Abstract
SARS-CoV-2 (SCV2) has posed significant global challenges, necessitating improved immunization strategies to enhance protection and mitigate disease severity. Combining Bacille Calmette-Guérin (BCG) with SCV2 vaccine shows potential due to BCG’s immunomodulatory properties and ability to induce trained immunity. This study evaluates the efficacy of a combined BCG and SCV2 vaccine regimen in enhancing immune responses, controlling viral load, and reducing lung pathology following a live SCV2 challenge in a Syrian hamster model. In this controlled, randomized study, hamsters were divided into six groups and immunized with various BCG and SCV2 vaccine regimens. Hamsters were randomized into six groups: Group A received a high dose of BCG (5 × 10⁶ CFU) plus SCV2 vaccine on Days 0 and 14; Group B (control) received PBS; Group C received BCG alone; Group D received SCV2 vaccine alone; Group E received a BCG/SCV2 combination on Day 0 and SCV2 booster on Day 14; Group F received a low BCG dose (5 × 10³ CFU) plus SCV2 on both days. Post-challenge, weight changes, lung histopathology, neutralizing antibody titers, and viral load were assessed using qRT-PCR, TCID50, and histological scoring to evaluate immunogenicity, viral control, and tissue damage. Post-challenge, Group A (Combined BCG [5 × 106 CFU] and SCV2 vaccine on Day 0 and Day 14 as a booster) demonstrated stable weights and the strongest neutralizing antibody titers, effectively suppressing viral replication and showing minimal lung pathology (P < 0.05). In contrast, the control group (B) exhibited significant weight loss, high viral loads, and severe lung damage. Groups C, D, E, and F showed varying degrees of immune responses and pathology, with Group F (BCG [5 × 103 CFU]) performing better than others but less effectively than Group A. The present investigation findings highlight the potential of a combined BCG and SCV2 vaccination strategy with an emphasis on dose to provide robust protection against severe COVID-19 outcomes and underscore the role of BCG as an immunological adjuvant to improve vaccine efficacy.
Similar content being viewed by others
Introduction
SARS-CoV-2 (SCV2), the causative agent of COVID-19, has posed significant global health challenges since its emergence, necessitating the rapid development of effective vaccines, immunization strategies, and treatments1,2. Although several vaccines have been developed, emerging variants and the variability of immune responses among different populations highlight the need for alternative or supplemental immunization strategies3. Numerous studies have highlighted that SCV2 vaccination may lead to adverse reactions in certain groups4,5. On the other hand, the requirement for multiple doses for most approved vaccines is a barrier to rapid, mass vaccination and has necessitated changes in dosing schedules in some countries to ensure sufficient vaccine coverage6. One promising approach to enhance immunity and improve vaccination performance against SCV2 involves using Bacille Calmette-Guérin (BCG), which has been traditionally used for tuberculosis prevention and is known for its broad immunomodulatory effects7,8,9,10. BCG vaccination has been shown to stimulate the innate immune system and potentially enhance immune responses to other pathogens, including viruses. Studies indicate that BCG can induce trained immunity, improving responses to various pathogens, including viruses11,12,13.
In this regard, recent studies have examined combining the Bacille Calmette-Guérin (BCG) vaccine with SCV2-specific vaccines to enhance immunity. Substantial evidence has shown that BCG can confer broad, non-specific immune benefits, offering protection against various pathogens, particularly respiratory infections14,15,16. Notably, BCG vaccination has been demonstrated to reduce the incidence of viral respiratory tract infections in older adults (over 65 years) without significant adverse effects17. This protective effect is attributed to BCG’s ability to induce “trained immunity,” a reprogramming of innate immune responses that provides cross-protection against various diseases12,18,19. Leveraging BCG’s immune-boosting properties in conjunction with targeted COVID-19 vaccines could potentially enhance the immune response, mitigate lung inflammation, and improve protection against SCV2 and its severe complications20. This approach may provide a complementary strategy to existing vaccines, which often require multiple doses and may not be effective against all variants21,22. While BCG shows potential, its effectiveness as a universal vaccine for SCV2 is not yet fully established, and further research is needed to clarify its long-term impact on immunity, viral control, immune response durability, and lung histopathology23,24.
The Syrian hamster model is widely recognized for its immunological similarity to humans, particularly in respiratory infections, making it a suitable and translationally relevant system for studying vaccine efficacy and viral pathogenesis25,26,27.
This study was designed to evaluate the efficacy of a combined BCG and inactivated SCV2 vaccine regimen, with a particular focus on histopathological and immunological outcomes in lung tissue following live SCV2 challenge. By comparing the effects of combined BCG/SCV2, BCG-only, SCV2-only, and Variety of combined vaccination, this study aims to determine whether the combination can enhance immune response, control viral load, and mitigate lung damage more effectively than either vaccine alone. The findings may offer insights into an optimized vaccination strategy to provide more robust, less risky, and more durable protection against SCV2.
