Abstract
Motor resonance (MoR) refers to the automatic activation of motor circuits during action observation, reflecting an internal simulation of the observed movement. This phenomenon is thought to arise from the activity of mirror neuron regions, which modulate primary motor cortex (M1) excitability via cortico-cortical pathways. MoR, which is believed to be involved in the mechanism underlying action understanding and motor learning, has been widely studied using visual stimuli and transcranial magnetic stimulation (TMS). However, the optimal form of movement presentation and TMS timing remains unclear. This study compared the effects of static photographs and videos on the MoR activation and explored the ideal timing for TMS. Participants observed abduction movements of the index finger (controlled by the first dorsal interosseous muscle, FDI) and the little finger (controlled by the abductor digiti minimi muscle, ADM) presented as photographs or videos. The task included three conditions: Photo (static images), Video (full movement videos), and Postvideo (post-movement period). TMS was applied over the primary motor cortex at 0, 320, or 640 ms from movement onset (Photo, Video) and at the same intervals from movement offset (Postvideo). Motor evoked potentials (MEPs) were recorded from FDI and ADM. The Postvideo condition yielded the strongest MEP modulations, with inhibition in the non-matching muscle and excitation in the muscle corresponding to the observed movement. In contrast, Photo and Video conditions showed time-dependent reductions in cortical excitability, especially in non-matching muscles. These findings suggest that applying TMS after movement observation provides a more accurate approach to studying MoR and highlights the role of motor surround inhibition in motor control.
Similar content being viewed by others
Introduction
The concept of mirror neurons has received considerable attention since their discovery, with numerous studies investigating their role in action perception and motor cognition. This effect, reliant on the observation of others’ activities, has been found to play a passive role in action comprehension and an active role in learning through imitation in animals and in humans1. Mirror neurons, firing during both action observation (AO) and execution, serve as the neuronal substrate for translating observed actions into our motor code, facilitating the link between action perception and execution2,3. In humans, the functional consequence of mirror neuron activity is reflected in motor resonance (MoR), the modulation of corticospinal excitability during AO. Functional magnetic resonance imaging (fMRI) studies in humans and intracranial neurophysiological recordings in monkeys have localized mirror neuron activity to the ventral premotor cortex (PMv, area F5) and the inferior parietal lobule (IPL)1,4,5. In humans, Non-invasive brain stimulation (NIBS) techniques, particularly transcranial magnetic stimulation (TMS), have been instrumental in the causal examination of MoR, offering precise targeting of cortical sensorimotor regions and eliciting measurable responses like the motor evoked potential (MEP)6,7,8.
Despite extensive research, critical questions remain unanswered regarding the most effective form of movement presentation and the optimal timing for TMS stimulation of the primary motor cortex (M1) in studies of MoR in humans. Here, we aim to address these questions by investigating the regulation of the MoR effect during AO through manipulation of visual stimuli and TMS timing.
To do that, we examined two widely employed techniques for presenting visual stimuli in hand movement studies: static photographs and videos. Both methods offer precise control over movement timing and execution, ensuring replicable presentation across trials. Additionally, we investigated commonly used stimulation times relative to movement onset in previous research7,9,10 and to movement offset, spanning from 0 to 640 ms. The static photographs do not induce a genuine perceptual motion effect but instead present an illusory representation of a completed action. Unlike videos, which allow observers to track movement as it unfolds, static photographs depict a single static frame of a fully executed action, without conveying an explicit sense of motion trajectory. This distinction is critical, as videos offer a continuous kinematic representation of movement, facilitating the study of MoR responses during action execution, whereas static photographs isolate a salient moment of the action, enabling the investigation of MoR responses without the confounding factor of movement tracking.
Importantly, this study does not aim to examine the precise time course of MoR. Instead, TMS was applied at three fixed time points (0, 320, and 640 ms) within each condition. This methodological approach ensures a controlled statistical design, allowing for meaningful comparisons across conditions. By maintaining consistent stimulation timings across different visual presentation modalities, our study seeks to provide a clearer understanding of how MoR varies in response to static, dynamic, and post-movement visual stimuli.
Research on MoR employs various methods of visual stimulus presentation7,8,10,11,12,13,14,15,16. While all methods demonstrate MoR effects, certain modalities of motor/movement stimuli visualization show higher reliability in replicability across trials, subjects, and the timing of TMS. Real-life movement presentation, conducted by a researcher during the experiment, offers greater control over stimulus presentation and higher ecological validity but has other limitations, such as difficulty repeating exact movements consistently and accurately timing the stimulation with respect to the movement8,12. While visual images presented on a screen are considered the most reliable approach for studying MoR due to their precise timing and replicability, the extent to which inhibitory mechanisms contribute to this effect remains less understood. Most research on MoR has focused on facilitatory mechanisms, with less attention given to the role of motor inhibition in refining observed action representations. Motor inhibition research has significantly advanced our understanding of the mechanisms involved in precise movement control17,18,19,20,21,22, particularly regarding action stopping and preparation18,20,23. One important inhibitory mechanism is motor surround inhibition (mSI), which suppresses activity in muscles not directly engaged in a given movement to fine-tune motor precision19,24,25,26. However, studies of mSI have typically used relatively high TMS intensities (e.g., 140% RMT) to elicit this effect5. In contrast, we employed 110% RMT, a level commonly used in MoR research7,27,28, allowing us to focus on the natural excitability changes occurring during AO. This decision was motivated by previous findings showing that lower TMS intensities are more effective at capturing MoR effects during AO29. Our results suggest that inhibitory responses, including mSI-like effects, can emerge even at lower TMS intensities, underscoring the robustness of these inhibitory mechanisms in shaping motor responses during observation.
While our primary objective was to explore the excitatory properties of MoR, our results revealed an additional inhibitory component that was not part of our initial hypothesis. Specifically, we observed an unexpected pattern of inhibition in muscles not directly engaged in the observed movement, an effect that aligns with known principles of mSI during motor execution19. These findings suggest that inhibitory mechanisms may contribute to AO effects in a way previously underexplored in the literature. In a similar vein, Aoyama et al.30 investigated the neural mechanisms behind selective finger movements, emphasizing the need for inhibitory neural activity to suppress unintended muscle activations during such movements. Their findings highlight the complexity of motor control, where the brain must often counteract inherent synergistic patterns to achieve precise and independent finger movements, a principle pivotal in contexts such as rehabilitation where retraining precise motor functions is critical. This underscores the relevance of their work to our investigation into how different visual stimuli, such as finger movements, and TMS timings can modulate motor cortex excitability and motor inhibition during AO.
