbidity and mortality worldwide. Furthermore, free radicals induced damage led to oxidative diseases such as cancer and diabetes.Therefore, finding safe and effective antimicrobial and antioxidant agents is vital. Pomegranate peel, constituting 60% of the total fruit’s weight, is largely disposed despite its known benefits in traditional medicine. The current study aimed to determine the chemical constituents and biological activities of the methanolic extracts of peels of three pomegranate varieties [wild (PPE-1), white (PPE-2), and red (PPE-3)]. Phytochemicals analysis were performed using UV-vis spectrophotometry, gas chromatography-mass spectroscopy (GC-MS), and high-performance liquid chromatography (HPLC). The antioxidant potentials were determined via 2,2-diphenyl-1-picrylhydrazyl scavenging and phosphomolybdate (ABTS) assay and In Vitro antidiabetic activity was assessed by α-amylase inhibition assay. The antibacterial activity was assesed against three most dominant pathogens including E. coli, Staphylococcus aureus and Staphylococcus epidermidis by disc diffusion. The total phenolics, flavonoid, and tannin contents were highest in PPE-1 followed by PPE-2 and PPE-3. Among the polyphenol, chlorogenic acid was found in substantial concentration in all extracts. The GC-MS analysis identified 13 distinct compounds with the notable presence of 5-(hydroxymethyl) furan-2-carbaldehyde. All varieties exhibited significant antioxidants, antibacterial and antidiabetics, but the PPE-1 showed the highest activities. Docking studies revealed that chlorogenic acid was a strong inhibitor of Cyclooxygenase–II (COX-II) indicating anti-inflammatory and anticancerous potential. The study revealed that pomegranate peels have promising biological potentials such as antioxidants, antidiabetic, and antimicrobial, suggesting their role in drug developments.
Similar content being viewed by others
Introduction
Infectious diseases are worldwide a major cause of morbidity and mortality1. The problem is compounded by the growing drug resistance exhibited by several human pathogens leading to disability and fatality worldwide2. The infections caused by most dominant bacterial strains such as Escherichia coli, Salmonella spp. and Staphylococcus aureus are widespread. In addition, the frequent use of antibiotics to treat infections is associated with many side effects, including hypersensitivity, immunosuppressant and allergic manifestations3,4. Further, noxious impact of free radicals on human health leading to devastating diseases including cancer, diabetes and cardiovascular is also a major concern of health practitioner. Cancer ranked second in the cause of mortality worldwide which occurs due to disturbance in cellular mechanisms mostly caused by free radicals. The problem is worsened by the severe side effects of synthetic drug therapies on human health. Therefore, there is a need to identify alternative drugs therapies from natural sources for the treatment of infectious and oxidative diseases with no or less side effects. Plant materials have been reported as a major source of natural antimicrobial, anticancerous and antioxidant agents. According to WHO, around 80% of the world’s population is dependent on traditional medicine and a major part of the traditional therapies involves the use of plant extracts or their active constituents5. Hence, the current study was conducted to explore the biological evaluations of different varieties of pomegranate peels extracts in terms of antimicrobial, antioxidant, antidiabetic and anticancerous agents which can help in the identification of best source of natural drugs.
Punica granatum (Pomegranate) is one of the oldest fruits in tropical and subtropical regions and has been distributed worldwide6. The fruits of Pomegranate have been extensively consumed due to their good flavor and nutritive values contributed by their biochemical substances such as vitamins, minerals, phenolics, flavonoids etc. It has been reported in a comprehensive review that the production of pomegranate is 8.1 million tons, 835,950 ha on planted area and expected to increase with time7,8. In 2022, the global total import of pomegranate was raised to $450 million where United State, China and Germany were at the top with an import value of 21.87%, 20.47%, and 6.86%, respectively. The market is expected to grow at a 5.3% annual rate from 2023 to 20309. Recently, biochemical diversity of wild and cultivated genotypes of pomegranate were reported10. In Pakistan, the best variety is red pomegranate (locally called Qandhari anar), whereas white and wild pomegranates are grown in different areas which are rarely consumed11 due to sour taste and small size. The fruit of pomegranate is widely used in different products while the peel is discarded12. The peel of pomegranate constitutes approximately 60% of the total weight of the fruit13. Therefore, a large amount of the fruit is not utilized despite having numerous medicinal benefits, highlighting the need to explore the chemical composition of the peel and their medicinal importance.
Previously, many studies reported the biological potential of pomegranate14,15,16,17, but the focus was on the flesh and seeds of pomegranate except few studies also reported the biological significance of peel. Moreover, in Pakistan the focus of mostly researcher was on biological potentials of red pomegranate but the limited study reported white and wild pomegranate. Thus, the current study was focused on assessing the phytochemical constituents and biological properties of peels of different varieties of pomegranate. The current study aimed to evaluate the phytochemical constituents and In Vitro antioxidants, antimicrobial, antidiabetic potentials of peels extracts of three pomegranate varieties. Furthermore, In Silco anticancerous and anti-inflammatory potentials of compounds identified from peels extracts were also performed against Cycolooxegenase-II (Cox-II) .
Materials and methods
Chemicals
All the chemicals used in this study were purchased from commercial sources. Acetic acid, acetonitrile, and sodium nitrite were purchased from Daejung (Siheung City, South Korea). Ammonium molybdate and aluminum chloride were purchased from BDH (Cambridge, UK). Folin-Ciocalteu reagent, dipotassium hydrogen phosphate, monosodium dihydrogen phosphate, rutin, and catechin were obtained from Merck (Darmstadt, Germany). Chlorogenic acid, gallic acid, , and cinnamic acids were purchased from Riedel de Haen (Seelze, Germany). Protocatechuic acid, 2,2-diphenyl-1-picrylhydrazyl (DPPH), ascorbic acid, benzoic acid, and hydroxytyrosol were purchased from Sigma-Aldrich (Steinheim, Germany).
Collection of pomegranate sample
Fresh fruits of three different varieties of pomegranate were collected from the Mardan District, KP, Pakistan from the same place and same time to avoid any environmental variations. The following notations were used for different varieties: PPE-1 for wild-type, PPE-2 for red, and PPE-3 for white. The plant samples were identified by Botanist at Department of Botany, Hazara University, Mansehra, Pakistan. The fruit peels were separated, rinsed with distilled water, and then dried in the shade at 15–20 0C. The dried peels were pulverized into fine powder and sieved through a 300 mm mesh size and stored in airtight bags.