Materials and methods
Materials
The Mycobacterium bovis bacilli Calmette-Guérin (BCG) (Pasteur strain) used in this study was sourced from the Pasteur Institute in Tehran, Iran. Wild-type SARS-CoV-2 strain (SCV2/Wuhan-1/2020) was obtained from the Razi Vaccine and Serum Research Institute (RVSRI) in Iran, cultured, and stored in single-use aliquots at temperatures below − 60 °C. The inactivated SCV2 vaccine, combined with the adjuvant Alhydrogel® (a 2% wet gel suspension of aluminum hydroxide from Brenntag Biosector, Denmark), was prepared at the RVSRI in Iran. Middlebrook 7H9 media was acquired from Becton Dickinson (BD), New Jersey, USA, to support BCG culture. Dulbecco’s Modified Eagle Medium (DMEM), used as the cell culture medium, was obtained from Thermo Fisher Scientific Gibco (cat. no. 11960-085). Phosphate-buffered saline (PBS) was sourced from Severn Biotech (cat. no. 207410) or an equivalent supplier.
For virus propagation and assay setups, Vero E6 cells were provided by the European Collection of Authenticated Cell Cultures (ECACC, cat. no. 85020206). Formalin solution, 40% (wt/vol), used for tissue fixation, was acquired from Sigma (cat. no. F8775). Sterile, 96-well, low-absorption, V-bottomed plates with lids (Thermo Fisher Scientific, cat. no. 249935) were used for the virus neutralization test (VNT) and other cell culture assays.
Study design
The study was structured as a controlled, randomized trial comprising six groups of Laboratory Animals: five experimental groups (Groups A, C, D, E, and F) and a control group (Group B) (Fig. 1). Each experimental group consisted of six animals, while the control group included twelve animals, allowing for enhanced statistical power in evaluating control outcomes. Animals in the experimental groups received distinct vaccination regimens to assess the immunostimulatory effects of BCG in combination with SCV2 vaccines, with variations in dosage and administration strategy (Fig. 1). In order to maintain blinding, all groups were randomly assigned and labeled to anonymize the treatment status of study personnel involved in the post-vaccination analysis.
For experimental groups, immunizations commenced on day zero, where animals received a primary intramuscular injection in the posterior thigh, with an injection volume of 50 µL. Each immunization involved either a combination of BCG and SCV2 vaccines or standalone BCG or SCV2 vaccines, as per each group’s protocol. A booster dose was administered on day 14, following the same regimen and injection protocol specific to each experimental group. The control group received a placebo injection of PBS on both day 0 and day 14 to serve as a baseline for comparative immunogenic response (Fig. 1). When animals received a combination of two vaccines (BCG & SCV2), the inoculum was prepared in endotoxin-free TE buffer.
On day 30, after the booster dose had ample time to elicit an immune response, all animals, including those in the control group, were challenged with a standardized inoculation of wild-type SCV2. The viral inoculum consisted of 5 × 106 TCID50 of SCV2 virus diluted in 100 µL of DMEM, with 50 µL administered intranasally to each nasal passage to maximize viral exposure.
Post-challenge clinical monitoring involved daily health observations and periodic weight measurements as health and immune response efficacy indicators. Weights were recorded on days 30, 33, 36, and 39 post-challenge to monitor changes indicative of immune status and disease progression. Significant weight loss in hamsters can suggest viral infection severity or insufficient immune response, marking weight as a valuable non-invasive marker of health status.
In order to evaluate immunogenicity and virological assessments (viral load), a necropsy was performed on three animals from each group on days 33, 36, and 39 post-challenge. To ensure the absence of pain and distress, the hamsters were euthanized under deep anesthesia with isoflurane by severing the spinal cord at the occipital region and collecting blood from the cervical veins. Isoflurane was used as the anesthetic agent, administered via inhalation at a concentration of 5% for induction, and maintained at 2–3% using a calibrated vaporizer with 100% oxygen flow. Once a surgical plane of anesthesia was confirmed by the absence of reflexes (e.g., pedal withdrawal reflex), a high dose of isoflurane was continued until euthanasia was complete. Under sterile conditions, lung tissues from each animal were carefully dissected and homogenized in equal weights to assess viral load. Additionally, lung tissue sections from each animal in each group were collected aseptically during necropsy and fixed in 10% neutral-buffered formalin for 24–48 h to facilitate histopathological examination, enabling precise assessment of viral presence and immune-induced pathological changes. This approach facilitated an accurate evaluation of the virus distribution and immune response within the body.
