Abstract
This study aimed to investigate risk factors associated with conversion from early laparoscopic cholecystectomy (ELC) to open cholecystectomy in patients diagnosed with acute calculous cholecystitis (ACC). A retrospective analysis was conducted on 3,191 ACC patients who underwent ELC at eight clinical centers between January 2013 and December 2023. To evaluate risk factors for conversion during ELC, least absolute shrinkage and selection operator (LASSO) regression with ten-fold cross-validation was employed to identify and select the most relevant variables. Subsequently, a binary logistic regression model was built using the variables selected from LASSO regression to develop a nomogram for prediction. The model’s performance was evaluated using external validation through receiver operating characteristic (ROC) curves for discrimination, Hosmer-Lemeshow test and calibration curves for calibration, and decision curve analysis (DCA) for clinical practicality. LASSO regression analysis identified five optimal variables from a total of twenty-nine for model development: preoperative C-reactive protein (CRP) level, anesthesia American Society of Anesthesiologists (ASA) classification, calculus location, Tokyo Guidelines 2018 (TG18) classification, and surgeon seniority. External validation of the model using the area under the curve (AUC) from ROC curves yielded moderate discrimination in both the training set (AUC = 0.868) and validation set (AUC = 0.833). Calibration plots indicated good agreement between predicted and observed probabilities, suggesting good calibration of the nomogram. Additionally, DCA analysis supported the model’s potential clinical usefulness. This study identified high preoperative CRP level, presence of gallbladder neck calculus, high grades in both anesthesia ASA and TG18 classifications, and junior surgeon as factors that can be used to predict the need for conversion to open surgery during ELC procedures for ACC patients.
Similar content being viewed by others
Introduction
Laparoscopic cholecystectomy (LC) has become the mainstay of treatment for gallbladder removal due to its association with low mortality and morbidity rates1,2. Compared to traditional open cholecystectomy, LC offers numerous advantages, including minimally invasive techniques, reduced postoperative complications, shorter hospital stays, and lower overall medical costs3,4. However, despite advancements in laparoscopic technology and improved management of intraoperative complications during LC, bile duct injuries during surgery and postoperative cystic duct leaks remain significant challenges. These complications are particularly prevalent in early laparoscopic cholecystectomy (ELC) for acute cholecystitis: according to Tokyo Guideline 2018’s criteria5, ELC for ACC was defined as performing laparoscopic cholecystectomy within 72 h of onset in patients. The extensive inflammation, adhesions, and increased bleeding associated with acute cholecystitis make laparoscopic dissection of Calot’s triangle and identification of the biliary anatomy difficult and hazardous2,6. To minimize the occurrence of these severe complications, surgeons are often forced to convert LC procedures to open cholecystectomy in cases encountering significant inflammation, adhesions, or unclear anatomical relationships around the gallbladder during surgery7,8. Therefore, a deeper understanding of preoperative risk factors associated with conversion to open surgery is crucial for safer procedures and more effective surgical planning. This study aims to investigate and assess the potential preoperative risk factors that could influence conversion rates in ELC for patients with acute calculous cholecystitis (ACC). By exploring these factors, the primary objective is to develop and externally validate a predictive model based on multicenter, cross-sectional research data. This model will aid in identifying patients at higher risk of conversion, allowing surgeons to tailor surgical approaches and optimize patient outcomes.
Methods
Patient data source
This multicenter, cross-sectional study utilized data extracted from the electronic medical records systems of eight hospitals in China. The study period spanned from January 2013 to December 2023. A total of 3191 patients with ACC who underwent ELC were included. Inclusion criteria were:1 first occurrence and diagnosis of ACC confirmed by the presence of fever and/or chills, laboratory evidence of inflammation and/or abnormal liver function, and imaging confirmation of gall bladder calculus9,2. Patients who underwent early laparoscopic cholecystectomy within 72 h of illness onset, according to the Tokyo Guidelines 2018 (TG18) criteria10. Exclusion criteria included:1 Patients with chronic cholecystitis: repeatedly and continuously chronic inflammation of the gallbladder;2 acute cholecystitis developed from the previous chronic cholecystitis;3 ACC complicated by acute pancreatitis;4 non-calculous acute cholecystitis, or choledocholithiasis requiring simultaneous choledocholithotomy and T-tube drainage during LC;4 gallbladder cancer cases were also excluded.