Preparation of extracts
The samples were extracted in methanol through maceration. 8% of the plant mixture was prepared in 60% methanol (20 g of peel powder in 250 mL of methanol), and the mixture was kept on shaking for 24 h in an orbital shaker (250 rpm). The extracts were filtered and the solvent from the filtrates was evaporated using a rotary evaporator (R-210, Buchi, Switzerland) for 40 min under optimized conditions, with a rotation speed of 120 rpm, a water bath temperature was maintained at 40 °C, and a vacuum pressure was set at 337 mbar. The concentrates were placed separately in a desiccator at room temperature to remove residual solvents until it is dried. The samples were extracted and analyzed in triplicates (n = 3).
Estimation of total phenolic contents (TPC)
The Folin-Ciocalteu (FC) reagent was used to estimate the total phenolic contents in all extracts18. The reaction mixture contained 0.2 mL sample, 0.5 mL FC reagent, 4 mL sodium carbonate (1 M), and distilled water was added to final volume of 10 mL. The sample was incubated for 30 min at room temp and the absorbance was measured at 765 nm using a spectrophotometer (UVD-3200, Labomed Inc., USA Tech). The TPC was quantified in milligrams of gallic acid equivalents per gram of dried extract (mg GAE/g).
Estimation of total flavonoid contents (TFC)
The aluminum chloride method was employed to check the total flavonoid contents19. The mixture contained 300 µL sample, 150 µL AlCl3 (0.3 M), 150 µL NaNO2 (0.5 M) and 1.4 mL 30% aqueous methanol. Then, 1 mL sodium hydroxide (1 M) was added and absorbance was recorded at 506 nm using a spectrophotometer (UVD-3200, Labomed Inc., USA Tech). The TFC was quantified as milligrams of rutin equivalent per gram of dried extract (mg RE/g).
Estimation of total condensed tannin contents (TCTC)
The total condensed tannin contents of pomegranate peels were determined using vanillin-sulfuric acid assay20. For the reaction mixture 1 mL of pomegranate peel sample was mixed with 2.5 mL of 1% vanillin solution and 2.5 mL of 25% sulfuric acid to facilitate the reaction between vanillin and polyphenols in the sample extracts. The mixture was incubated for 15 min and absorbance was measured at 500 nm. The results were calculated using standard calibration curve of vanillin with R² = 0.976 and expressed in milligram equivalent of vanillin (mg VE/g) dried extracts of pomegranate peels.
Identification of polyphenols by HPLC
Eight previously reported phenolic and flavonoids standards include quercetin, benzoic acid, cinnamic acid, gallic acid, e-hydroxy tyrosol, protocatechuic acid, catechin and chlorogenic acid were prepared to identify the compounds in the samples. The sample and standard were filtered through 0.45 μm Millipore HPLC filter and then 5 µl sample was injected. Chromatographic separation was conducted at 35 oC on a reverse-phase column (Agilent Eclipse plus C18, 3.5 μm, 2.1 × 150 mm). The mobile phase was used in concentration gradients such as 100% A (5% acetonitrile: 94.9% water: 0.5% acetic acid) to 100% B (4.9% water: 95% acetonitrile: 0.5% acetic acid) for over 25 min for polyphenols. The gradient was used as follows: 0 min-0% B; 1 min-0% B; 5 min-20% B; 20 min-100% B following the 5 min post run-time, the flow rate was 0.25 ml/min21.
Identification of compounds by GC-MS
The volatile chemical constituents of the pomegranate peel extracts were identified using GC-MS analysis (Agilent Technologies, US). The initial instrument temperature was held at 60 °C for 2 min, then ramped at a rate of 4 °C/min to the final detector temperature of 240 °C. The sample (1 µL) was injected; helium was used as carrier gas at flow rate of 1 mL/min; the mass spectrometer was operated at 70 eV22. The presence of compounds in extracts were identified by comparison with the NIST 05 spectral library (Gaithersburg, MD, US).
Determination of total antioxidant capacity
The total antioxidant capacity (TAC) was determined as previously described phosphomolybdate assay23. The phosphomolybdate reagent was prepared by mixing equal volumes of 4 mM ammonium molybdate (NH4)6Mo7O24), 0.6 M H2SO4, and 28 mM sodium phosphate (Na3PO4). In test tube 300 µL of the sample or standard was mixed with 3 mL phosphomolybdate reagent then, covered with aluminum foil and incubated in water bath for 90 min at 95 oC. After cooling the mixture at room temperature, the absorbance was measured at 765 nm using a spectrophotometer (UVD-3200, Labomed Inc., USA Tech). The methanol was used as a blank and total antioxidant capacity of the sample was quantified as micrograms ascorbic acid equivalent/g (µg/g of AAE).
Determination of free radical scavenging activity
The free radical scavenging activity of peel extracts was determined using the DPPH assay24. 0.1 mM DPPH solution was mixed with sample in equimolar concentration and incubated for 30 min at 37 °C. then absorbance was recorded at 517 nm. Asocorbic acid was used as a positive control while DPPH as a negative control. The formula below was used to calculate % antioxidant potential.
Where, As is the sample absorbance and Ac is the absorbance of the blank.