Laboratory animals, housing conditions, and ethics
In this study, pathogen-free male Syrian hamsters (Mesocricetus auratus), specifically golden Syrian hamsters (6–8 weeks of age), were used. The animals were obtained from the RVSRI in Iran. The hamsters were housed under controlled environmental conditions in pathogen-free ventilated cages at a temperature range of 22–24 °C and relative humidity of 45–55%. A 12-hour light-dark cycle was maintained to minimize circadian variations. Each hamster was provided with ad libitum access to sterilized water and a diet formulated to meet the specific nutritional needs of Syrian hamsters. All animals underwent a 30-day acclimatization period before the start of the experiment to ensure stability in physiological and immune responses.
Following acclimatization, the hamsters were transferred to a biosafety level 3 (BSL-3) containment laboratory at RVSRI, which allowed for secure handling and manipulation of SCV2. All experimental protocols were approved by the Ethics Committee of the RVSRI (Ethics code: IR.RVSRI.REC.1399.001). The methods employed in this study were conducted in accordance with the ARRIVE guidelines (https://arriveguidelines.org) and complied with all relevant animal welfare regulations to ensure ethical and humane treatment of laboratory animals28,29. During the studies, all animals were monitored daily by the animal resources center or laboratory staff.
Immunological assessments
Blood samples were collected from the animals on the 14 th day before receiving the booster and again on the 30 th day before the viral challenge. Following the challenge, additional blood samples were taken on days 33, 36, and 39 from three selected animals each day prior to euthanasia. These samples provided the serum necessary for immunometric evaluations.
The QuantiVac ELISA (enzyme-linked immunosorbent assay IgG) kit (Euroimmun, Germany) was used to measure the titration of anti-S1 IgG (receptor-binding domain [RBD]) antibodies in serum samples prepared on the 30 th day. Additionally, a microneutralization assay (MNA) was conducted according to the method of Bewley et al. using the wild strain of SCV2 to determine serum-neutralizing antibody titers, assessing the animals’ immunological response to the virus and vaccine efficacy30.
Preparation of BCG vaccine suspension
The BCG vaccine was prepared by culturing M. bovis BCG (strain Pasteur) at 37 °C in Middlebrook 7H9 liquid media supplemented with 0.5% glycerol, 0.02% Tyloxapol, and 10% albumin-dextrose-catalase (ADC) or on solid Middlebrook 7H11 media with oleic acid–ADC supplement. The growth conditions facilitated optimal bacterial proliferation necessary for vaccination purposes. To prepare single-cell suspensions suitable for accurate dosing, cultures in the exponential growth phase (OD600 = 0.6) were harvested, washed in PBS, and then subjected to mechanical processing. This process included ten successive passages through a 27-gauge syringe, followed by brief sonication to further disaggregate bacterial clumps. A low-speed centrifugation step was conducted for 10 min to separate and discard any remaining bacterial aggregates, ensuring a uniform suspension.
The prepared BCG suspensions were aliquoted and stored at − 80 °C in PBS containing 20% glycerol to maintain viability. For vaccine administration, colony-forming units (CFU) were determined by plating on supplemented Middlebrook 7H11 agar plates. Two doses of 5 × 106 CFU and 5 × 103 CFU were prepared for vaccination.
Preparation of Wild-Type SCV2 strain for challenge
The SCV2 wild strain was propagated in Vero E6 maintained in DMEM supplemented with 10% fetal bovine serum (FBS) and 1% Pen/Strep to support optimal viral replication. The cells were incubated at 37 °C in a 5% CO₂ atmosphere. At approximately 72 h post-infection, the virus culture supernatant was collected. This timing coincided with peak viral replication, as indicated by a pronounced cytopathic effect (CPE) visible under microscopy, signifying a high viral titer. The supernatant was then clarified by low-speed centrifugation to remove cellular debris and filtered through a 0.22 μm filter to ensure purity, thus yielding a clean viral suspension suitable for the experimental challenge. The viral titer was quantified using the TCID50 assay, following the Reed and Muench method. This approach allowed for precise determination of the tissue culture infectious dose required to produce a CPE in 50% of the inoculated wells. Serial dilutions of the virus were prepared and applied to Vero E6 cells in a 96-well plate format, with results recorded based on the presence or absence of CPE. The Reed and Muench calculation was then applied to determine the TCID50/ml31, ensuring accurate dosage (5 × 106 TCID50) for the in vivo challenge experiments.
Histopathology examination
Following fixation of the lung tissue in 10% neutral-buffered formalin for 24–48 h, the fixed tissues were then processed by dehydration through graded alcohols, cleared in xylene, and embedded in paraffin wax. Paraffin-embedded tissue blocks were sectioned at a thickness of 4–5 micrometers using a rotary microtome. The sections were mounted on glass slides and stained with hematoxylin and eosin (H&E) to allow a detailed microscopic evaluation of tissue architecture and inflammatory changes. The lung sections were examined under a light microscope at varying magnifications to assess the presence and severity of histopathological lesions, such as inflammatory cell infiltration, alveolar damage, hemorrhage, and edema, which are indicative of SCV2 infection and immune response.