Measurement outcomes
Demographic characteristics, corresponding physical examination findings, serum biomarkers, transabdominal ultrasound measurements, disease severity grading, and surgical data were collected for all patients. Demographic characteristics included gender, age, body mass index (BMI), hypertension status, diabetes mellitus, chronic obstructive pulmonary disease (COPD) status, and history of abdominal surgery. Physical examinations included temperature and mean arterial pressure (MAP). Serum biomarkers included white blood cell (WBC) count, C-reactive protein (CRP), procalcitonin (PCT), platelet count, creatinine, international normalized ratio (INR), alanine aminotransferase (ALT), and aspartate aminotransferase (AST). Transabdominal ultrasound characteristics included gallbladder wall thickness, length and width, number of calculi (single or multiple), calculus location (gallbladder body or neck), calculus diameter (≥ 3 cm or < 3 cm), presence of gallbladder polyps, and pericholecystic effusion. Patients’ preoperative condition were carefully assessed, we graded severity of ACC and risk of the operation using the TG18 classification11 and the American Society of Anesthesiologists (ASA) classification system respectively. Surgical data included time from symptom onset to surgery, surgeon seniority (categorized as junior: ≤5 years of surgical experience, intermediate: >5 but ≤ 10 years of experience, or senior: >10 years of experience), the presence of anatomical variations in the bile ducts or hepatic arteries intraoperatively, the use of bailout procedures(subtotal cholecystectomy) during the surgery, and whether conversion to open surgery occurred during the laparoscopic procedure. In order to obtained the anatomical variations in the bile ducts or hepatic arteries intraoperatively, the surgeons meticulously examined the anatomical structures of both bile ducts and hepatic arteries during the procedure, noting variations in branching patterns, diameters, and positional relationships with surrounding tissues. Additionally, intraoperative ultrasound was utilized to assess the bile ducts and hepatic arteries in real-time, providing high-resolution images that helped identify subtle anatomical variations, such as accessory ducts or aberrant arterial branches. The main reason for conversion in this study was prophylactical to prevent postoperative complications, and conversions also performed due to other situations such as uncontrolled bleeding, bile duct injury, gallbladder perforation or unclear anatomical visualization of Calot’s triangle intraoperatively.
Surgical procedures
The main procedures of laparoscopic cholecystectomy are as follows. After the anesthesia takes effect, we disinfect the surgical field of patients and lay sterile surgical drapes. Under normal conditions, three surgical entrances have been identified, there are umbilical region, 1 ~ 2 cm below the xiphoid process and the right subcostal, respectively. We insert the trocar through these surgical entrances and establish artificial carbon dioxide pneumoperitoneum with a pressure of 12 ~ 14mmHg, and then insert laparoscopic surgical instruments through the trocars. We dissect the Calot’ triangle and separate the gallbladder artery, then clamp gallbladder artery with an absorbable clip, and disconnect it. We find and confirm the cystic duct, to avoid damaging the common bile duct, the position of the absorbable clip to clamp the cystic duct during surgery is determined intraoperatively based on anatomical variation, and disconnected the cystic duct at the distal end. Finally, gallbladder is peeled off from the gallbladder bed completely. When the anatomy of the Calot’ triangle is unclear, intraoperative bleeding is difficult to control, or bile duct injury occurs, we transfer laparoscopic surgery into open surgery promptly.
Model establishment
In this study, patients from the leading institution, Huadu District People’s Hospital of Guangzhou, were included in the training set. Within the training set, we used Least Absolute Shrinkage and Selection Operator (LASSO) regression to perform variable shrinkage and selection with ten-fold cross-validation. This analysis was conducted to identify which of the thirty-one aforementioned outcome variables were most relevant for predicting conversion to open cholecystectomy during ELC in ACC patients. Based on the criterion of lambda.1SE, variables that were not shrunk to zero by LASSO regression were selected for model development. Using these selected variables, we then employed binary logistic regression to construct a predictive model and a nomogram.
Model external validation
To assess the predictive performance of the nomogram, external validation was employed. Data for ACC patients from the remaining seven clinical centers in this study were used as the validation set. During external validation, ROC curves and their corresponding AUCs were used to evaluate the nomogram’s discrimination ability. Calibration curves and the Hosmer-Lemeshow test were employed to assess the calibration of the prediction model. Finally, to evaluate the model’s clinical practicality and net benefits, DCA curves were generated, and the area under the DCA curve (AUDC) was calculated.
Ethics statement
This retrospective cross-sectional study adhered to ethical principles outlined in the Declaration of Helsinki and its subsequent revisions. The study design did not involve any intervention in patients’ existing treatment plans, and the researchers ensured complete confidentiality of all collected patient information. This study has been reported in line with the STROCSS criteria12.