Determination of α-amylase inhibition
3,5-dinitrosalicylic acid method was used to check the inhibitory effects of peel extracts on α- amylase25. First the extract was prepared in 10% DMSO solution and then subsequently diluted in a buffer (0.02 M of Na2HPO4 and 0.006 M NaCl at pH 6.9) to achieve final concentrations ranging from 10 to 1000 µg/mL. 200 µl of peel extract was treated with 200 µL of α-amylase (2 units/mL) and allowed for 10 min incubation at 30 °C. Then, 200 µL of 1% starch solution (w/v in water) was added to each tube, and the tubes were incubated for 30 min to allow the reaction to occur. The reaction was terminated by adding 200 µL of DNS reagent which was prepared by mixing 12 g of sodium potassium tartrate tetrahydrate in 8 mL of 2 M NaOH and 20 mL of 96 mM 3,5-dinitrosalicylic acid. The mixture was then heated in a water bath at 85 °C to 90 °C for 10 min and then cooled at room temperature. The mixture was further diluted with 5 mL of distilled water and UV-Vis spectrophotometer was employed to record the absorbance at 540 nm. The blank solution was prepared by using 200 µL of buffer instead of plant extract. Acarbose was used as a positive control, using the same procedure. The inhibitory activity was calculated as percentage of inhibition using the formula provided below. The % inhibition of α-amylase was then plotted against the extract concentrations, and the IC50 values were determined from the plotted graph. % α-amylase inhibition = ((Abs of control-Abs of sample)/(Abs of control)) x 100
Determination of antibacterial activity
The antibacterial activity of each peel extract was investigated against three Gram positive and Gram-negative bacterial strain including E. coli, Staphylococcus aureus and Staphylococcus epidermidis using the agar well diffusion method26. Bacterial cultures were swabbed onto agar nutrient media petri dishes and incubated for 24 h at 37 oC. 6 mm wells were punched through sterile cork borer in the media plates inoculated with selected bacterial strains and then 50 µL of each extract was loaded into each well. The petri plates were incubated for 24 h at 37 ℃ to allow the sample to diffuse in the media and kill bacterial strains. After incubation the zone of inhibition was measured around the well in mm. Antibiotic drugs such as ciprofloxacin and erythromycin were used as positive controls and methanol (extract solvent) was used as a negative control.
Docking study: Retrieval and preparation of target protein
The COX-II protein structure was obtained from the Protein Data Bank27. The Modeler software was used to add missing residues28. Prior to docking, the structure was prepared using the Molecular Operating Environment (MOE)29. All the heteroatoms along with molecules of water that have no specific roles within the structure were deleted. The compounds were retrieved in SDF format and converted to 3D format using Avogadro Software30 and were optimized. Partial charges were added using the protonate 3D and MMFF94X force-fields, and the molecules were added to the MOE ligand database for docking31. A library was created using compounds obtained from PubChem32.
Molecular Docking and analysis of ligand‒receptor interaction
The previously described structure of the protein helped in the identification of the protein pocket residues, which were additionally confirmed using site finder tools in molecular operating environment (MOE) software. Docking of the selected compounds against the targeted protein was performed using same software. The triangular algorithm was used to investigate the finest postures and the London DG function offered additional comprehensive information related to the re-scored simulated poses. Based on the energy score, hydrogen bonding and other hydrophobic interactions were selected for docking analysis.
Discovery Studio33 was used to analyze the interaction of the docked complexes and develop 2D plots of the receptor‒ligand interactions, with an emphasis on hydrogen bonds, interactions of electrostatic and non-electrostatic, and other interactions. These interactions demonstrated the attachment of compounds on the active protein site. Chimera and Discovery Studio were used to display the 3D structures34,35.
Statistical analysis
The three biological replicates (n = 3) were used to calculate the mean and standard deviation (mean ± SD). The IBM SPSS software (SPSS v.21; IBM, Armonk, New York, NY, USA) was used to perform statistical analysis. The one way analysis of variance (ANOVA) and Post hoc Tukey test was performed to compare the variations. The probability values were used at p < 0.05.
Results
Total phenolic, flavonoid, and tannin contents
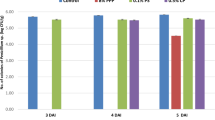
In the current study analysis of total phenolic content (TPC), flavonoid content (TFC), and total condensed tannin content (TCTC) in the methanolic extracts of peels from the three different pomegranate varieties revealed interesting findings. The wild pomegranate variety (PPE-1) exhibited the highest TPC (710.23 mg GAE/g), indicating a rich concentration of phenolic compounds, followed by PPE-3 (639.77 mg GAE/g) and PPE-2 (570.88 mg GAE/g), as illustrated in Fig. 1(a). Similarly, the trend observed for the TFC mirrored that of the phenolic content, with the highest levels in PPE-1 (59.88 mg RE/g DW), followed by PPE-3 and a lowest content in PPE-2 (Fig. 1(b). A similar pattern of total condensed tannin contents was seen in all varieties (PPE1(50.47 VE/g) > PPE3 (45.23 VE/g) > PPE2 (42.43 VE/E) depicted in Fig. 1(c).
Phytochemicals identified by HPLC
Seventeen distinct chemical compounds peaks were revealed in the peel methanolic extracts of different pomegranate varieties. The presence and percentage composition of various phenolic compounds in the methanolic extracts of the different varieties (PPE-1, PPE-2, and PPE-3) are displayed in Tables S1, S2, and S3 in the Supplementary Materials, respectively. Ten compounds were common to all three methanolic peel varieties. Among them, eight compounds were matched with the standard compounds and identified based on the retention time (RT), listed in Table 1. Chlorogenic acid was the most abundant and common compound among all the pomegranate varieties. Among the varieties PPE-1 and PPE-2 contained high concentrations of cinnamic acid and benzoic acid, whereas PPE-3 contained high concentrations of benzoic acid and gallic acid. Quercetin was found in very low quantities in PPE-2 but was comparatively high in PPE-1 and PPE-3. Interestingly, two unknown peaks were detected in all three varieties in a substantial amount that could attributed to the abundant pomegranate compound such as punicalagins and punicalins. Seven other compounds were present in one variety but absent in the other (Table 1).
Phytochemicals identified by GC-MS
Volatile bioactive compounds present in the methanolic extracts of the peels of three varieties of pomegranate (PPE-1, PPE-2, and PPE-3) were identified through GC-MS and the results are shown in Table 2. Thus far, several compounds have been identified. 2-Furancarboxaldehyde-5-(hydroxymethyl) (syn. 5-(hydroxymethyl) furfural) was the major component present in all three varieties. 3,5-dihydroxy-2,3-dihydro-4H-pyran-4-one, oleic acid, and vitamin E were present in all samples. Furthermore, some of the compounds were found in one variety but were absent in another; however, their abundance may be low and could be beyond the limit of detection. The structures of other identified compounds are shown in Figure S1 in the Supplementary Materials. Among the identified compounds, all three pomegranate varieties commonly showed the presence of several other bioactive volatile compounds such as 1-hexadecanol-2-methyl, 1-tetradecene, pentadecanoic acid-14-methyl-methyl ester, oleic acid, and hexadecanoic acid. The white variety uniquely contained 1-hexadecene, 1-hexadecanol-2-methyl, 2-methyl-3-tetradecene-1-ol acetate, and estra-1,3,5(10)-trien-17-beta-ol. The red variety was characterized by the presence of 1,6-octadien-3-ol-3,7-dimethy and methyl 6-oxoheptanoate. Finally, the wild variety contained unique compounds, including 1-dodecanol-3,7,11-trimethyl, 2,2,4-trimethyl-3-cyclohexanol, and propanoic acid-2(3-acetoxy-4,4,14-trimethylandrost-8-en-17-yl).