To date, research on MoR effects induced by TMS has shed light on the importance of these systems in AO and imitation. However, a gap exists in our understanding of how the presentation of visual stimuli and the timing of TMS influence these effects, particularly regarding the potential contribution of inhibitory mechanisms, including mSI. Our study uniquely addresses this gap by examining both excitation and inhibition in response to different stimulation timings during AO, providing insights into how the motor system processes observed movements beyond simple facilitation effects. By targeting M1 during movement initiation, movement execution, and the post-stimulus offset phase with TMS stimulation, we engage higher levels of motor processing, providing insights into the temporal dynamics of motor response during AO. Importantly, we explore the extent to which inhibitory mechanisms shape MoR, rather than solely focusing on facilitatory effects.
Materials and methods
Subjects
Thirty right-handed volunteers (17 females, age range: 20–35, average age: 25.93) participated in the study. Remaining twenty-eight volunteers after exclusions (15 females, age range: 20–35, average age: 26.25). They reported no personal or family history of neurological or psychiatric disorders. Participants provided informed written consent and received financial compensation for their time in the lab. The study adhered to the ethical guidelines of the Declaration of Helsinki and was approved by the Ethical Committee of the National Research University-Higher School of Economics (HSE), Moscow. During the experiment, participants sat comfortably in a reclining chair, keeping their right arm relaxed and orthogonal to the presentation screen, with instructions not to move their hands.
Transcranial Magnetic Stimulation (TMS)
Neuronavigated TMS was administered over the left primary motor cortex (M1) using a MagPro X100 stimulator (MagVenture, Farum, Denmark) with a C-B60 butterfly induction coil. A frameless neuronavigation system (Localite TMS Navigator, Localite GmbH, Sankt Augustin, Germany) facilitated MRI-guided navigation based on individual T1 weighted MR scans, ensuring accurate coil positioning. After manually locating the hotspot with the C-B60 coil, the device was switched to an analogous coil with the same outer diameter of 75 mm, a C-B85 butterfly induction coil attached to an Axilum TMS Cobot System (Brainbox Ltd, United Kingdom). This system maintained the same neuronavigation session and hotspot position. During the sessions, biphasic single-pulse TMS (spTMS) was applied over the left M1 using a figure-of-eight coil. The coil was positioned tangentially to the scalp, with the handle oriented 45° backward and laterally from the midline, approximately perpendicular to the central sulcus, to optimize the posterior–anterior current flow in the brain31,32,33. This orientation was chosen based on previous findings demonstrating that the lowest motor threshold is achieved when the induced electric current flows perpendicular to the central sulcus, thereby facilitating transsynaptic activation of corticospinal neurons34,35.
The coil was systematically adjusted to identify the optimal scalp position (OSP), defined as the location where TMS at slightly suprathreshold intensity elicited the largest motor-evoked potentials (MEPs) from both the first dorsal interosseous (FDI) and abductor digiti minimi (ADM) muscles, which are responsible for index and little finger movements, respectively36. The coil’s position and orientation ensured consistent stimulation of the corticospinal pathway, maximizing the reliability of the recorded MEP responses37,38. Both muscles (FDI and ADM) MEPs were measured simultaneously from the same hotspot. The optimal hotspot was identified after exploring the motor cortex and selecting the location that produced the highest and most stable MEPs for both muscles, with an approximate ratio of 2/3 of ADM with respect to FDI. Resting motor threshold (rMT) was defined as the minimum stimulation intensity required to generate reliable MEPs (≥ 50 μV peak-to-peak amplitude) in a relaxed muscle in at least five out of ten consecutive trials. The rMT was determined for the muscle with the lower threshold to avoid the loss of any differential modulations involving the less excitable muscle37,38,39. The intensity adopted for spTMS during the experimental tasks was then fixed at 110% of the left hemisphere (dominant) rMT. The 110% intensity of rMT was selected following the example of previous studies in motor resonance (MoR)7,27,28,37 and the specific recommendations form the study of Loporto et al.29. Surface EMG activity was recorded from the right FDI and ADM muscles using disposable adhesive surface electrodes (EB Neuro S.p.A., Florence, Italy) and a BrainAmp ExG amplifier with a bipolar EXG AUX module (Brain Products GmbH, Munich, Germany) (sampling rate: 5 kHz). EMG recording was performed with a belly-tendon montage for FDI and ADM muscles with reference electrodes placed on the corresponding knuckle bone (index finger and little finger respectively) and a common ground electrode was used for both muscles to further reduce signal artifacts. The EMG activity with triggers from the TMS was collected and stored on a computer for offline analysis.
Task and stimulation protocol
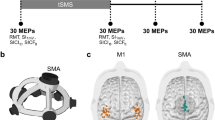
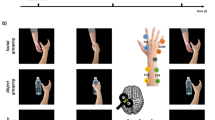
Participants’ elbows were positioned at approximately 90 degrees, ensuring that the hand remained parallel to the screen to minimize potential confounding effects of spatial alignment with the observed actions, as highlighted in previous studies40. Hand images were presented from a first-person viewpoint based on the evidence indicating that this perspective elicits parietal activation in the hemisphere contralateral to the observed hand, as if the action were being mimicked with the same anatomical limb. In this view, anatomical and specular processing strategies align, leading to the predominant activation of contralateral brain regions responsible for motor control of the observed limb. This perspective enhances the natural correspondence between observed and executed actions, reinforcing sensorimotor mechanisms41,42. The standard protocol included baseline measurement recordings (resting state) before the main task. For this purpose, a black screen with a white fixation cross was presented while 27 motor evoked potentials (MEPs) were collected. The main task then comprised three conditions: Photo, Video, and Postvideo (Fig. 1a–c).
Conditions: (a) Photo: Presentation modality using static photos of the finished movements, with TMS stimulation beginning at the onset of the movement stimuli presentation. (b) Video: Presentation modality using videos of the full movements, with TMS stimulation beginning at the onset of the movement stimuli presentation. (c) Postvideo: Presentation modality using videos of the full movements, with TMS stimulation beginning at the offset of the movement stimuli presentation (equivalent to onset of the post-movement presentation).