The scoring system for lesion severity was as follows: a score of 0 indicated normal tissue with no detectable lesions, a score of 1 represented mild minimal lesions, a score of 2 indicated mild but more extensive lesions, a score of 3 represented moderate lesions, and a score of 4 indicated severe, extensive lesions.
Viral load quantification
Quantitative reverse transcription polymerase chain reaction (qRT-PCR) was utilized to determine the viral load in lung tissue samples. This method enabled precise quantification of SCV2 RNA, providing insights into viral replication and distribution within the tissues.
Homogenized tissue samples were initially processed for RNA extraction using the SinaPure Viral kit (SinaClon Co., Iran), following the protocol provided by the manufacturer. The quality and concentration of the extracted RNA were assessed using a NanoDrop spectrophotometer to ensure adequate purity and yield for subsequent qRT-PCR analysis. The extracted RNA was reverse-transcribed into complementary DNA (cDNA) using the SinaClon First Strand cDNA Synthesis Kit (Iran).
Viral load quantification was conducted using qRT-PCR with the COVID-19-RdRp/spike [S] assay, as previously described32,33. SCV2 RNA detection was performed with the QuantiNova Probe RT-PCR kit (Qiagen, Germany) on a LightCycler 480 real-time PCR system (Roche, Basel, Switzerland). This assay enabled precise quantification of viral RNA levels, allowing for the assessment of viral replication in lung tissue samples.
The TCID50 was calculated to determine viral infectivity in lung tissue samples. Serial tenfold dilutions of viral supernatants were prepared and inoculated into Vero E6 cell cultures in 96-well plates. Following incubation, the presence or absence of CPE in each well was recorded. TCID values were calculated using the Reed and Muench method, which estimates the viral dose required to infect 50% of the cultured cells34,35. This quantification provides a reliable measure of viral load and infectivity.
Statistical analysis
Data were analyzed to assess the impact of each vaccine regimen on immune responses, lung pathology, and viral load in lung tissue. Histopathology scores on days 33, 36, and 39 post-challenge were analyzed using one-way ANOVA with Tukey’s post-hoc test to determine differences across groups and time points, presented as mean ± standard deviation (SD). Neutralizing antibody titers (FRNT50) measured on days 14, 30, 33, 36, and 39 were analyzed using repeated measures ANOVA for within-group comparisons and two-way ANOVA with Bonferroni correction for between-group comparisons to assess immune response levels. Real-time PCR cycle threshold (CT) values, indicating viral load in lung tissue on days 3, 6, and 9 post-challenge, were analyzed with two-way ANOVA to compare viral control across groups and over time. Viral titers, expressed as TCID50/mL and measured at the same time points, were log-transformed and analyzed using one-way ANOVA with Tukey’s test for between-group differences and repeated measures ANOVA for within-group analysis. Significance was set at P ≤ 0.05, with individual data points shown alongside mean ± SD or SEM. Analyses were conducted in GraphPad Prism (GraphPad Software, San Diego, CA, USA).
Results
Weight changes in study groups Post-Challenge
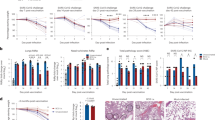
Baseline weights recorded on day 30 before the challenge showed similar averages across all groups, indicating uniformity in initial health status. Following the viral challenge on day 30, distinct trends in weight changes emerged among groups. Daily weight monitoring revealed that hamsters vaccinated with the combined BCG and SCV2 regimen (Group A) maintained stable weights over six days, experiencing no significant weight loss (P < 0.05). In contrast, the control group (Group B) and other experimental groups displayed a marked weight reduction, with a loss of approximately 20% by day six (Fig. 2).
Statistical analysis further indicated that Group E experienced the most pronounced weight loss among all groups. At the same time, Group A and F exhibited the least, reinforcing the potential protective effect of the combined vaccine regimen. Additionally, while all groups experienced some degree of weight fluctuation post-challenge, Group A displayed a mild overall weight gain, particularly noticeable in subsequent days. Group F, meanwhile, showed an initial weight decline by the third-day post-challenge, followed by a recovery and an overall weight increase by the sixth and ninth days. Nonetheless, these results highlight that weight changes post-challenge varied among groups, with Group A exhibiting the most consistent weight stability. These findings suggest that BCG/SCV2-vaccinated hamsters may experience better protection against SCV2.
Trends in average body weight across study groups (A-F) before and after the SCV2 challenge show differential responses to infection. Group A (BCG/SCV2-vaccinated) maintained stable body weight post-challenge. At the same time, the control group and other experimental groups exhibited varying degrees of weight loss, with significant reductions in Groups B and E by day six.