DPPH free-radical scavenging activity
The antioxidant potential of the methanolic extracts of the three distinct pomegranate varieties was investigated by assessing the free-radical scavenging capacity using the DPPH assay. Remarkably, the extracts of all pomegranate varieties (PPE-1, PPE-2, and PPE-3) demonstrated significant scavenging activities (Fig. 2(b)). Furthermore, the calculated IC50 values (Fig. 2(a)) indicated that the peel extracts were more potent in antioxidant activity than standard ascorbic acid. These findings suggest that the methanolic extracts of pomegranate possess robust antioxidant properties, which can be endorsed to the existence of bioactive compounds with free-radical scavenging capabilities. The superior antioxidant activity of all three pomegranate peel extracts highlights the promising role of the plants as natural sources of antioxidants that can be used to achieve health-promoting benefits.
Total antioxidant capacity (TAC)
The total antioxidant capacity (TAC) of the methanolic extracts of the peels of the various pomegranates was determined via colorimetric assay using phosphomolybdate assay (Fig. 3). Interestingly, PPE-1 exhibited the highest antioxidant activity among the pomegranate peel extracts, followed by PPE-3 and PPE-2, indicating differences in the antioxidant potential across the pomegranate varieties. PPE1 has been found to be a key natural antioxidants source with promising applications in food, pharmaceutical, and cosmetic products.
Inhibition of α-amylase
The inhibitory effects of pomegranate peel extracts of all selected varieties on α-amylase were evaluated and compared with standard of acarbose, and the % inhibitions and IC50 values are shown in Fig. 4. It has been observed that PPE-1 exhibited the lowest IC50 value of 16.66 while PPE-2 and PPE-3 revealed 20.11 and 25.37 respectively. PPE-1 revealed almost the same inhibition as positive control of acarbose while the PPE-2 and PPE-3 showed lower inhibition of α-amylase compared to standard drug.
Antibacterial activity
The antibacterial potential of the methanolic extracts of the peels of the three distinct pomegranate varieties was investigated, as presented in Fig. 5. Among the three varieties the wild pomegranate exhibited the most potent antibacterial activity against all the three tested microbial strains, demonstrating its remarkable efficacy in inhibiting bacterial growth. Interestingly, the antibacterial activity of all pomegranate varieties against Staphylococcus epidermidis was significantly higher than standard antibiotic erythromycin. This finding suggests that the methanolic extracts of pomegranate peel possess superior antibacterial properties compared to the commonly used erythromycin when targeting S. epidermidis. The enhanced antibacterial activity of the wild pomegranate variety against all three microbial strains highlights the potential of this variety as a natural and effective alternative to synthetic antibiotics. These results underscore the importance of exploring the antibacterial potential of pomegranate peels, particularly the PPE-1 variety, which could be used in the isolation and identification of novel antimicrobial agents withhigh efficiency and lower side effects.
Docking studies of COX-II-chlorogenic acid and Furan complex
The selected structure of human COX-II consisted of two chains. Each chain is shown in a different color. The structure was retrieved from the protein databank using PDB ID 51 KT, as shown in Figure S2 in the Supplementary Materials36. The two utmost abundant compounds in the peels varieties of pomegranate were selected for the docking studies. The structures of the selected compounds (furan-2-carbaldehyde and chlorogenic acid) are shown in Fig. S3(a) and S3(b) in Supplementary Materials.
The chlorogenic acid ligand interacted with 16 residues of the COX-II active site with an energy score of ‒12.2639 kcal/mol. The Arg120 residue formed hydrogen bonds with the carbonyl group of chlorogenic acid, with a bond length of 2.00 Å and underwent an attractive interaction (electrostatic interaction), with a bond length of 3.04 Å. The ligand also interacted with nine residues (His90, Leu93, Tyr355, Tyr115, Ser119, Lys83, Leu531, Ser353, and Leu359) via van der Waals interactions, and formed alkyl π-alkyl interactions with three residues (Ala527, Val359, and Val89). Furthermore, the ligand underwent an amid π-stacking interaction with Leu352 and formed a C-H bond with Val116. In the 3D interaction diagram, the orange color represents the ligand (chlorogenic acid), cyan represents the Arg120 residue of COX-II, the green dotted line denotes hydrogen bonds, and the orange dotted line revealed an attractive charge, as shown in Fig. 6(a). In the 3D interaction diagram in Fig. 6(b), the Arg120 residue and all other active-site residues are labeled. The orange color indicates the chlorogenic acid ligand, and the black line indicates the hydrogen bond with Arg120. The 2D interaction diagram is displayed in Supplementary Fig. S4(a).
The furan ligand interacted with 7 residues of the COX-II active site, with an energy score of − 7.26993 kcal/mol. Arg120 formed a hydrogen bond and π‒cation interaction with a bond distance of 1.25 and 4.06 Å, respectively. Furthermore, furan interacted with four residues (Leu93, Val89, Tyr355, and Leu531) by van der Waals interactions, and with Val116 and Ala527 by π‒alkyl interactions. In the 3D interaction diagram in Fig. 7(a), orange represents the ligand (furan), cyan represents the Arg120 residue of COX-II, dotted green lines indicate the hydrogen bonding, and the π‒cation interaction is represented by orange dotted line. In the 3D interaction diagram in Fig. 7(b), the Arg120 residue and all other active-site residues are labeled. The orange color indicates the ligand (furan) and the black line indicates a hydrogen bond with Arg120. The 2D interaction diagram is shown in Supplementary Material Fig. S4(b).