For the Photo condition: (a) a white fixation cross over a black screen was presented during 1500 ms; (b) then a static hand in neutral position was presented previous to the movement stimuli for a randomised time of 800, 1600, or 2400 ms to avoid predictability of the incoming stimulus; (c) from the neutral position hand the image transition to one of the movements as a static image of the finished movement lasting 1000 ms from the movement onset. Resembling stimuli used in the study from Catmur et al.7: either Index finger movement, Little finger movement, or Neutral hand (no movement), one per trial. Each hand movement was presented as a static image of the finished movement in 54 trials, the order of the trials for the three hand movements were randomised for every participant; (d) TMS pulses were applied at 0, 320, or 640 ms from the beginning of the visualisation of the movement onset, one per trial, a total of 54 trials each, and in a randomised order; and the trial was finished with a (e) black screen of 1500 ms (Fig. 1a).
For the Video condition: for each trial (a) a white fixation cross over a black screen was presented during 1500 ms; (b) then a static hand in neutral position was presented previous to the movement stimuli for a randomised time of 800, 1600, or 2400 ms to avoid predictability of the incoming stimulus; (c) from the neutral position hand the image transition to one of the movements as a video of the full movement lasting 1000 ms from the movement onset. Either Index finger movement, Little finger movement, or Neutral hand (no movement), one per trial. Each hand movement was presented as a video movement in 54 trials (movement lasting 1000 ms), the order of the trials for the three hand movements were randomised for every participant; (d) TMS pulses were applied at 0, 320, or 640 ms from the beginning of the visualisation of the movement onset, one per trial, a total of 54 trials each, and in a randomised order; (e) for the post-movement phase, the position of the finished movement remained for 1000 ms as an static image; and the trial was finished with a (f) black screen of 1500 ms (Fig. 1b).
For the last condition, Postvideo: for each trial (a) a white fixation cross over a black screen was presented during 1500 ms; (b) then a static hand in neutral position was presented previous to the movement stimuli for a randomised time of 800, 1600, or 2400 ms to avoid predictability of the incoming stimulus; (c) from the neutral position hand the image transition to one of the movements as a video of the full movement lasting 1000 ms from the movement onset. Either Index finger movement, Little finger movement, or Neutral hand (no movement), one per trial. Each hand movement was presented as a video movement in 54 trials (movement lasting 1000 ms), the order of the trials for the three hand movements were randomised for every participant; (d) for the post-movement phase, the position of the finished movement remained for 1000 ms as an static image; (e) TMS pulses were applied at 0, 320, or 640 ms from the beginning of the post-movement phase of the visual presentation, one per trial, a total of 54 trials each, and in a randomised order; and the trial was finished with a (f) black screen of 1500 ms (Fig. 1c).
The three conditions—Photo, Video, and Postvideo—were similar in terms of the presentation timing, with TMS applied at identical intervals (0, 320, 640 ms) across all conditions. Each condition aimed to evaluate motor responses to visual stimuli under controlled circumstances, ensuring consistency in the experimental setup and allowing for direct comparisons between the different types of visual input. In our experimental setup, participants observed isolated finger movements, specifically, index and little finger abductions. These movements, typically part of broader hand actions, were presented as discrete events to examine the cortical responses to perceived independent movements. This methodological choice aligns with Aoyama et al.30, who demonstrated the brain’s capability to selectively inhibit synergistic muscle actions to facilitate independent motor actions. This approach allowed us to probe the specific cortical activity associated with each observed finger movement, shedding light on the mechanisms of MoR and inhibition during action observation.
All stimuli were presented using E-Prime 3.0 software (Psychology Software Tools, Pittsburgh, PA). A total of 162 trials with stimuli were presented, 54 of each hand movement, and TMS pulses were delivered at randomised times using the Trigger Station (BrainTrends, Rome, Italy) connector. A total of 189 pulses were delivered during the experimental protocol for each condition including baseline and presentation, and the correspondent MEPs were recorded for both FDI and ADM.
Each experimental condition (Photo, Video, Postvideo) was conducted on separate days, with a minimum interval of four days between sessions to prevent carryover effects from TMS. The order of conditions was counterbalanced and randomly assigned across subjects. Within each session, all stimulus presentations (index and little finger movements, as well as neutral stimuli) and their corresponding TMS timing were fully randomized using the built-in randomization feature of E-Prime 3. This ensured that no systematic order effects influenced the results. Since it was a passive task, no learning effect was expected between sessions. Prior to the commencement of each session, subjects underwent a training period where they were instructed and well-trained to passively observe the stimuli. They were explicitly directed to focus their attention on the fixation cross and the visual presentation of hand movements, particularly the specific movements of the fingers. Participants were expressly asked to refrain from imitating, imagining, or actively thinking about the movements they observed, to ensure that the neural responses measured were purely attributable to action observation. This instruction was crucial to prevent contamination of results by motor imagery or anticipatory cognitive processes. The randomized presentation of stimuli further supported this objective, ensuring participants could not predict or prepare for specific movements, thereby maintaining the integrity of the experimental conditions.
Data processing
A 10-Hz high-pass filter and a notch filter (50 Hz) to remove power-line noise were applied during the sessions for online visualization. In order to reduce the variability of MEPs amplitude we conducted an analysis of background EMG activity across all trials and conditions for all subjects, excluding trials with background EMG activity exceeding 100 μV in the 50 ms pre-TMS43. Trials with MEPs containing artifacts, excessive latency jitter or a peak-to-peak maximal amplitude of lower than 50 µV were excluded. The raw amplitude data from the FDI and ADM muscle MEPs was then calculated as a median value for each condition according to the stimuli type and time of stimulation and then normalised based on the baseline (pre-stimuli) for each independent muscle (i.e. FDI, ADM) on each session (i.e. Photo, Video, Postvideo). Normalisation was performed due to variability on raw MEPs between subjects results during the stimuli presentation. The effects on the size of the MEPs recorded during the experimental part (hand movements presentation) were analysed as percentage changes in the mean peak-to-peak amplitude of the baseline (100%) for both the FDI and ADM muscles11,12.
Each combination of experimental factors could include a maximum of 18 trials per condition for stimulus presentation (e.g., MEPs recorded from the FDI muscle during index finger movement with TMS applied at 0 ms in the Photo condition), while baseline measurements consisted of 27 trials. To ensure data reliability, exclusion criteria were applied as follows: participants with fewer than 14 MEPs in the baseline condition (< 50% of total baseline trials) or fewer than 9 out of 18 MEPs in any factor combination across the three experimental conditions were excluded from the analysis. Following these exclusion criteria, an average of 17.29 trials per condition was retained, including baseline trials (16.46 trials if baseline was excluded). The final dataset consisted of a minimum of 10 and a maximum of 27 trials per condition when baseline trials were included (10 to 18 trials when baseline trials were excluded).