Statistical analysis
Data analysis and statistical plots were generated using R(version 4.1.3, https://www.r-project.org/). To address missing values in this cross-sectional study and ensure data analyzability, multiple imputation was performed using the “mice” package with Predictive Mean Matching (PMM) as the primary imputation method for continuous variables. For categorical variables (e.g., binary or ordinal), we employed logistic regression or polytomous regression imputation, depending on the variable type. The choice of PMM was motivated by its robustness in preserving the original data distribution, particularly for variables that may deviate from normality. Imputation validation included visual inspection of density plots (observed vs. imputed) and statistical comparison of summary measures (mean, variance). For continuous variables with normal distribution, descriptive statistics were expressed as mean ± standard deviation. Median (upper and lower quartiles) was used for non-normally distributed data. Categorical variables were described using frequencies and their corresponding percentages. To compare groups, the Chi-square test was employed for categorical variables. Student’s t-tests were used for normally distributed continuous variables, while Mann-Whitney U tests were used for non-normally distributed continuous variables.
To identify optimal variables for model construction, LASSO regression was performed using the “glmnet” package for variable shrinkage and selection. Subsequently, the “glm” function was used to fit a logistic regression model based on the selected variables. With the fitted model, a nomogram was constructed using the “nomogram” function from the “rms” package. For model validation, discrimination was assessed using the area under the receiver operating characteristic curve (AUC) calculated with the “pROC” package. Calibration, which reflects the agreement between predicted and observed risks, was evaluated using a calibration plot and Hosmer-Lemeshow test performed with the “rms” package. Finally, decision curve analysis (DCA) was performed using the “ggDCA” package to assess the net benefit of the model across different risk thresholds in the ACC cohort. Additionally, the area under the decision curve was calculated. To assess whether the relationships between CRP, ASA classification, TG18 classification, calculus location, and surgeon experience varied according to operative time, interaction terms were incorporated into the analysis. A p-value of less than 0.05 was considered statistically significant, except for interaction analyses where a p-value of less than 0.10 was used.
Results
Data acquisition and splitting
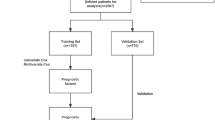
Following the defined inclusion and exclusion criteria, a total of 3,191 patients with ACC who underwent ELC during their hospitalization at eight participating hospitals were included in this study. Among 3191 ACC patients who performed ELC, 263 cases(8.24%) switched to open cholecystectomy during the operation. Theoretically, the original dataset encompassed 98,921 data points considering 3,191 cases and 31 variables. However, due to the cross-sectional nature of the study, 1,678 missing values (1.69%) were identified during data extraction. To ensure data integrity for statistical analysis, missing values were imputed using multiple imputation with the “mice” package in R software. The training set for model development comprised patients from the leading institution(n = 1,658). The validation set included 1,533 patients from the remaining seven clinical centers. The training and validation sets were split in a ratio of approximately 1:0.92. A flowchart depicting the data acquisition and splitting process is presented in Fig. 1.
Baseline and univariate analysis of the study cohort
Out of the 3,191 patients included in this cohort study, 1,658 (51.96%) were assigned to the training set, and 1,533 (48.04%) were allocated to the validation set. Baseline analysis revealed no significant differences between the training and validation sets regarding any of the investigated variables. Among the 3,191 ACC patients who underwent ELC, 263 (8.24%) experienced intraoperative conversion to open surgery, while 2,928 (91.76%) successfully completed laparoscopic surgery. Univariate analysis was conducted to explore factors associated with conversion to open cholecystectomy surgery. The results of the baseline analysis for both sets and the univariate analysis comparing patients with and without conversion are presented in Table 1. In order to validate weather the data of this study was subject to historical bias, we controlled the time variable factor and divided the dataset into 2013–2017 group and 2018–2023 group respectively, and then compared the variables between two groups one by one. The result indicated that under the controlling of time factor, all of the including variables of this study had no significantly difference(P > 0.05) between the two groups, which demonstrated that the study was not obviously subject to historical bias.(Supplementary Table 1).
Predictive model establishment
LASSO regression with ten-fold cross-validation was employed to identify the optimal variables for model construction from the original variables listed in Table 1. Five variables were selected: CRP level, ASA classification, calculus location, TG18 classification, and surgeon seniority (Fig. 2A,B). Using the backward step approach, binary logistic regression analysis of these five predictors revealed with the least value of AIC(Akaike Information Criterion) and confirmed that they were independent of each other(Supplementary Table 2). Based on these five predictors, we developed a conversion risk nomogram for ELC patients (Table 2). The nomogram not only displays the scoring system for each independent risk factor and the total points but also visually depicts the risk stratification and the estimated probability of conversion for each individual case. For example, an ACC patient undergoing ELC with a TG18 classification of III, ASA classification of III, preoperative CRP of 100 mg/ml, a calculus located in the gallbladder neck, and surgery performed by a junior surgeon would be predicted to have a high risk of conversion with an estimated probability of 85% (Fig. 3).
Variables shrinkage and selection using LASSO regression. (A) Pathway diagram of variables after shrinkage. Five variables, including CRP, ASA classification, calculus position, TG18 classification, and surgeon seniority, were selected based on the optimal lambda value. (B) Ten-fold cross-validation chart. After identifying the optimal parameter, LASSO was used with lambda equal to 1 standard error (1.SE) to perform variable shrinkage and selection.