Discussion
Recently, there has been a tremendous demand in the food industry for additives such as antioxidants and preservatives from natural rather than conventional sources to avoid toxicity. The ability of these natural compounds to boost the immune system and provide resistance toward pathogens in humans is an established fact. This trend has been magnified by the coronavirus (COVID-19) pandemic and is anticipated to remain high in the post-pandemic era37,38. Phenolic and flavonoids have garnered significant attention and are actively utilized in various industries, including the food, pharmaceutical, health, and cosmetics sectors due to their diverse potential. Analysis of phenolic and flavonoids in plants and identifying the rich sources could be significant addition in medical sciences. Therefore, the purpose of this present investigation was to evaluate the antioxidant and antibacterial potential of peels of different varieties of pomegranate to explore its beneficial effects on humans. To valorize pomegranate peels, it is important to scientifically investigate this part of the pomegranate, which is generally discarded as a waste. A large amount of pomegranate fruit is cultivated and consumed worldwide, producing millions of tons of peels. Several varieties of pomegranate have been identified in Pakistan but only the red pomegranate locally known as qndhari anar is used while white and wild (locally known as Darunna) varieties are completely ignored. Therefore, the current study focused on evaluation of chemical constituents and biological activities of wild (PPE-1) and white pomegranate peels (PPE-2) and compared their outcome with red pomegranate peels (PPE-3). Interestingly, wild variety was found to be the best variety in terms of their phytochemical constituents and biological potential as it showed highest TPC, TFC and TCTC followed by the white and red variety. The high concentration of phenolics and flavonoids in pomegranate extracts has been validated through numerous published studies on different varieties of pomegranate39,40,41,42. Sweidan et al. reported a relatively low TPC contents in Jordanian pomegranate ethanolic peels, possibly due to environmental variations and soil conditions43. Fourati and colleagues also reported almost the same total flavonoid content in the ethyl acetate extract of pomegranate peel, whereas they found a relatively higher concentration in the methanol, butanol, and water extracts44. Similar findings were reported by a few studies where TCTC were found a difference in different varieties of pomegranate peels45,46. The presence of these compounds in plant extracts revealed their medicinal potential because these exhibited various bioactivities, including antioxidants, antimicrobial, and anticancer activity37,47 suggesting that methanolic extracts of pomegranate peels potentially have significant health benefits. These results highlight the importance of the wild pomegranate variety and underscore its potential as a valuable source of bioactive compounds with health-promoting effects.
Furthermore, all the varieties of pomegranate peels also showed substantial biological activities including antioxidant antimicrobial and antidiabetic and anti-inflammatory. Among the peel extracts PPE 1 possessed significantly high biological potential compared to PPE 2 and PPE 3. The high antioxidant and antidiabetic potential of peels, especially PPE 1, showed its potential application in medicine and food. In medicines it could be used to inhibit the degenerative effects of free radicals and reactive oxygen species (ROS) and counteract aging and oxidative damages24,48. Malviya et al. evaluated the antioxidant potential of pomegranate peel by utilizing different solvent systems and reported the significance of the peel extract49. Similarly, Mansic and colleagues also reported total antioxidant potentials of pomegranate peel extracts50. Furthermore, PPE1 has been found stronger inhibitor of α-amylase than standard drug acarbose making it a promising candidate for the management of diabetes. The study aligned to previous study where methanolic peel extract of Indian pomegranate showed significant α-amylase inhibitory potential51.
The antioxidant potential of pomegranate peels aligned with TPC and TFC contents which has been reported for their strong antioxidant potential52,53 indicated that these phytochemicals may contribute at least partly to antioxidants. Moreover, HPLC analysis also revealed important polyphenols including chlorogenic acid, benzoic acid, cinnamic acid and gallic acid which have been reported for their antioxidant potential54. Two unknown peaks not validated against the standards may be assumed the presence of most abundant and important phytochemical of pomegranate punicalagins and punicalins in all peel extracts. A study reported polyphenol in pomegranates peels from different cultivars of China showed punicalagins and punicalins were most abundant polyphenols55.
There were only a few studies that reported the volatiles of pomegranate peels. A study reported 2 H-Pyran-2-one, hexadecanoic acid, ethyl ester were most abundant compounds in the peel extracts of Iraqian pomegranate56. Similarly, another study reported propanoic acid, benzenedicarboxylic acid, methoxypropionic acid and methyl amine were the major constituents in two varieties of Saudi pomegranates57. The variations in the biochemical constituents may be due to different genotypes of pomegranate and environmental and climatic differences.
The peel samples displayed remarkable antimicrobial potential against E. coli, S. aureus, and S. epidermidis. The peel extracts were particularly potent against S. epidermidis, showing higher activity than the standard erythromycin and ciproflaxacin. The study revealed the potential of pomegranate extracts contained potential phytochemical which may contribute to antibacterial activity against Gram positive (S. epidermidis) and Gram negative (E. coli) bacterial strains. Previously, a study on pomegranate peels of Ganesh variety were also analyzed against Staphylococcus aureus, Enterobacter aerogenes, Salmonella typhi and Klebsiella pneumoniae and which showed remarkable antibacterial potential against all the selected bacterial strains48. Further, pomegranate peel of Jordanian origin was tested against gentamicin-resistant Pseudomonas aeruginosa demonstrated promising antibacterial potential. The studies evidenced that peel extract showed broad spectrum potential against range of bacterial strains and could be used to treat many infectious diseases where other antibiotics are not effective.
GC-MS analysis of the peel extracts of the pomegranate varieties identified many compounds. Some of the compounds were commonly found in all varieties while some were unique to only one variety. 2-Furancarboxaldehyde-5-(hydroxymethyl) was detected in the highest concentration in all extracts (63.6‒82.9%). 5-hydroxymethylfurfural is an important antiviral agent by upregulation of the retinoic acid-inducible gene I (RIG-I) and mediated type I interferon (IFN) as a response to viral infection58. Furthermore, 5-hydroxymethylfurfural has been used as quality control in food59. Further, the compound has been reported for its anti-inflammatory and protective efficacy in lipopolysaccharide -induced acute lung injury, and by its action against NLRP3 inflammasome-related inflammatory disorders60. The reported literature on 5-hydroxymethylfurfural suggested the medicinal importance of pomegranate peels.
The docking study showed that chlorogenic acid is a strong inhibitor of COX-II. Most of the identified compounds by HPLC including chlorogenic acid possessed antioxidant and anticancerous potential61. Their presence in pomegranate peel supports the use of peel extracts as antioxidants and free-radical scavenging agents. In the current study In Silco docking study of chlorogenic acid against COX-II revealed its anti-inflammatory as well as anticancerous potentials. Previously, many studies evidenced the medicinal importance of chlorogenic acid which supports the biological importance of peel extracts, most specifically the wild variety.