Statistical analysis
In this study, we opted not to use a MoR index, as it would obscure the distinct excitatory and inhibitory components of MoR by collapsing the activity of both FDI and ADM muscles into a single measure. Instead, we analyzed these muscles separately to accurately capture the differential modulation of motor excitability during action observation (AO). This approach is essential for distinguishing the excitatory responses of muscles directly involved in the observed movement from the inhibitory suppression of non-related muscles, which reflects the motor surround inhibition (mSI) mechanism. By maintaining separate analyses for FDI and ADM, we ensured a more precise characterization of MoR effects, particularly the interplay between facilitation and inhibition in response to different movement conditions.
After correction based on the exclusion criteria, we ran a four-way repeated measures ANOVA with the independent factors: Condition (Photo, Video, Postvideo), Movement (Index finger, Little finger, Neutral), Muscle (FDI, ADM), and Time (0, 320, 640 ms).
After conducting the main statistical analysis on normalized data and reviewing the results, we performed an additional analysis to highlight values for Index and Little finger movements. These values were adjusted using the Neutral stimulus at each specific time by subtracting the normalized MEP values of the Neutral condition from the Index and Little finger movement values. This step was decided upon due to the excitatory effect produced by the Neutral stimulus, allowing a more realistic and detailed comparison as well as a better visualisation of results. This excitatory effect on both muscles FDI and ADM during the Neutral stimulus presentation can be, hypothetically, the result of a preparatory effect on M1, a consequence of the expectation generated for an incoming movement when presenting a hand image. After data correction, we ran an additional four-way repeated measures ANOVA with the independent factors: Condition (Photo, Video, Postvideo), Movement (Index finger, Little finger), Muscle (FDI, ADM), and Time (0, 320, 640 ms).
The Greenhouse–Geisser correction was applied where necessary to compensate for the violation of the assumption of sphericity. In the presence of significant interactions and due to the exploratory nature of the study, corrected pairwise comparisons were performed using Bonferroni test to limit the experiment-wise error rate to α and to maximise the power of the test for detecting pairwise differences. The level of significance was set at p = 0.05.
Results
Four-way repeated measures ANOVA for normalized MEPs
Interaction effects
A four-way repeated measures ANOVA was conducted to analyze median motor evoked potentials (MEPs) normalized to baseline, incorporating the factors of Condition (Photo, Video, Postvideo), Movement (Index Finger, Little Finger, Neutral), Muscle (FDI, ADM), and Time (0, 320, 640 ms). The analysis revealed significant three-way interactions between Condition, Movement, and Muscle [F(1.377, 37.188) = 4.02, MSE = 17,344.8, p = 0.04, partial η2 = 0.13], as well as between Movement, Muscle, and Time [F(3.054, 82.45) = 4.03, MSE = 4231.06, p = 0.01, partial η2 = 0.13].
Additionally, a significant two-way interaction was identified between Movement and Muscle [F(1.332, 35.969) = 8.55, MSE = 32,016.81, p = 0.003, partial η2 = 0.24].
Finally, a significant main effect was observed for the factor Time [F(2, 54) = 5.33, MSE = 8315.16, p = 0.008, partial η2 = 0.17], highlighting the complex temporal modulation of MEP responses across different phases of stimulus presentation.
Post-hoc comparisons
Three-way interactions
Interaction of Condition, Movement, and Muscle (p = 0.04):
The results revealed a motor resonance (MoR) effect, characterized by an increase in MEPs for muscles involved in the observed abduction movement (FDI for the index finger; ADM for the little finger). Notably, high MEPs in the Neutral stimulus condition suggest that differences between muscles across movements are also driven by inhibition of the non-involved antagonistic muscle (ADM for index finger movement; FDI for little finger movement).
In the Photo condition, the FDI muscle exhibited greater excitability during Index finger (p = 0.005) and Neutral (p < 0.001) movements compared to Little finger movement (Fig. 2a). A similar effect was observed in the Postvideo condition, with significantly higher FDI activation during Index finger (p = 0.002) and Neutral (p = 0.004) movements compared to Little finger movement (Fig. 2c). Additionally, in the Postvideo condition, ADM excitability increased significantly during Neutral movement compared to Index finger movement (p = 0.014) (Fig. 2c). The analysis of Video condition revealed no significant effects (Fig. 2b).
Interaction of muscle and movement for each condition: (a) FDI MEPs increase for Index finger and Neutral movements compared to Little finger on Photo condition. (b) No effects for Video consition. (c) FDI MEPs increase for Index finger and Neutral movements compared to Little finger and ADM excitability increased during Neutral movement compared to Index finger on Postvideo condition. Asterisks: *p ≤ .05, **p ≤ .01, *** p ≤ .001.
Furthermore, in the Postvideo condition, observation of Little finger movement led to a significant increase in ADM excitability alongside a concomitant reduction in FDI activation (p = 0.049), reinforcing the inhibitory effect on the non-involved muscle.
Interaction of Movement, Muscle, and Time (p = 0.01):
The results indicate that MoR develops progressively from movement onset, with inhibitory components becoming more pronounced at later time points.
For the FDI muscle, significant differences between movement were found at 0 ms and 640 ms. At 0 ms, FDI activation was greater during Index finger (p = 0.037) and Neutral (p = 0.049) movements compared to Little finger movement (Fig. 3a). By 640 ms, FDI inhibition was evident during Little finger movement compared to both Index finger (p = 0.001) and Neutral (p = 0.034) conditions (Fig. 3a). In contrast, the ADM muscle exhibited significant modulation only at 640 ms, with greater activation during Neutral compared to Index finger movement (p = 0.015) (Fig. 3b). During observation of Little finger movement, ADM excitation increased significantly compared to FDI at 320 ms (p = 0.047), while at 640 ms, FDI inhibition became more pronounced relative to ADM activation (p = 0.016). Temporal differences were also evident within each movement. For Index finger movement, ADM activation decreased significantly at 640 ms compared to 0 ms (p = 0.004) (Fig. 4a). Similarly, during Little finger movement, FDI inhibition was significantly stronger at 640 ms compared to both 0 ms (p = 0.026) and 320 ms (p = 0.01) (Fig. 4b). The analysis of neutral stimulus revealed no significant effects (Fig. 4c), reinforcing the notion that the observed motor resonance effects were specifically driven by finger movement observation.