Immunological results
The analysis of neutralizing antibody titers (FRNT50) revealed notable differences among the study groups (Fig. 3). In summary, Groups A and F, and to a lesser extent Group E, demonstrated the strongest neutralizing antibody responses both on day 30 and after the challenge, indicating that their respective vaccine regimens effectively stimulated the immune system. Groups B (control) and C exhibited limited responses, with Group B showing the weakest immune response, as expected for an unvaccinated group.
On the 14 th day (before the booster), neutralizing antibody titers were uniformly low across all groups, establishing a baseline with minimal immune activation. Following the booster dose, on the 30 th day, Groups A, F, D, and E, respectively, demonstrated significant increases in antibody titers. Group A showed the highest and most favorable titers, indicating a strong and consistent immune activation within this group due to the combined BCG/SCV2 vaccine regimen. Group F also exhibited a robust titer on the 30 th day, reflecting strong immune activation. Groups E and D showed comparable and moderate increases in titers, suggesting effective but significantly lower immune responses compared to Groups A and F. In contrast, the control group, Group B, as expected in the absence of vaccination, showed no neutralizing antibody titers against live SCV2 from day 14 to day 30. Similarly, Group C, which received only the BCG vaccine, exhibited a response similar to the control group on both the 14 th and 30 th days (Fig. 3).
The ELISA test conducted on day 30 pre-boost showed a clear distinction in neutralizing antibody titers among the study groups (Fig. 4). Groups A and F, which received the combined BCG and SCV2 vaccine regimen, exhibited the highest antibody titers, with median values approaching 1.25, indicating a strong immune response even prior to the booster dose. Group A showed a slightly broader range, suggesting some variability within the group, but overall displayed a robust response. In contrast, Groups C, D, E, and B had markedly lower antibody titers. Groups C (BCG-only) and B (control) displayed the weakest titers, with median values near 0.25, reflecting minimal immunogenicity. Groups D and E had slightly higher titers, around 0.5, but still significantly lower than Groups A and F.
Following the SCV2 challenge, additional variations in immune response dynamics were observed. By day 3 post-infection, Group A maintained the highest titers, showing a rapid and robust immune response to the viral challenge, which remained elevated through days 36 and 39. Group E had lower antibody levels than Group F on day 33 but did not maintain high antibody levels on days 36 and 39. Group F exhibited high titers with a slightly delayed response, showing a moderate increase in titers from day 36 to 39; however, its performance was still inferior to that of Group A. Group D displayed a moderate antibody response post-challenge, though it was not as pronounced as the responses observed in Groups A, E, and F. The control group, Group B, along with Group C, consistently displayed low titers throughout the post-challenge period, indicating a minimal immune response (Fig. 3).
Results of viral load and qRT-PCR analysis in lung tissue
The analysis of viral load in lung tissue using qRT-PCR and TCID50 titers across the six study groups revealed distinct differences in the ability of each vaccination regimen to control SCV2 replication post-challenge (Fig. 3). In summary, Groups A and F demonstrated the strongest control over viral replication in lung tissue, with Group A achieving complete suppression of detectable viral load, highlighting its superior efficacy. Groups B, C, D, and E showed progressively higher viral titers, indicating less effective immune responses, with Group B exhibiting the highest viral loads due to the absence of vaccination.
Group A, which received the combined BCG/SCV2 vaccine, showed consistently high CT values at 3, 6, and 9 days post-challenge, indicating no detectable viral load in the lung tissue. This suggests that the combined vaccine was highly effective in preventing viral replication, potentially achieving viral clearance and offering long-term protection. The low or undetectable viral titers in this group reinforce the strong immunogenic response induced by this regimen. In contrast, the control group, Group B, demonstrated low CT values across all time points, corresponding to high viral loads in lung tissue. The lack of vaccination in this group resulted in uncontrolled viral replication, with progressively increasing viral titers, highlighting the extent of viral proliferation when no immune defense was provided.
Group C, which received only the BCG vaccine, showed moderately low CT values and relatively high viral titers at each time point. This indicates that while the BCG vaccine provided some degree of protection, it was insufficient to fully prevent SCV2 replication, resulting in substantial viral load within the lung tissue. Group D, receiving the SCV2 vaccine alone, displayed moderate CT values and corresponding viral loads. Although this group showed an intermediate level of control, the absence of broader immune stimulation, such as that potentially offered by BCG, limited its effectiveness in completely suppressing viral replication. Group E, which received a BCG/SCV2 vaccine with an additional SCV2 booster, exhibited CT values that were generally lower than those of Groups A and F, indicating higher viral loads. While the booster provided some level of viral control, it was not as effective as the primary BCG/SCV2 combined regimen seen in Group A, resulting in moderate viral proliferation within lung tissue. Group F, receiving a low-dose BCG/SCV2 vaccine, also exhibited relatively high CT values, though slightly lower than Group A, suggesting effective but partial control of viral replication. The low viral titers observed indicate that while protection was not as robust as in Group A, viral proliferation was still significantly limited.