Conclusions
This study presented a comparison of the biochemical constituents and biological activities of the methanolic extracts of pomegranate peel. Substantial amounts of phenolics, flavonoids, and condensed tannin content were present in all varieties, with PPE-1 exhibiting the highest content. Chlorogenic acid and furan-2-carabldehyde were the major compounds identified in all pomegranate varieties. Antioxidant and antimicrobial potentials were notable in all pomegranate varieties, with PPE-1 displaying the most significant activity against the tested bacterial strains, surpassing that of the standard erythromycin against Staphylococcus epidermidis. This confirms the hypothesis that the chemical profile of the methanolic extracts of pomegranate peel from different varieties differ, affecting the antioxidant and antimicrobial potentials. These findings suggest that pomegranate peel, particularly PPE-1, is a promising source of antioxidants and antimicrobial agents. Furthermore, molecular docking studies demonstrated significant binding energies and interactions between chlorogenic acid and COX-II, supporting the potential anti-inflammatory properties of chlorogenic acid. These results underscore the potential therapeutic value of pomegranate peel extracts and warrant further exploration for drug development and healthcare.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Naghavi, M. et al. Global burden associated with 85 pathogens in 2019: a systematic analysis for the global burden of disease study 2019. Lancet Infect. Dis. 24 (8), 868–895. https://doi.org/10.1016/ (2024).
Ahmed, S. K. et al. Antimicrobial resistance: impacts, challenges, and future prospects. J. Med. Surg. Public. Health. 2, 100081. https://doi.org/10.1016/j.glmedi.2024.100081 (2024).
Ben, Y. et al. Human health risk assessment of antibiotic resistance associated with antibiotic residues in the environment: A review. Environ. Res. 169, 483–493. https://doi.org/10.1016/j.envres.2018.11.040 (2019).
Khan, I. U. et al. Green synthesis and synergistic antibacterial activity of iron oxide and aluminum oxide nanoparticles using Cymbopogon citratus extract. Pak J. Bot. 57 (5). https://doi.org/10.30848/PJB2025-5(8) (2025).
World Health Organization. WHO Global Report on Traditional and Complementary Medicine 2019 (World Health Organization, 2019).
da Silva, J. A. T. et al. Pomegranate biology and biotechnology: A review. Sci. Hortic. 160, 85–107. https://doi.org/10.1016/j.scienta.2013.05.017 (2013).
Ferrara, G., Mazzeo, A., Colasuonno, P. & Marcotuli, I. Production and growing regions. In The Fig: Botany, Production and Uses 47–92 (CABI, 2022).
Behrooznia, L., Khojastehpour, M. & Hosseinzadeh-Bandbafha, H. A comprehensive assessment of the environmental footprint of pomegranate juice production system by life cycle assessment approach. Environ. Sustain. Indic. 22, 100398. https://doi.org/10.1016/j.indic.2024.100398 (2024).
Zhang, M. et al. Pomegranate: A comprehensive review of functional components, health benefits, processing, food applications, and food safety. J. Agric. Food Chem. 73, 5649–5665. https://doi.org/10.1021/acs.jafc.4c05428 (2025).
Melgarejo-Sánchez, P. et al. Pomegranate variety and pomegranate plant part, relevance from bioactive point of view: A review. Bioresour Bioprocess. 8, 1–29. https://doi.org/10.1186/s40643-020-00351-5 (2021).
Shilpa, P. et al. Pomegranate genetic resources: conservation and utilization. Fruit Nut Crops. 529–570. https://doi.org/10.1007/978-981-99-5348-6_18#DOI (2024). Springer Nature Singapore.
Faria, G. M. L. & Silva, E. K. Pulsed electric field, ultrasound and microwave heating based extraction techniques for valorization of pomegranate Peel by-products: A review. J. Environ. Chem. Eng. 113078. https://doi.org/10.1016/j.jece.2024.113078 (2024).
Dardona, Z. Literature review: Punica granatum (pomegranate) with an emphasis on its anti-parasitic activity. GSC Biol. Pharm. Sci. 23 (2), 100–114. https://doi.org/10.30574/gscbps.2023.23.2.0192 (2023).
Ge, S. et al. A unique Understanding of traditional medicine of pomegranate, Punica granatum L. and its current research status. J. Ethnopharmacol. 271, 113877. https://doi.org/10.1016/j.jep.2021.113877 (2021).
Karami, S., Faraji, S., Basaki, T. & Ghanaei, S. Assessment of Yield-based drought tolerance indices and physiological traits for screening pomegranate (Punica granatum L.) genotypes. Int. J. Hortic. Sci. Technol. 11 (3), 317–330. https://doi.org/10.22059/ijhst.2023.363604.680 (2024).
Hu, Y., Zhang, X., Shan, L., Liu, L. & Chen, J. The antiviral activity of currently used medicinal plants in aquaculture and structure–activity relationship of their active ingredients. Rev. Aquacult. 16 (1), 154–173. https://doi.org/10.1111/raq.12825 (2024).
Azmat, F. et al. Phytochemical profile, nutritional composition of pomegranate Peel and Peel extract as a potential source of nutraceutical: A comprehensive review. Food Sci. Nutr. 12 (2), 661–674. https://doi.org/10.1002/fsn3.3777 (2024).
Kupina, S., Fields, C., Roman, M. C. & Brunelle, S. L. Determination of total phenolic content using the Folin-C assay: Single-laboratory validation, first action 2017.13. J. AOAC Int. 102 (1), 320–321. https://doi.org/10.5740/jaoacint.2017.13 (2019).
Singh, S. K., Patel, J. R. & Dangi, A. Physicochemical, qualitative and quantitative determination of secondary metabolites and antioxidant potential of Kalanchoe pinnata (Lam.) Pers. Leaf extracts. J. Drug Deliv Ther. 9 (1), 220–224. https://doi.org/10.22270/jddt.v9i1.2225 (2019).
Sun, B., Ricardo-da-Silva, J. M. & Spranger, I. Critical factors of Vanillin assay for catechins and proanthocyanidins. J. Agric. Food Chem. 46 (10), 4267–4274. https://doi.org/10.1021/jf980366j (1998).