General interaction of muscle and movement factors over time: (a) FDI manifested its better representation and control with clear inhibition during Little finger compared to Index and Neutral at 0 and 640 ms. (b) ADM showed a later inhibitory effect due to merging of the three conditions and overall less precise excitation/inhibition control, with inhibition during Index finger compared to Neutral only at 640 ms. Asterisks: *p ≤ .05, ** p ≤ .01.
Two-way interactions
Interaction of Movement and Muscle (p = 0.003):
The FDI muscle exhibited significant inhibition during Little finger movement compared to Index finger (p < 0.001) and Neutral (p = 0.003) stimuli. Moreover, during Little finger movement, FDI activation was significantly lower than ADM activation (p = 0.099), reinforcing the presence of a strong inhibitory effect on the non-involved muscle during action observation.
Main effects
Time (0, 320, 640 ms) (p = 0.008):
MEPs recorded at 0 ms (p = 0.014) and 320 ms (p = 0.032) were significantly greater than those at 640 ms, further emphasizing the progressive development of inhibitory MoR over time.
Four-way repeated measures ANOVA for neutral-adjusted MEPs
To account for the inherent excitatory effect triggered by hand presentation—likely due to preparatory motor mechanisms—an additional analysis was performed with MEP values adjusted relative to the Neutral condition. This adjustment enabled a clearer comparison of AO responses by isolating excitatory and inhibitory components of MoR. A four-way repeated measures ANOVA was conducted on median MEPs normalized to baseline and adjusted to Neutral movement. Significant interaction effects were observed for Condition, Movement, and Muscle [F(1.146, 30.949) = 4.57, MSE = 20,003.12, p = 0.036, partial η2 = 0.15], as well as for Movement, Muscle, and Time [F(2, 54) = 7.43, MSE = 5659.04, p = 0.001, partial η2 = 0.22]. A significant two-way interaction was identified between Movement and Muscle [F(1, 27) = 9.92, MSE = 42,059.9, p = 0.004, partial η2 = 0.27]. Additionally, a significant main effect was found for Condition [F(2, 54) = 4.99, MSE = 18,843.4, p = 0.01, partial η2 = 0.16].
Post-hoc comparisons (neutral-adjusted MEPs)
Three-way interactions
Interaction of Condition, Movement, and Muscle (p = 0.036):
As in the unadjusted analysis, mirror resonance effects were observed with excitation of task-relevant muscles (FDI for index finger movement; ADM for little finger movement) and inhibition of non-involved antagonistic muscles (ADM for Index finger; FDI for Little finger). These effects were most pronounced in the Photo and Postvideo conditions.
For Index finger movement, ADM inhibition was significantly stronger in Postvideo compared to Video (p = 0.003) (Fig. 5a). Similarly, for Little finger movement, FDI inhibition was significantly greater in Photo (p = 0.005) and Postvideo (p = 0.009) compared to Video (Fig. 5b).
In Photo and Postvideo conditions, FDI activity was significantly lower during Little finger movement than during Index finger movement (p = 0.002, p = 0.001, respectively) (Fig. 6a,c). The analysis of Video condition revealed no significant effects (Fig. 6b). These findings highlight how the interaction between the factors Movement and Muscle varies across Conditions, with the most pronounced mirror responses observed in the FDI muscle. The excitatory and inhibitory components of MoR are differentially modulated depending on the presentation type, with the Postvideo condition eliciting the clearest effects.
Further significant differences were identified within the Photo condition. Specifically, during Index finger movement, ADM exhibited significantly lower activation compared to FDI (p = 0.043), whereas during Little finger movement, FDI showed significantly lower activation than ADM (p = 0.017). In the Video condition, Index finger movement resulted in significantly higher FDI activation compared to ADM (p = 0.043). In contrast, within the Postvideo condition, Index finger movement produced significantly lower ADM activation compared to FDI (p = 0.002).
These results reinforce the notion that MoR is predominantly characterized by the inhibition of muscles not directly involved in the observed movement. Notably, the Postvideo condition consistently yielded the strongest and most distinct effects, further supporting its efficacy in eliciting clear MoR responses. The increased statistical significance of findings in the Postvideo condition suggests that presenting an observed movement as a completed action may enhance the differentiation between task-relevant and non-relevant motor representations.
Interaction of factors Movement, Muscle, and Time (p = 0.001):
A significant interaction was observed among Movement, Muscle, and Time, indicating a dynamic modulation of MEPs over time. For the FDI muscle, MEP values were significantly lower during Little finger movement compared to Index finger movement at 0 ms (p = 0.012), 320 ms (p = 0.038), and 640 ms (p < 0.001) (Fig. 7a). Similarly, for the ADM muscle, MEP values were significantly lower during Index finger movement compared to Little finger movement at 320 ms (p = 0.046) and 640 ms (p = 0.029) (Fig. 7b).
General variation over time for muscle and movement: (a) FDI showed a clear differentiation with excitation and inhibition during movements where it acts as effector and non-effector at all three time. ADM had similar results but based only on inhibition of unrelated movement at times 320 and 640 ms. (b) During Index finger movement ADM was inhibited over time, same as FDI for Little finger, with the stronger inhibition at times 320 and 640 ms. Asterisks: *p ≤ .05, ***p ≤ .001.
These results provide strong evidence for mSI as the primary mechanism underlying MoR. The temporal profile of these effects reveals a progressive decrease in MEP values for muscles that were not directly involved in the observed abduction movement. This inhibitory effect was present at all stimulation times for the FDI muscle and became particularly pronounced at the later time points (320 and 640 ms) for the ADM muscle. In contrast, the muscles responsible for the observed movement exhibited a clear increase in activation, reinforcing the specificity of the MoR response.
Further analysis of Index finger movement revealed that ADM activation was significantly lower than FDI activation at 320 ms (p = 0.021) and 640 ms (p = 0.003) (Fig. 8a). The Little finger stimulus exhibited a distinct, albeit non-significant, inhibitory effect on the FDI muscle (Fig. 8b). This pattern reflects a precise balance between inhibition of non-task-related muscles and excitation of task-relevant muscles during action observation. Additionally, the growing significance of these differences over time suggests a temporal accumulation of inhibitory effects, supporting the idea that mSI strengthens as the observed movement progresses.
Two-way interactions
Interaction of Movement and Muscle (p = 0.004):
Significant FDI inhibition was observed during Little finger movement compared to Index finger movement (p < 0.001), reinforcing the presence of mSI. Additionally, ADM exhibited significantly lower activation during Index finger movement for ADM compared to FDI (p = 0.003), suggesting a combined effect of inhibition and excitation.