Comparison of model building methods with machine learning
In order to investigate the advantage of predicted value for model construction approach, we made a comparison between the Logistic regression and seven machine learning models named Random Forest, Boost Tree, Decision Tree, Naïve Bayes, Nearest Neighbor, Support Vector Machine and Multi-Layer Perceptron. Via ROC and accuracy analysis, we could understand that either accuracy or AUC value of ROC from Logistic regression was the highest among these models, which manifested that Logistic regression nomogram of this study having certain advantages.(Supplementary Fig. 1A,B).
Comparison between nomogram and previous predicted models
For the sake of comparing the nomogram developed in this study with the previous models predicting conversion to open surgery, several models from previous studies were constructed. We defined the previous models as Model.A, from Goonawardena J et al.13; Model.B, from Licciardello A et al.14; Model.C, from Ibrahim S et al.15 and the TG18 model. We compared these models with the nomogram and assessed their performance using the Net Reclassification Index (NRI) and Integrated Discrimination Improvement Index (IDI) indices. The results showed that both the NRI and IDI indices of the nomogram were significantly higher compared to the previous models, suggesting that the nomogram demonstrated a positive improvement in efficacy compared to the previous models, highlighting its superiority in predicting conversion to open surgery. (Supplementary Table 3)
Collinearity and interaction analysis
To assess the presence of collinearity among the included variables in the predictive model, the variance inflation factor (VIF) was calculated for each variable. Results indicated that all VIF values were below 5.0, suggesting no significant collinearity concerns (Supplementary Fig. 2). We further explored potential interaction effects between the included covariates. The analysis revealed interaction effects between calculous position and ASA grading, as well as between ASA grading and surgeon seniority. (Supplementary Fig. 3). To investigate how the impact of varying calculous position stratification on outcomes differed across subgroups with distinct characteristics, we performed a subgroup analysis. Patients were stratified based on calculous in gallbladder body or calculous in gallbladder neck. The analysis demonstrated that, regardless of the calculous position stratification, p-values for interaction terms involving other covariates were all greater than 0.1 (Fig. 4). This suggests that while significant interactions exist between calculous position and ASA grading, ASA grading and CRP, surgeon seniority and ASA grading (p-values for interaction were 0.0130, 0.0706 and 0.0369, respectively), stratifying by calculous position did not substantially alter the associations with serum CRP level, ASA classification,, TG18 classification, and surgeon seniority (Supplementary Table 4). Collectively, the findings from the collinearity and interaction analyses indicate that the predictive model is not prone to overfitting and that the relationships between variables remain consistent across different strata. This suggests good stability and reliability of the fitted model.
Model external validation
The receiver operating characteristic (ROC) curves for model discrimination yielded AUC values of 0.868 and 0.833 in the training and validation sets, respectively (Fig. 5A,B). These AUC values indicated moderate discriminatory ability of the predictive model. Evaluation of model calibration, utilizing calibration curves, revealed a high degree of concordance between the predicted probabilities and the actual observed probabilities within both the training and validation datasets. The calibration curves exhibited a close correspondence to the optimal reference lines (Fig. 5C,D). This observation was further confirmed by the Hosmer-Lemeshow test, which yielded high p-values (P = 0.9124 and P = 0.8537 for the training and validation sets, respectively). Both the calibration plots and the Hosmer-Lemeshow test demonstrated favorable calibration of the model. DCA was employed to assess the net benefit and clinical practicality of the nomogram. As shown in Fig. 6A,B, the threshold probability for the prediction model ranged from 1 to 81% in the training set and 1–75% in the validation set. Additionally, the AUDC was calculated, with values of 0.0293 and 0.0213 for the training and validation sets, respectively. Overall, DCA revealed that the predictive model offers higher net benefit and demonstrates good clinical practicality.
Model discrimination and calibration both in training set and external validation set. (A) ROCs and their corresponding AUCs of the nomogram and each independent risk factor in training set. (B) ROCs and their corresponding AUCs of the nomogram and each independent risk factor in validation set. (C) Calibration curve and Hosmer-Lemeshow test in training set. (D) Calibration curve and Hosmer-Lemeshow test in validation set.