Page, M., Sultana, N., Paszkiewicz, K., Florance, H. & Smirnoff, N. The influence of ascorbate on anthocyanin accumulation during high light acclimation in Arabidopsis Thaliana: further evidence for redox control of anthocyanin synthesis. Plant. Cell. Environ. 35 (2), 388–404. https://doi.org/10.1111/j.1365-3040.2011.02369.x (2012).
Rehman, S., Sultana, N., Sultana, T. & Ahmad, D. Comparative GC-MS analysis of nine different seasonal flowers growing in selected region of Pakistan. J. Chem. Soc. Pak. 41 (5), 893–902 (2019).
Baig, H., Ahmed, D., Zara, S., Aujla, M. I. & Asghar, M. N. Vitro evaluation of antioxidant properties of different solvent extracts of Rumex acetosella leaves. Orient. J. Chem. 27 (4), 1509 (2011).
Umamaheswari, M. & Chatterjee, T. Vitro antioxidant activities of the fractions of Coccinia grandis L. leaf extract. Afr. J. Tradit Complement. Altern. Med. 5 (1), 61–73. https://doi.org/10.4314/ajtcam.v5i1.31258 (2008).
Miller, G. L. Use of Dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31 (3), 426–428 (1959).
Valgas, C., Souza, S. M. D., Smânia, E. F. & Smânia Jr, A. Screening methods to determine antibacterial activity of natural products. Braz J. Microbiol. 38, 369–380. https://doi.org/10.1590/S1517-83822007000200034 (2007).
Burley, S. K. et al. Protein data bank (PDB): the single global macromolecular structure archive. Protein Crystallogr. Methods Protoc. 627–641. https://doi.org/10.1007/978-1-4939-7000-1_26 (2017).
Schuhmann, F., Korol, V. & Solov’yov, I. A. Introducing Pep McConst-A user-friendly Peptide modeler for biophysical applications. J. Comput. Chem. 42 (8), 572–580. https://doi.org/10.1002/jcc.26479 (2021).
Vilar, S., Cozza, G. & Moro, S. Medicinal chemistry and the molecular operating environment (MOE): application of QSAR and molecular Docking to drug discovery. Curr. Top. Med. Chem. 8 (18), 1555–1572. https://doi.org/10.2174/156802608786786624 (2008).
Hanwell, M. D. et al. Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 4, 1–17 (2012).
Scholz, N. et al. The adhesion GPCR Latrophilin/CIRL shapes mechanosensation. Cell. Rep. 11 (6), 866–874. https://doi.org/10.1016/j.celrep.2015.04.008 (2015).
Kim, S. et al. PubChem substance and compound databases. Nucleic Acids Res. 44 (1), 1202–1213. https://doi.org/10.1093/nar/gkv951 (2016).
Jejurikar, B. L. & Rohane, S. H. Drug designing in discovery studio. (2021). https://doi.org/10.5958/0974-4150.2021.00025.0
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25 (13), 1605–1612. https://doi.org/10.1002/jcc.20084 (2004).
Yuan, S., Chan, H. S. & Hu, Z. Using PyMOL as a platform for computational drug design. Wiley Interdisciplinary Reviews Comput. Mol. Sci. 7 (2), 1298. https://doi.org/10.1016/j.apjtb.2015.05.006 (2017).
Orlando, B. J. & Malkowski, M. G. Substrate-selective Inhibition of cyclooxygeanse-2 by Fenamic acid derivatives is dependent on peroxide tone. J. Biol. Chem. 291 (29), 15069–15081. https://doi.org/10.1074/jbc.M116.725713 (2016).
Ahmed, D., Saeed, R., Shakeel, N., Fatima, K. & Arshad, A. Antimicrobial activities of methanolic extract of Carissa Opaca roots and its fractions and compounds isolated from the most active Ethyl acetate fraction. Asian Pac. J. Trop. Biomed. 5 (7), 541–545. https://doi.org/10.1016/j.apjtb.2015.05.006 (2015).
Galanakis, C. M. The food systems in the era of the coronavirus (COVID-19) pandemic crisis. Foods 9 (4), 523. https://doi.org/10.3390/foods9040523 (2020).
Jiménez-Moreno, N., Esparza, I., Bimbela, F., Gandía, L. M. & Ancín-Azpilicueta, C. Valorization of selected fruit and vegetable wastes as bioactive compounds: opportunities and challenges. J. Crit. Rev. Envi Sci. Tech. 50 (20), 2061–2108. https://doi.org/10.1080/10643389.2019.1694819 (2020).
Benítez-Correa, E., Bastías-Montes, J. M., Acuña-Nelson, S. & Muñoz-Fariña, O. Effect of choline chloride-based deep eutectic solvents on polyphenols extraction from cocoa (Theobroma Cacao L.) bean shells and antioxidant activity of extracts. Curr. Res. Food Sci. 7, 100614. https://doi.org/10.1016/j.crfs.2023.100614 (2023).
Obluchinskaya, E. D. & Pozharitskaya, O. N. The efficacy of two methods for extracting fucoidan from frozen Arctic algae Thalli: chemical composition, kinetic study and process optimization. Appl. Phycol. 1–20. https://doi.org/10.1007/s10811-023-03178-7 (2024).
Rupp, M., Walter, N., Brochhausen, C. & Alt, V. Fracture related Infection-Challenges in definition and diagnosis. J. Orthop. 49, 38–41. https://doi.org/10.1016/j.jor.2023.11.050 (2024).
Sweidan, N., Abu Rayyan, W., Mahmoud, I. & Ali, L. Phytochemical analysis, antioxidant, and antimicrobial activities of Jordanian pomegranate peels. PLoS One. 18 (11), 0295129. https://doi.org/10.1371/journal.pone.0295129 (2023).
Fourati, M. et al. Multiresponse optimization of pomegranate Peel extraction by statistical versus artificial intelligence: predictive approach for foodborne bacterial pathogen inactivation. Evid. -based Complement. Altern. Med. 2019 https://doi.org/10.1155/2019/1542615 (2019).
Saad, H. et al. Characterization of pomegranate peels tannin extractives. Ind. Crop Prod. 40, 239–246. https://doi.org/10.1016/j.indcrop.2012.02.038 (2012).