Main effects
Condition (p = 0.01):
MEP values in Postvideo were significantly lower compared to Video (p = 0.03), indicating that mSI effects were more robustly captured in Postvideo, highlighting its efficacy in eliciting clear MoR.
The results demonstrate that mirror resonance is driven predominantly by inhibitory mechanisms rather than excitation alone, as evidenced by stronger inhibition of non-involved muscles (mSI). The Postvideo condition elicited the clearest MoR responses, highlighting its potential as an optimal paradigm for studying MoR dynamics. Furthermore, inhibitory components of the response grew stronger over time, reinforcing the idea that mSI is a fundamental aspect of MoR rather than a secondary modulation.
Discussion
Our findings suggest that the optimal condition for eliciting a robust and temporally stable motor resonance (MoR) response involves stimulating the primary motor cortex (M1) after observing a completed intransitive movement (Figs. 2c, 5, 6c). This condition, termed Postvideo (Fig. 1c), represents a novel approach that uniquely combines video observation with delayed transcranial magnetic stimulation (TMS), applied after the movement is completed. This differs from conventional approaches where TMS is applied during movement observation (Video) (Fig. 1b) or when observing a static image of the completed movement (Photo) (Fig. 1a). The stronger and more consistent MoR effect observed in the Postvideo condition suggests that motor resonance induced by action observation (AO) is maximized after the full execution of an action, rather than during movement execution or static image observation. This finding highlights the importance of the post-movement phase, emphasizing the need to carefully select the timing of TMS stimulation to optimize MoR responses. Moreover, it introduces a novel paradigm for assessing MoR activity, offering a more precise approach to studying MoR dynamics that has not been extensively explored in prior research. Our data suggest that motor resonance is more strongly engaged after the action has been fully observed, likely reflecting a neural process of evaluating or simulating the action’s completion rather than focusing on the action in progress (Figs. 2, 5, 6). Understanding these temporal dynamics is crucial for advancing research on motor learning, imitation, and social cognition. By specifically targeting the post-movement phase, we open new avenues for studying the functional role of MoR in motor rehabilitation.
Motor resonance, surround inhibition, and synergy in action observation
In our analysis of motor evoked potentials (MEPs) elicited during finger movement observation, we found activation patterns that align with the principles of motor surround inhibition (mSI), as described by Sohn and Hallett19 and further explored by Kassavetis et al.44. Additionally, our results shed light on the interplay between mSI and muscle synergy during AO. These researchers have emphasized that motor precision is achieved through a balance of activating specific muscles while strategically inhibiting adjacent ones, refining motor actions. This is particularly relevant in hand movements, where multiple muscles coordinate to execute complex actions.
Sohn and Hallett19 described how the human motor system employs surround inhibition to regulate interactions between synergistic muscle groups, ensuring precision in voluntary movements. Similarly, Kassavetis et al.44 highlighted that surround inhibition is not always strictly uniform—the degree of inhibition varies significantly across different movement contexts. Our findings suggest that these inhibitory mechanisms may also be engaged during AO, helping to refine and disambiguate observed movements (Figs. 2, 5).
Emergence of motor surround inhibition
The inhibitory effects observed in our study were not originally hypothesized, but rather emerged as a robust finding during data analysis. This unexpected result highlights an important but previously underexplored aspect of MoR. Specifically, we found that during AO, the motor cortex appears to actively inhibit non-effector muscles, which may serve to sharpen the representation of observed actions.
Our results suggest that the motor system not only mirrors observed actions but also actively regulates motor output by inhibiting muscles that are not directly engaged in the observed movement. This finding expands our understanding of how motor resonance and inhibitory mechanisms interact during AO, suggesting that the functional role of MoR extends beyond simple motor facilitation (Figs. 5, 6, 7, 8).
Excitation-inhibition balance in motor resonance
The key contribution of this study is the identification of a dual mechanism in motor resonance (Figs. 4, 7, 8):
-
1.
Excitatory activation of cortical representations of muscles involved in the observed movement.
-
2.
Inhibitory suppression of non-effector muscles (e.g., increased FDI activation during index finger movements, concurrent with ADM inhibition, and vice versa for little finger movements).
As highlighted by Aoyama et al.30, cortical-mediated inhibition is critical for managing unintended muscle activity during selective finger movements. Our findings extend this principle to action observation, demonstrating that similar inhibitory mechanisms are recruited even when movements are passively observed. Interestingly, our findings show that inhibitory responses were more pronounced than excitatory responses, suggesting that inhibition plays a dominant role in motor resonance. This is consistent with the concept of mSI, a mechanism that ensures precision in motor control by suppressing non-essential muscles21,22,24,26,45. By extending this concept to AO, our results indicate that motor resonance not only mirrors observed actions but also simulates precise motor inhibition processes, mirroring the mechanisms underlying real movement execution.
Although our study was not originally designed to investigate mSI, our TMS coil orientation was chosen based on MoR research protocols. The posterior-anterior (PA) current direction, which is widely used in MoR studies, is also known to be optimal for eliciting surround inhibition46. This fortuitous alignment reinforces the validity of our findings—the observed inhibition was not an artifact of stimulation parameters but rather a meaningful neurophysiological effect.
Temporal dynamics of motor resonance and inhibition
While our study was not designed to investigate the precise time course of MoR, the inclusion of fixed TMS timings (0, 320, and 640 ms) allowed for structured statistical comparisons across conditions. Within this framework, we observed distinct temporal patterns, suggesting that mSI during AO exhibits unique temporal characteristics compared to motor execution.
-
In motor execution, mSI typically occurs before or during movement onset19.
-
In action observation, we found that mSI was strongest after the observed movement had ended (Postvideo condition) (Figs. 2, 5, 6).
This discrepancy suggests that the role of inhibition in motor resonance is different from its role in movement execution. While mSI in execution prevents unwanted movement, mSI in AO may serve a post-movement function—helping encode and refine action representations. Our findings align with predictive coding models, which suggest that the brain first simulates observed actions, then engages inhibition in a later phase to optimize motor representations. The delayed onset of inhibition may also be linked to cortical circuits (M1, premotor cortex) rather than subcortical inhibitory circuits (e.g., basal ganglia-thalamic loops)47,48,49.
Methodological considerations and future directions
While our study offers novel insights into motor resonance, several methodological limitations should be acknowledged. One important consideration is the data exclusion criteria, as trials with MEPs below 50 µV were discarded. While this threshold was chosen to ensure reliable MEP recordings, it may have inadvertently led to an underestimation of strong inhibitory effects, particularly in muscles where inhibition was prominent.