Discussion
LC is currently considered the gold standard treatment for cholecystitis16,17. Advancements in laparoscopic technology and growing surgical experience have expanded the indications for LC, even for conditions previously deemed unsuitable, such as early-stage acute cholecystitis18,19. Despite its established role as a standard surgical procedure for ACC, LC is not without complications. Intraoperative bile duct injuries, bleeding, postoperative bile leaks, and bile duct stenosis remain significant concerns17,20,21. The risk of accidental injury to the bile duct or blood vessels is particularly high in cases involving severe gallbladder inflammation, a highly edematous gallbladder wall, unclear anatomy of Calot’s triangle, or the presence of Mirizzi syndrome. These complications can significantly impact a patient’s quality of life and prognosis after LC. Therefore, during challenging LC procedures, timely identification of conversion risk factors and the prompt decision to switch to open cholecystectomy are crucial. Prior studies have shown that timely conversion during difficult LCs can significantly reduce complications like bile duct injury and bile leakage22,23. By understanding preoperative risk factors for conversion, clinicians can optimize patient care and minimize the risk of unnecessary damage and complications. However, limitations exist in previous research on preoperative risk factors for conversion from LC to open cholecystectomy. These limitations include relatively small sample sizes, single-center designs, and the absence or lack of external validation for the developed models23,24,25.
This study leveraged multicenter participation, big data analysis, and external validation to establish a model for analyzing risk factors associated with conversion from laparoscopic to open cholecystectomy. Following a comprehensive evaluation of model collinearity and interaction effects between covariates, a stable and clinically predictive model was developed. To further validate the model’s accuracy, we performed multicenter external validation based on discrimination, calibration, and clinical practicality. Our findings identified preoperative CRP level, calculus location in the gallbladder neck based on imaging results, TG18 classification, anesthesia ASA classification, surgeon experience, and operative duration as significant risk factors for conversion to open surgery. The external validation results demonstrated high predictive value in terms of both discrimination and calibration for the model on both the training and validation sets. Additionally, the DCA analysis indicated a high net benefit of the model, highlighting its clinical practicality.
CRP is a biomarker that reflects the inflammatory response in the body. During the early stages of biliary inflammatory diseases like acute cholecystitis or cholangitis, serum CRP levels typically exhibit varying degrees of elevation26. Studies have shown that for patients with ACC undergoing ELC, the primary reason for conversion to open cholecystectomy is closely linked to the severity of gallbladder inflammation14,27,28. CRP plays a significant role in predicting this increased risk of conversion29,30. Severe gallbladder inflammation, characterized by substantial edema and congestion of the gallbladder wall, can obscure the anatomical landmarks of Calot’s triangle and lead to uncontrollable intraoperative bleeding. In such scenarios, surgeons may opt to switch to open cholecystectomy. A ten-year retrospective study revealed that unrecognizable anatomical structures surrounding the gallbladder were the main reason for conversion to open surgery during LC procedures (approximately 50%)31. Furthermore, obstruction of the gallbladder duct caused by impacted calculi lodged in the gallbladder neck is another critical factor contributing to severe gallbladder inflammation. A normally unobstructed gallbladder duct prevents retrograde bacterial infections from the intestine. However, when blocked by calculi, the duct becomes susceptible to retrograde bacterial infection, potentially leading to complications like gallbladder empyema, gangrene, or even perforation, which significantly increase the risk of conversion to open surgery32,33.
The ASA classification system, based on a patient’s physical health, serves as an assessment tool for surgical risk during anesthesia34,35. Similarly, the TG18 grading system provides severity assessment criteria for acute cholecystitis9,36. The Tokyo Guidelines 2018 for Surgical Management of Acute Cholecystitis explicitly state a close association between preoperative ASA score and the risk of conversion to open surgery during ELC for patients with grade II or III acute cholecystitis10. This finding aligns with our own study results. Furthermore, a prior study by Michael Rosen et al.7. demonstrated that an ASA classification greater than 2 (OR = 5.3, P = 0.01) predicted conversion in patients undergoing LC, substantiating that preoperative ASA classification is a risk factor for conversion from LC to open surgery. In summary, the association between ASA classification and conversion risk likely reflects the interplay of comorbidities, chronic inflammatory changes, and risk-averse surgical decision-making. As shown in recent studies37, ASA ≥ III patients are more susceptible to intraoperative instability during prolonged laparoscopy, which may compel conversion to open surgery for expedited completion.
The current study also demonstrated that surgeon specialization and skill played a critical role in minimizing unnecessary conversions. Senior surgeons with over ten years of experience were significantly associated with lower conversion rates compared to junior surgeons with less than five years of experience. These findings highlight that, in addition to preoperative laboratory and imaging results, surgeon expertise is another important factor influencing the decision to convert LC to open surgery22,38,39,40. In addition, due to the fact that surgical operation is a collaborative process within a teamwork, weather transfer into conversion can also be influenced by the experience and judgment of the supervising assistant, a previous retrospective study indicated that even in less complex operations, complication rate from the younger supervising assistant was significantly higher than that from the well-experienced senior, which showed the importance of supervising assistant in decision-making process during surgery41.