Mottaghipisheh, J. et al. Total anthocyanin, flavonoid, polyphenol and tannin contents of seven pomegranate cultivars grown in Iran. Acta Sci. Pol. Technol. 17 (3), 211–217. https://doi.org/10.17306/J.AFS.0584 (2018).
Haider, R., Mehdi, A., Zehra, A., Das, G. K. & Ahmed, Z. Antibacterial activity of naturally occurring compounds from selected plants. Int. J. Sci. Multidisciplinary Res. 2 (4), 337–362. https://doi.org/10.55927/ijsmr.v2i4.8630 (2024).
Siddiqui, S. A., Singh, S. & Nayik, G. A. Bioactive compounds from pomegranate peels-biological properties, structure–function relationships, health benefits and food applications-A comprehensive review. J. Funct. Foods. 116, 106132. https://doi.org/10.1016/j.jff.2024.106132 (2024).
Malviya, S., Arvind, Jha, A. & Hettiarachchy, N. Antioxidant and antibacterial potential of pomegranate Peel extracts. J. Food Technol. 51, 4132–4137. https://doi.org/10.1007/s13197-013-0956-4 (2014).
Mandić, K. N. et al. Antioxidative potential of pomegranate Peel extract: In vitro and in vivo studies. Scr. Med. 54 (1), 9–18. https://doi.org/10.5937/scriptamed54-43453 (2023).
Chukwuma, C. I., Mashele, S. S. & Akuru, E. A. Evaluation of the In vitro ⍺-amylase inhibitory, antiglycation, and antioxidant properties of Punica granatum L.(pomegranate) fruit Peel acetone extract and its effect on glucose uptake and oxidative stress in hepatocytes. J. Food Biochem. 44 (5), 13175. https://doi.org/10.1111/jfbc.13175 (2020).
Rasouli, H., Farzaei, M. H. & Khodarahmi, R. Polyphenols and their benefits: A review. Int. J. Food Prop. 20 (2), 1700–1741. https://doi.org/10.1080/10942912.2017.1354017 (2017).
Călinoiu, L. F. & Vodnar, D. C. Whole grains and phenolic acids: A review on bioactivity, functionality, health benefits and bioavailability. Nutrients 10 (11), 1615. https://doi.org/10.3390/nu10111615 (2018).
Huang, J., Xie, M., He, L., Song, X. & Cao, T. Chlorogenic acid: A review on its mechanisms of anti-inflammation, disease treatment, and related delivery systems. Front. Pharmacol. 14, 1218015. https://doi.org/10.3389/fphar.2023.1218015 (2023).
Man, G., Xu, L., Wang, Y., Liao, X. & Xu, Z. Profiling phenolic composition in pomegranate Peel from nine selected cultivars using UHPLC-QTOF-MS and UPLC-QQQ-MS. Front. Nutr. 8, 807447. https://doi.org/10.3389/fnut.2021.807447 (2022).
Al-Tai, A. A. & Al-Mayyahi, T. F. A chemical study by using GC-Mass spectrometry of the Peel and seeds of Punica Granatum L. plant. Sys Rev. Pharm. 12 (1), 1414–1421 (2021).
Al-Huqail, A. A., Elgaaly, G. A. & Ibrahim, M. M. Identification of bioactive phytochemical from two Punica species using GC–MS and Estimation of antioxidant activity of seed extracts. Saudi J. Biol. Sci. 25 (7), 1420–1428. https://doi.org/10.1016/j.sjbs.2015.11.009 (2018).
Zou, H. et al. 5-Hydroxymethylfurfural enhances the antiviral immune response in macrophages through the modulation of RIG-I-mediated interferon production and the JAK/STAT signaling pathway. ACS Omega. 6 (42), 28019–28030. https://doi.org/10.1021/acsomega.1c03862 (2021).
Martins, F. C., Alcantara, G. M., Silva, A. F. S., Melchert, W. R. & Rocha, F. R. The role of 5-hydroxymethylfurfural in food and recent advances in analytical methods. Food Chem. 395, 133539. https://doi.org/10.1016/j.foodchem.2022.133539 (2022).
Zhang, H., Jiang, Z., Shen, C., Zou, H., Zhang, Z., Wang, K., … Xie, T., 2021. 5-hydroxymethylfurfural alleviates inflammatory lung injury by inhibiting endoplasmic reticulum stress and NLRP3 inflammasome activation. Front. Cell Dev. Biol. 9, 782427. https://doi.org/10.3389/fcell.2021.782427.
Bender, O. & Atalay, A. Polyphenol chlorogenic acid, antioxidant profile, and breast cancer. In Cancer 311–321. Academic Press. (2021). https://doi.org/10.1016/B978-0-12-819547-5.00028-6
Acknowledgements
The authors of this study extend their appreciation to the Ongoing Research fund Program (Project number ORF-2025-378)), King Saud University, Riyadh, Saudi Arabia.
Funding
The financial support for the study is provided from Ongoing Research fund program (Project number ORF-2025-378) by , King Saud University, Riyadh, Saudi Arabia, (RSP2025R378).
Author information
Authors and Affiliations
Contributions
S.M.B: Conceptualization, Methodology, Fromal analysis, Writing and Visualization, N.S: Supervision, Project Administration, Validation, Writing and Data Curation, M.B. Al-R: Funding Acquisition KS. L: Writing, Data Curation and Validation, W.S, A.H and Z.T: Visualization and Validation, D.A: Resources and Fromal analysis, J.H and B.K: Investigation, Validation, Data Analysis, F U. R: Review. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Permission to collect pomegranate
All research activities conducted in this study, including the collection of pomegranates (Punica granatum) samples, complied with relevant institutional, national, and international guidelines and legislation. Prior written permissions/licenses were obtained from the appropriate authorities, private property owners to collect plant material. The study was approved from the Department of Biochemistry, Hazara University, Mansehra, Kp, Pakistan. Some of the analysis are conducted in collaboration of Department of Chemical Engineering and Materials Science/Environmental Technology Research Center, Yuan Ze University, Chung-Li District, Taoyuan City 32003, Taiwan.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Badshah, S.M., Sultana, N., Al-Rawi, M.B.A. et al. Phytochemical profiling, biological potential and In Silico identification of anticancerous compound from Pakistani pomegranate Peel. Sci Rep 15, 26689 (2025). https://doi.org/10.1038/s41598-025-03596-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-03596-2