Histopathological evaluation results
The histopathological analysis of lung tissues across the six groups demonstrated levels of inflammatory responses and tissue damage post-SCV2 challenge. The histopathological scores (Fig. 3 – (b, c, and d)) highlight Group A as having the least lung pathology among vaccinated groups, demonstrating the efficacy of the combined BCG/SCV2 vaccine in reducing inflammation. In contrast, Groups B and E showed higher levels of tissue damage, with Group B (control) displaying the most severe pathology due to the absence of vaccination. Group A, which received the combined BCG and SCV2 vaccine on day 0 and a booster of the same on day 14, exhibited mild inflammation. Observations on days 33 and 36 showed focal bronchitis and minimal interstitial pneumonia, suggesting limited immune-mediated lung damage. By day 39, inflammation had significantly reduced, with some samples showing no pathological findings, suggesting effective resolution of inflammation. Additional observations included occasional mild perivasculitis and minimal arterial endothelial leukocyte margination. The majority of samples scored between 1 and 2, reflecting the lowest pathology among all groups (Fig. 5 - Group A).
The control group (Group B) exhibited severe inflammatory reactions, with pronounced interstitial pneumonia, bronchiolitis, bronchitis neutrophilia, vasculitis, perivascular edema, and arterial endothelial leukocyte margination, particularly evident on days 36 and 39. On day 33 post-challenge, the lung tissue exhibited signs of bronchiolitis, interstitial pneumonia, and arterial endothelial leukocyte margination, with accompanying vasculitis, bronchitis, and perivascular edema. Additional findings included perivascular edema and frequent hemorrhages, indicative of extensive tissue damage and uncontrolled immune response. Scores in this group ranged from 3 to 4, demonstrating significant lung pathology in the absence of vaccination (Fig. 5 - Group B). Mild to moderate inflammation was observed in Group C. On day 33, histopathological findings included bronchiolitis, minimal interstitial pneumonia, perivasculitis, and interstitial pneumonia. However, by days 36 and 39, inflammation intensified with additional observations of vasculitis, bronchiolitis, severe interstitial pneumonia, mild hemorrhage, arterial endothelial leukocyte margination and neutrophilia, reflecting an active but partial immune response. The scores for this group were predominantly in the range of 2 to 3, suggesting moderate tissue involvement (Fig. 5 - Group c).
Pathological examinations for Group D revealed moderate to severe inflammation, with an increase in severity from day 33 to day 39. Histopathological findings included pulmonary edema, frequent hemorrhages, and extensive interstitial pneumonia, indicating progressive inflammatory damage. Bronchiolitis and arterial endothelial leukocyte margination were also noted, reflecting a strong but unregulated immune response. Scores ranged from 2 to 3, indicative of moderate to significant pathology. (Fig. 5 - Group D). Group E showed moderate to severe inflammation. On day 33, moderate interstitial pneumonia and bronchiolitis were observed, which progressed to severe inflammation by days 36 and 39. Additional findings included extensive perivascular edema and hemorrhages, suggesting a robust but less controlled immune response. Scores averaged around 3, indicating considerable lung tissue damage (Fig. 5 - Group E). In Group F, Mild pathology was initially observed, with minimal findings such as small foci of perivascular mononuclear cells on day 33. However, by day 39, severe interstitial pneumonia and vascular involvement, including hemorrhages and arterial endothelial leukocytic margination, were noted in some animals, indicating a late but significant inflammatory response. Scores in this group ranged from 1 to 3, reflecting mild to moderate lung damage (Fig. 5 - Group F).
Evaluation of histopathological and immunological parameters in SCV2-challenged hamster groups (A–F) at multiple time points. (a) The animal study timeline shows vaccination, booster, and challenge schedules alongside sampling points. (b–d) Lung histopathology scores on days 33, 36, and 39 post-challenge: Group A consistently demonstrated the least tissue damage, with the lowest histopathological scores, while Group B (control) exhibited the most severe pathology. (e–f) Neutralizing antibody titers (FRNT50) before and after booster vaccination (days 14 and 30) and post-challenge (days 33, 36, and 39), highlighting robust immune responses in Groups A and F. (g) Real-time PCR CT values for viral titers in lung tissue on days 3, 6, and 9 post-challenge: Higher CT values in Groups A and F reflect effective viral control, with Group B displaying the lowest CT values and highest viral loads. (h) Viral titers (TCID50) in lung tissue at days 3, 6, and 9 post-challenge: Group A showed the lowest titers, demonstrating superior viral suppression compared to other groups. Abbreviations: CT – Cycle Threshold; FRNT50 – Focus Reduction Neutralization Test, 50% endpoint; TCID50 – Tissue Culture Infectious Dose, 50% endpoint. Each circle represents an individual data point, while error bars (mean ± SD or SEM) depict data variation within each group. Statistical tests assessed significant differences between groups across time points.