With regard to the real-world application of this prediction model, when patients identified as high-risk switching, clinicians can use the results of this model to adjust treatment strategies to reduce complications and improve surgical success rates. Specifically, when a patient is predicted to be at high risk, clinicians may consider the following aspects to influence clinical decision-making:1Optimal operation opportunity: prioritize early laparoscopic cholecystectomy (ELC) after inflammation control, as ELC is associated with higher success rates than delayed approaches (DLC)42. However, delayed surgery may be safer in unstable patients or severe inflammation2. Surgical approach: consider planned open cholecystectomy when justified by patient stability, inflammation severity, and surgical expertise to avoid unplanned conversions43,3. Preoperative preparation: Enhance imaging/laboratory evaluations and ensure transparent communication with patients/families regarding conversion risks44.
As with any study, this research has limitations. Firstly, its retrospective nature may limit the generalizability of the results to other settings. Secondly, while our study identified several risk factors for conversion from ELC to open cholecystectomy, the causal relationships between these factors remain largely unknown. In clinical practice, surgeons encounter a complex interplay of risk factors during ELC decision-making. Zhang et al.45 suggest that reliable structural modeling with inverse probability weighting (IPW) could be a valuable tool for inferring causality from observational data like ours. Thirdly, although surgeon seniority emerged as a risk factor, our analysis using collinearity and stratified subgroups did not reveal evidence of overfitting or interaction effects within the predictive model. Notably, our definition of surgeon seniority solely considered professional years. A surgeon’s experience encompasses other factors like educational background, training history, and the number of independently performed operations. Additionally, model construction and validation of this study is base on an Asian cohorts due to the limitations of data acquisition conditions. And the model lacks validation across the entire population especially in western populations, which implied that there may be involve in potential bias. These additional aspects could be explored in future studies to refine the model through international cooperation in the future. Despite these limitations, our study provides valuable clinical insights. We look forward to future research that investigates the causal associations between identified risk factors and conversion during ELC for ACC patients.
Conclusion
This study identified several risk factors associated with conversion from ELC to open cholecystectomy in patients with ACC. These factors include high preoperative CRP levels, gallbladder neck calculus, higher grades in both ASA classification and TG18 classification, and surgeon seniority (junior surgeons). It is important to emphasize that conversion to open surgery during ELC should not be viewed as a surgical failure. Instead, it represents a crucial and potentially life-saving intervention that can minimize complications and mortality rates.
Data availability
Data is provided within the supplementary information files.
References
Strasberg, S. M. & Brunt, L. M. Rationale and use of the critical view of safety in laparoscopic cholecystectomy. J. Am. Coll. Surg. 211 (1), 132–138 (2010).
Jin, Y. et al. Critical view of safety in laparoscopic cholecystectomy: A prospective investigation from both cognitive and executive aspects. Front. Surg. 9, 946917 (2022).
Pontarelli, E. M. et al. Regional cost analysis for laparoscopic cholecystectomy. Surg. Endosc. 33 (7), 2339–2344 (2019).
Mannam, R. et al. Laparoscopic cholecystectomy versus open cholecystectomy in acute cholecystitis: A literature review. Cureus 15 (9), e45704 (2023).
Okamoto, K. et al. Tokyo guidelines 2018: flowchart for the management of acute cholecystitis. J. Hepatobiliary Pancreat. Sci. 25 (1), 55–72 (2018).
Mencarini, L., Vestito, A., Zagari, R. M. & Montagnani, M. The diagnosis and treatment of acute cholecystitis: A comprehensive narrative review for a practical approach. J. Clin. Med. 13 (9). (2024).
Magnano San Lio, R. et al. Preoperative risk factors for conversion from laparoscopic to open cholecystectomy: A systematic review and meta-analysis. Int. J. Environ. Res. Public. Health 20 (1). (2022).
Chin, X., Mallika Arachchige, S., Orbell-Smith, J. & Wysocki, A. P. Preoperative and intraoperative risk factors for conversion of laparoscopic cholecystectomy to open cholecystectomy: A systematic review of 30 studies. Cureus 15 (10), e47774 (2023).
Kiriyama, S. et al. Tokyo guidelines 2018: diagnostic criteria and severity grading of acute cholangitis (with videos). J. Hepatobiliary Pancreat. Sci. 25 (1), 17–30 (2018).
Wakabayashi, G. et al. Tokyo guidelines 2018: surgical management of acute cholecystitis: safe steps in laparoscopic cholecystectomy for acute cholecystitis (with videos). J. Hepatobiliary Pancreat. Sci. 25 (1), 73–86 (2018).
Yokoe, M. et al. Tokyo guidelines 2018: diagnostic criteria and severity grading of acute cholecystitis (with videos). J. Hepatobiliary Pancreat. Sci. 25 (1), 41–54 (2018).
Mathew, G. et al. STROCSS 2021: strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Int. J. Surg. 96, 106165 (2021).