Another limitation concerns temporal resolution, as additional TMS time points could provide a more detailed characterization of how motor resonance unfolds over time. Future studies incorporating a broader range of stimulation delays would allow for a finer-grained analysis of the temporal evolution of excitation and inhibition during AO.
Furthermore, our findings indicate that FDI exhibited clearer excitatory and inhibitory effects compared to ADM, a pattern consistent with previous studies. This suggests that the differential sensitivity of these muscles to motor resonance effects should be carefully considered when interpreting results19. Investigating additional hand muscles in future studies may provide a more comprehensive understanding of motor resonance dynamics.
A further limitation is the lack of behavioral or neurophysiological correlates, such as social cognition measures, which could help establish a direct link between motor resonance responses and individual differences. Prior research has demonstrated significant correlations between corticospinal excitability modulation during AO and social traits such as Empathy Quotient (EQ) and Autism Spectrum Quotient (AQ) scores50. Future studies incorporating these measures could provide new insights into the functional significance of motor resonance in social processing.
Finally, while the number of trials included in our analysis aligns with standard TMS protocols, previous studies suggest that recording more than 20 MEPs per condition improves reliability30. However, increasing the number of trials must be balanced against the potential for mental fatigue due to repetitive task execution, which could impact participants’ engagement and the consistency of motor resonance responses51. Future studies should carefully optimize trial numbers to maximize data robustness while minimizing participant fatigue.
Conclusion
Our findings propose a new framework for understanding motor resonance during AO, emphasizing both excitatory and inhibitory components. The Postvideo condition, which integrates video-based movement observation with post-movement stimulation, provides a novel and effective paradigm for studying MoR.
By demonstrating strong inhibitory components in motor resonance, our study refines current models of MoR and suggests that inhibition plays a crucial role in shaping motor responses during AO. Future research should further explore the temporal and neural mechanisms of motor resonance to optimize its clinical and cognitive applications.
Data availability
All data for this study is included in the Supplementary Information files.
References
Rizzolatti, G. & Craighero, L. The mirror-neuron system. Annu. Rev. Neurosci. 27, 169–192 (2004).
Oztop, E., Kawato, M. & Arbib, M. A. Mirror neurons and imitation: A computationally guided review. Neural Netw. 19, 254–271 (2006).
Umiltà, M. et al. I know what you are doing. A neurophysiological study. Neuron 31, 155–165 (2001).
Fogassi, L. et al. Space coding by premotor cortex. Exp. Brain Res. 89, 686–690 (1992).
Di Pellegrino, G., Fadiga, L., Fogassi, L., Gallese, V. & Rizzolatti, G. Understanding motor events: A neurophysiological study. Exp. Brain Res. 91, 176–180 (1992).
Fadiga, L., Fogassi, L., Pavesi, G. & Rizzolatti, G. Motor facilitation during action observation: A magnetic stimulation study. J. Neurophysiol. 73, 2608–2611 (1995).
Catmur, C., Walsh, V. & Heyes, C. Sensorimotor learning configures the human mirror system. Curr. Biol. 17, 1527–1531 (2007).
Bianco, J. et al. Bi-hemispheric effects on corticospinal excitability induced by repeated sessions of imagery versus observation of actions. Restor. Neurol. Neurosci. 30, 481–489 (2012).
Catmur, C., Walsh, V. & Heyes, C. Associative sequence learning: The role of experience in the development of imitation and the mirror system. Philos. Trans. R. Soc. B 364, 2369–2380 (2009).
Barchiesi, G. & Cattaneo, L. Early and late motor responses to action observation. Soc. Cogn. Affect. Neurosci. 8, 711–719 (2013).
Rossini, P. M., Rossi, S., Pasqualetti, P. & Tecchio, F. Corticospinal excitability modulation to hand muscles during movement imagery. Cereb. Cortex 9, 161–167 (1999).
Feurra, M. et al. State-dependent effects of transcranial oscillatory currents on the motor system during action observation. Sci. Rep. 9, 12858 (2019).
Taschereau-Dumouchel, V. et al. BDNF Val66Met polymorphism influences visuomotor associative learning and the sensitivity to action observation. Sci. Rep. 6, 1–10 (2016).
Ubaldi, S., Barchiesi, G. & Cattaneo, L. Bottom-up and top-down visuomotor responses to action observation. Cereb. Cortex 25, 1032–1041 (2015).
Errante, A. & Fogassi, L. Activation of cerebellum and basal ganglia during the observation and execution of manipulative actions. Sci. Rep. 10, 1–15 (2020).
Press, C. et al. fMRI evidence of ‘mirror’ responses to geometric shapes. PLoS ONE 7, e51934 (2012).
Beck, S. & Hallett, M. Surround inhibition is modulated by task difficulty. Clin. Neurophysiol. 121, 98–103 (2010).
Duque, J., Greenhouse, I., Labruna, L. & Ivry, R. B. Physiological markers of motor inhibition during human behavior. Trends Neurosci. 40, 219–236 (2017).
Sohn, Y. H. & Hallett, M. Surround inhibition in human motor system. Exp. Brain Res. 158, 397–404 (2004).
Wessel, J. R. & Aron, A. R. Unexpected events induce motor slowing via a brain mechanism for action-stopping with global suppressive effects. J. Neurosci. 33, 18481–18491 (2013).
Wessel, J. R. & Aron, A. R. On the globality of motor suppression: Unexpected events and their influence on behavior and cognition. Neuron 93, 259–280 (2017).
Jahanshahi, M., Obeso, I., Rothwell, J. C. & Obeso, J. A. A fronto–striato–subthalamic–pallidal network for goal-directed and habitual inhibition. Nat. Rev. Neurosci. 16, 719–732 (2015).
Wessel, J. R. & Aron, A. R. It’s not too late: The onset of the frontocentral P 3 indexes successful response inhibition in the stop-signal paradigm. Psychophysiology 52, 472–480 (2015).
Kaji, R. Basal ganglia as a sensory gating device for motor control. J. Med. Investig. 48, 142–146 (2001).
Mink, J. W. The basal ganglia: Focused selection and inhibition of competing motor programs. Prog. Neurobiol. 50, 381–425 (1996).
Mink, J. W. The basal ganglia and involuntary movements: Impaired inhibition of competing motor patterns. Arch. Neurol. 60, 1365–1368 (2003).