Neutralizing antibody ELISA titers across study groups measured 30 days post-vaccination boost (dpv). Box plots illustrate the distribution and variability of ELISA titers within each group, indicating the degree of immune response elicited by each vaccination regimen. Groups A and F, which received a combined BCG and SCV2 vaccine, demonstrated the highest median titers (approaching 1.25), suggesting strong immunogenicity. Group A exhibited a wider range of responses, reflecting greater variability among individuals. In contrast, Groups C (BCG-only), B (control), D, and E showed markedly lower titers, with medians closer to 0.25, indicating weaker or minimal immune responses. These findings underscore the enhanced antibody production associated with the BCG/SCV2 combination strategy.
Representative histopathology images of lung tissue from groups A to F on days 33, 36, and 39 post-challenge. Each row corresponds to a different study group (A–F), with columns showing the progression of histopathological changes over time. Group A. No significant pathologic lesion to mild interstitial pneumonia and congestion. In contrast, group B. (control) moderate to severe interstitial pneumonia, hemorrhage, and bronchitis. Group C. mild intra alveolar wall thickness, congestion to mild inflammatory infiltration. Group D. moderate to severe interstitial pneumonia, hemorrhage to pulmonary consolidation. Group E. No significant pathologic lesion to mild interstitial pneumonia and hemorrhage. Group F. mild to moderate interstitial pneumonia, hemorrhage, and bronchitis. Staining: Hematoxylin and eosin (H and E), magnification ×40.
Discussion
Ensuring global access and distribution of vaccines to low- and middle-income countries is essential for controlling the COVID-19 pandemic. Vaccines need to provide strong protective immunity, be cost-effective to produce, and avoid causing adverse effects in recipients, and research such as the present study offers insights that could help address these challenges7. The current investigation, conducted in a Syrian hamster model, provides valuable insights into the effectiveness of a combined BCG/SCV2 vaccination strategy against SCV2 infection. The present study findings suggest that the BCG/SCV2 immunization approach, especially in Groups A and F, offers robust protection, limits viral spread, and significantly reduces pulmonary pathology, morbidity, and mortality associated with SCV2 infection. Both Groups A and F, which received BCG and SCV2 vaccines, exhibited strong immune responses, though Group A, which received a higher BCG dose, demonstrated the most favorable outcomes with fewer unwanted side effects. This highlights a critical dose-dependent aspect of the BCG vaccine’s effectiveness in enhancing protection against SCV2. The findings emphasize the importance of dosage in BCG’s efficacy, with Group A showing the most favorable outcomes.
BCG may induce trained immunity, enhancing the innate immune response against SCV236. In the study by Singh et al., immunization with two BCG/SCV2 vaccines increased the recruitment of immune cells, including alveolar macrophages and T cells, contributing to stronger lung immunity37. BCG has been demonstrated to reprogram myeloid cells and natural killer (NK) cells through mechanisms collectively known as “trained immunity.” Once phagocytosed by macrophages, BCG initiates epigenetic and metabolomic changes that elevate the immune readiness of these cells upon re-exposure to different antigens, including viruses38,39. BCG-trained macrophages exhibit enhanced cytokine production and adopt M1-like phenotypes upon re-challenge with heterologous antigens. These processes are linked to the activation of B and T lymphocytes, elevated antibody levels40, and the expansion of unconventional T cells such as innate lymphoid cells (ILCs) and mucosa-associated invariant T (MAIT) cells41,42. Furthermore, BCG exposure leads to an increase in “trained” hematopoietic stem cells (HSCs) and multipotent progenitors (MPPs) in the bone marrow, providing enhanced defense against future pathogen challenges43,44.
The elevated levels of neutralizing antibodies observed in Groups A and F further support the immunogenic potency of the BCG/SCV2 regimen. The significantly higher antibody titers in these groups indicate a stronger humoral response compared to other groups, underscoring the potential of BCG to synergize with SCV2 vaccination in inducing a broad and durable immune response. The improved immunogenicity in Groups A and F suggests that the combined BCG/SCV2 approach may bolster both innate and adaptive immunity, potentially offering more comprehensive protection than SCV2 vaccination alone. In contrast, Groups C (BCG-only) and B (control) showed minimal immune response, with higher viral titers and extensive lung pathology, underscoring the insufficiency of BCG alone or the absence of vaccination in controlling SCV2 infection. While BCG alone has some immunomodulatory effects, it lacks the specificity required to provide substantial protection against SCV2, as evidenced by the absence of significant neutralizing antibody production in Group C. The poor performance in these groups emphasizes the need for a targeted SCV2 component to achieve effective immune protection.