Goonawardena, J., Gunnarsson, R. & de Costa, A. Predicting conversion from laparoscopic to open cholecystectomy presented as a probability nomogram based on preoperative patient risk factors. Am. J. Surg. 210 (3), 492–500 (2015).
Licciardello, A. et al. Preoperative risk factors for conversion from laparoscopic to open cholecystectomy. Eur. Rev. Med. Pharmacol. Sci. 18 (2 Suppl), 60–68 (2014).
Ibrahim, S. et al. Risk factors for conversion to open surgery in patients undergoing laparoscopic cholecystectomy. World J. Surg. 30 (9), 1698–1704 (2006).
Mora-Guzmán, I., Di Martino, M., Gancedo Quintana, A. & Martin-Perez, E. Laparoscopic cholecystectomy for acute cholecystitis: is the surgery still safe beyond the 7-Day barrier?? J. Gastrointest. Surg. 24 (8), 1827–1832 (2020).
Kim, S. S., Donahue, T. R. & Laparoscopic Cholecystectomy JAMA ;319(17):1834. (2018).
Bundgaard, N. S., Bohm, A., Hansted, A. K. & Skovsen, A. P. Early laparoscopic cholecystectomy for acute cholecystitis is safe regardless of timing. Langenbecks Arch. Surg. 406 (7), 2367–2373 (2021).
Gurusamy, K. S., Davidson, C., Gluud, C. & Davidson, B. R. Early versus delayed laparoscopic cholecystectomy for people with acute cholecystitis. Cochrane Database Syst. Rev. (6), CD005440. (2013).
Fu, J-N., Liu, S-C., Chen, Y., Zhao, J. & Ma, T. Analysis of risk factors for complications after laparoscopic cholecystectomy. Heliyon 9 (8), e18883 (2023).
Sato, M., Endo, K., Harada, A. & Shijo, M. Risk factors of postoperative complications in laparoscopic cholecystectomy for acute cholecystitis. JSLS 24 (4). (2020).
Ali, A., Saeed, S., Khawaja, R., Samnani, S. S. & Farid, F. N. Difficulties in laparoscopic cholecystectomy: conversion versus Surgeon’s failure. J. Ayub Med. Coll. Abbottabad. 28 (4), 669–671 (2016).
Warchałowski, Ł. et al. The analysis of risk factors in the conversion from laparoscopic to open cholecystectomy. Int. J. Environ. Res. Public Health 17 (20). (2020).
Malla, B. R., Shakya, Y. R., Rajbhandari, N. & Karki, B. Laparoscopic cholecystectomy: conversion rate and associated factors for conversion. Kathmandu Univ. Med. J. (KUMJ). 17 (67), 241–244 (2019).
Vaccari, S. et al. Laparoscopic cholecystectomy: which predicting factors of conversion? Two Italian center’s studies. Minerva Chir. 75 (3), 141–152 (2020).
Díaz-Flores, A., Cárdenas-Lailson, E., Cuendis-Velázquez, A., Rodríguez-Parra, A. & Trejo-Ávila, M. E. C-Reactive protein as a predictor of difficult laparoscopic cholecystectomy in patients with acute calculous cholecystitis: A multivariate analysis. J. Laparoendosc Adv. Surg. Tech. A. 27 (12), 1263–1268 (2017).
Vardar, Y. M. & Akturk, O. M. Can we predict the risk of conversion in elective laparoscopic cholecystectomy? Ann. Ital. Chir. 91, 181–186 (2020).
Philip Rothman, J., Burcharth, J., Pommergaard, H-C., Viereck, S. & Rosenberg, J. Preoperative risk factors for conversion of laparoscopic cholecystectomy to open Surgery - A systematic review and Meta-Analysis of observational studies. Dig. Surg. 33 (5), 414–423 (2016).
Bouassida, M. et al. C-reactive protein is the best biomarker to predict advanced acute cholecystitis and conversion to open surgery. A prospective cohort study of 556 cases. J. Gastrointest. Surg. 24 (12), 2766–2772 (2020).
Yigit, B., Cerekci, E., Baran, E. & Citgez, B. Simple blood tests May be used to predict the increased risk of conversion in elective laparoscopic cholecystectomy surgery. J. Laparoendosc Adv. Surg. Tech. A. 32 (4), 408–412 (2022).
Ravendran, K. et al. Converting from laparoscopic cholecystectomy to open cholecystectomy: A systematic review of its advantages and reasoning. Cureus 16 (7), e64694 (2024).
Amin, A. et al. Preoperative and operative risk factors for conversion of laparoscopic cholecystectomy to open cholecystectomy in Pakistan. Cureus 11 (8), e5446 (2019).
Le, V. H., Smith, D. E. & Johnson, B. L. Conversion of laparoscopic to open cholecystectomy in the current era of laparoscopic surgery. Am. Surg. 78 (12), 1392–1395 (2012).