Puglisi, G., Leonetti, A., Cerri, G. & Borroni, P. Attention and cognitive load modulate motor resonance during action observation. Brain Cogn. 128, 7–16 (2018).
Cracco, E., De Coster, L., Andres, M. & Brass, M. Mirroring multiple agents: Motor resonance during action observation is modulated by the number of agents. Social Cognit. Affect. Neurosci. 11(9), 1422–1427 (2016).
Loporto, M., Holmes, P. S., Wright, D. J. & McAllister, C. J. Reflecting on mirror mechanisms: Motor resonance effects during action observation only present with low-intensity transcranial magnetic stimulation. PLoS ONE 8(5), e64911 (2013).
Aoyama, T., Kaneko, F., Ohashi, Y. & Kohno, Y. Neural mechanism of selective finger movement independent of synergistic movement. Exp. Brain Res. 237, 3485–3492 (2019).
Kaneko, K., Kawai, S., Fuchigami, Y., Morita, H., & Ofuji, A. (1996). The effect of current direction induced by transcranial magnetic stimulation on the corticospinal excitability in human brain. Electroencephalogr. Clin. Neurophysiol./Electromyogr. Motor Control. 101(6), 478–482.
Amoruso, L. & Urgesi, C. Contextual modulation of motor resonance during the observation of everyday actions. Neuroimage 134, 74–84 (2016).
Di Lazzaro, V. et al. The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin. Neurophysiol. 115(2), 255–266 (2004).
Brasil-Neto, J. P. et al. Optimal focal transcranial magnetic activation of the human motor cortex: Effects of coil orientation, shape of the induced current pulse, and stimulus intensity. J. Clin. Neurophysiol. 9(1), 132–136 (1992).
Mills, K. R., Boniface, S. J. & Schubert, M. Magnetic brain stimulation with a double coil: the importance of coil orientation. Electroencephalogr. Clin. Neurophysiol./Evoked Potentials Sect. 85(1), 17–21 (1992).
Lazzaro, V. D. et al. Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Exp. Brain Res. 119, 265–268 (1998).
Sartori, L., Bucchioni, G. & Castiello, U. When emulation becomes reciprocity. Social Cognit. Affect. Neurosci. 8(6), 662–669 (2013).
Cavallo, A., Heyes, C., Becchio, C., Bird, G. & Catmur, C. Timecourse of mirror and counter-mirror effects measured with transcranial magnetic stimulation. Soc. Cogn. Affect. Neurosci. 9, 1082–1088 (2014).
Rossini, P. M. et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: Basic principles and procedures for routine clinical application Report of an IFCN committee. Electroencephalogr. Clin. Neurophysiol. 91, 79–92 (1994).
Alaerts, K., Swinnen, S. P. & Wenderoth, N. Is the human primary motor cortex activated by muscular or direction-dependent features of observed movements?. Cortex 45(10), 1148–1155 (2009).
Vingerhoets, G. et al. Cerebral lateralization of praxis in right-and left-handedness: Same pattern, different strength. Hum. Brain Mapp. 33(4), 763–777 (2012).
Maeda, F., Kleiner-Fisman, G. & Pascual-Leone, A. Motor facilitation while observing hand actions: Specificity of the effect and role of observer’s orientation. J. Neurophysiol. 87, 1329–1335 (2002).
Barchiesi, G. et al. Sharing motor plans while acting jointly: A TMS study. Cortex 151, 224–239 (2022).
Kassavetis, P. et al. Motor ‘surround inhibition’is not correlated with activity in surround muscles. Eur. J. Neurosci. 40(3), 2541–2547 (2014).
Rodriguez-Sabate, C., Gonzalez, A., Perez-Darias, J. C., Morales, I. & Rodriguez, M. The causal interaction in human basal ganglia. Sci. Rep. 11, 12989 (2021).
Thirugnanasambandam, N., Khera, R., Wang, H., Kukke, S. N. & Hallett, M. Distinct interneuronal networks influence excitability of the surround during movement initiation. J. Neurophysiol. 114(2), 1102–1108 (2015).
Beck, S. & Hallett, M. Surround inhibition in the motor system. Exp. Brain Res. 210, 165–172 (2011).
Cho, H. J., Panyakaew, P., Thirugnanasambandam, N., Wu, T. & Hallett, M. Dynamic modulation of corticospinal excitability and short-latency afferent inhibition during onset and maintenance phase of selective finger movement. Clin. Neurophysiol. 127, 2343–2349 (2016).
Stagg, C. J. et al. Relationship between physiological measures of excitability and levels of glutamate and GABA in the human motor cortex. J. Physiol. 589, 5845–5855 (2011).
Lepage, J. F., Tremblay, S. & Théoret, H. Early non-specific modulation of corticospinal excitability during action observation. Eur. J. Neurosci. 31(5), 931–937 (2010).
Rozand, V., Lebon, F., Papaxanthis, C. & Lepers, R. Effect of mental fatigue on speed–accuracy trade-off. Neuroscience 297, 219–230 (2015).
Acknowledgements
This work/article is an output of a research project implemented as part of the Basic Research Program at the National Research University Higher School of Economics (HSE University) and was carried out using HSE Automated system of non-invasive brain stimulation with the possibility of synchronous registration of brain activity and registration of eye movement.
Author information
Authors and Affiliations
Contributions
C.ND.: Main author responsible for the study design, data collection, and analysis. He drafted the manuscript and integrated feedback from co-authors. A.R.: Contributed to data collection and analysis, and assisted in the interpretation of results. She provided critical revisions to the manuscript. G.P.: Contributed data analysis and interpretation, offering insights into the statistical methods used. He also contributed to manuscript editing. T.P.: Involved in the experimental design and methodology development. He contributed to the discussion of findings and manuscript revisions. O.S.: Assisted in data analysis and interpretation, offering insights into the statistical methods used. He also contributed to manuscript editing. V.M.: assisted in data analysis and contributed to manuscript editing. M.F.: Leader of the research group, overseeing the overall project direction and ensuring alignment with the group’s research goals. Matteo played a key role in the development of the research framework and methodology, and he contributed to the interpretation of results. His extensive expertise and leadership were instrumental in navigating challenges and maintaining the integrity of the research process. Additionally, he provided critical feedback on the manuscript, enhancing its rigour and impact.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Nieto-Doval, C., Ragimova, A., Perevoznyuk, G. et al. Motor resonance and inhibitory mechanisms in action observation as revealed by corticospinal excitability. Sci Rep 15, 23245 (2025). https://doi.org/10.1038/s41598-025-03989-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-03989-3