Voney, G. et al. Interrelation of peri-operative morbidity and ASA class assignment in patients undergoing gynaecological surgery. Eur. J. Obstet. Gynecol. Reprod. Biol. 132 (2), 220–225 (2007).
Nelson, G. et al. Guidelines for perioperative care in gynecologic/oncology: enhanced recovery after surgery (ERAS) society recommendations-2019 update. Int. J. Gynecol. Cancer. 29 (4), 651–668 (2019).
Luo, Y. et al. An acute general surgical unit (AGSU) negates the impact of the Tokyo guidelines 2018 (TG18) diagnostic criteria for the treatment of acute cholecystitis. World J. Surg. 43 (11), 2762–2769 (2019).
Takada, T. & Tokyo Guidelines 2018: updated Tokyo guidelines for the management of acute cholangitis/acute cholecystitis. J. Hepatobiliary Pancreat. Sci. 25 (1), 1–2 (2018).
Donkervoort, S. C. et al. Outcome of laparoscopic cholecystectomy conversion: is the Surgeon’s selection needed? Surg. Endosc. 26 (8), 2360–2366 (2012).
Bartlett, A. & Parry, B. Cusum analysis of trends in operative selection and conversion rates for laparoscopic cholecystectomy. ANZ J. Surg. 71 (8), 453–456 (2001).
Ahmed, S., Ali, A. A., Hasan, M. & Awal, A. Problems leading to conversion in laparoscopic cholecystectomy. Mymensingh Med. J. 22 (1), 53–58 (2013).
Lee, A. et al. The surgical outcomes of pedicle Subtraction osteotomy per different first assistant: retrospective analysis of 312 cases. J. Am. Acad. Orthop. Surg. 32 (1), e33–e43 (2024).
Lyu, Y., Cheng, Y., Wang, B., Zhao, S. & Chen, L. Early versus delayed laparoscopic cholecystectomy for acute cholecystitis: an up-to-date meta-analysis of randomized controlled trials. Surg. Endosc. 32 (12), 4728–4741 (2018).
Nassar, A. H. M., Zanati, H. E., Ng, H. J., Khan, K. S. & Wood, C. Open conversion in laparoscopic cholecystectomy and bile duct exploration: subspecialisation safely reduces the conversion rates. Surg. Endosc. 36 (1), 550–558 (2022).
Cruz, W. W. B. & Han, H-S. Preoperative evaluation and management of acute cholecystitis: optimal timing and surgical approach. In: (ed Di Carlo, I.) Difficult Acute Cholecystitis: Treatment and Technical Issues. Cham: Springer International Publishing; 53–63. (2021).
Zhang, Z. et al. Causal inference with marginal structural modeling for longitudinal data in laparoscopic surgery: A technical note. Laparosc. Endoscopic Robotic Surg. 5 (4), 146–152 (2022).
Acknowledgements
We acknowledge Guangzhou Red Cross Hospital, The First Affiliated Hospital/School of Clinical Medicine of Guangdong Pharmaceutical University, Guangzhou Nansha Central Hospital, Qingyuan Municipal People’s Hospital, People’s Hospital of Qianxi of Guizhou Province, Second People’s Hospital of Huadu District of Guangzhou City and Guangzhou First People’s Hospital for providing us with the clinical data of this study. And we also acknowledge funding support from the Internal Medicine Research Fund and the Construction of Major Subject of Huadu District People’s Hospital of Guangzhou.
Funding
This study was supported by funding from Internal Medicine Research Fund(Grant Number: 2019A01) and the Construction of Major Subject of Huadu District People’s Hospital of Guangzhou(Grant Number: YNZDXK202201, 2022–2025).
Author information
Authors and Affiliations
Contributions
H.W, K.M and B.L designed the study, analyzed and interpreted the data, and wrote the manuscript; T.J and Z.Z analyzed and interpreted the clinical data; Y.Y, J.Y, H.Y, Y.L and Y.Z collected patient samples and clinical data; G.H and W.G were responsible for the statistics of data and processing the figures and tables; T.C conceptualized and designed the study, supervised the project, and revised the paper. All authors have approved the final version and agreed to publish the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
The studies involving humans were approved by Ethics Committee of Huadu District People’s Hospital of Guangzhou. The Registration Number: 2019050. All patients (or their guardians) included in the study were provided with an informed consent form, which they read and understood before signing to indicate their full understanding of the research.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wu, H., Ma, K., Liao, B. et al. Multicenter external validation of a nomogram predicting conversion to open cholecystectomy during laparoscopic surgery for acute calculous cholecystitis: a cross-sectional study. Sci Rep 15, 18481 (2025). https://doi.org/10.1038/s41598-025-03687-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-03687-0