In comparing the present study with the study by Singh et al., several key differences and similarities emerge. Both studies utilized golden Syrian hamsters as the experimental model and employed a similar SCV2 challenge method to evaluate the efficacy of BCG vaccination. In the study by Singh et al., BCG vaccination was administered intravenously, whereas in the current study, it was delivered via intramuscular injection in the posterior thigh. However, while Singh et al. focused on a single dose of BCG37, the present study explored a more diverse set of experimental conditions, including six distinct groups with varied vaccination regimens, such as combined BCG and SCV2 vaccines (Groups A and F) and two different BCG doses. This diversity allowed for a broader evaluation of the combined vaccine approach and its dose-dependent effects on immune responses and pathology.
The observed weight changes in hamsters following the viral challenge revealed notable differences in resilience among the various vaccination regimens. Hamsters in the combined BCG and SCV2 vaccination group (Group A) maintained stable body weights throughout the study, indicating enhanced protection against SCV2. In contrast, the control group (Group B) and other experimental groups exhibited significant weight loss, suggesting a lack of sufficient immune protection. The stability in Group A may be attributed to the immunomodulatory effects of BCG, which has been shown to reduce inflammation and improve immune responses, thereby mitigating disease severity and preserving overall health45,46,47.
A key finding in the present study was the reduction in viral load and lung pathology in Groups A and F. The limited viral replication and fewer pulmonary lesions observed in these groups suggest that the combined vaccine regimen effectively prevented extensive tissue damage and immune overreaction, such as cytokine storms. Notably, Group A demonstrated a more pronounced reduction in inflammatory markers, including cytokine levels and neutrophil infiltration, compared to Group F. This finding aligns with the hypothesis that the dose of BCG directly influences its immunomodulatory capacity, helping prevent excessive inflammatory responses that are typically associated with severe SCV2 outcomes. BCG vaccination can reverse T cell lymphopenia and reduce lung granulocyte levels, indicating a protective role against immunopathology48,49. The results of the current study expand upon Singh et al.‘s findings by showing that the combined BCG/SCV2 approach can produce a more comprehensive immune response, with substantial reductions in viral load and pulmonary damage. While Singh et al. demonstrated BCG’s ability to modulate immune cell recruitment and transcriptional activity, favoring repair, the current study illustrates that adding SCV2 to the BCG regimen enhances both humoral and cellular immunity. The combined approach not only led to early antibody production but also demonstrated dose-dependent immune modulation, underscoring the potential benefits of an optimized BCG/SCV2 strategy for effective and sustained protection against SCV250. A study by Ravichandran et al. found that administering two doses of vaccines such as COVAXIN (ChAdOx1 nCoV-19) and COVISHIELD, was effective in preventing severe lung involvement, highlighting the crucial role of vaccination in controlling respiratory infections51.
Singh et al. reported that a single dose of BCG reduced lung viral load and bronchopneumonia while promoting an immune response characterized by increased alveolar macrophages, Th1 and Th17 cells, and reduced lung granulocytes. Additionally, they observed transcriptional shifts favoring immune tolerance and lung repair. In contrast, our study’s combined BCG/SCV2 approach yielded even more robust protection, with significantly lower viral titers and reduced lung pathology in Groups A and F. The higher dose of BCG in Group A correlated with an enhanced immunogenic response, fewer inflammatory lesions, and fewer side effects, demonstrating the potential added benefit of dose optimization and combination with SCV2 for enhanced protection. Overall, while both studies highlight BCG’s potential against SCV2, the present study expands on Singh et al.‘s findings by providing evidence that combined BCG and SCV2 regimens, especially with higher doses of BCG, can yield superior immunoprotection and minimize SCV2 pathogenesis more effectively37.
An important observation from the current study is the limited ability of neutralizing antibodies alone to fully control SCV2 infection in certain groups. While neutralizing antibodies are critical for virus neutralization, our findings suggest that cellular immune responses, particularly those induced by BCG, play a crucial role in controlling infection and mitigating inflammatory damage. Neutralizing antibodies are essential for blocking viral entry and replication. However, their effectiveness can vary across different demographic groups, indicating a need for additional immune strategies. Combining BCG with specific SCV2 vaccine components could optimize protection and improve clinical outcomes11. This highlights the unique role of BCG in inducing “trained immunity” through reprogramming innate immune cells, which may provide a broader immune defense against the virus. The failure of BCG alone to prevent severe outcomes, as observed in Group C, underlines the necessity of combining BCG with a specific SCV2 vaccine component for optimal protection.
In comparing the present study with that of Ramírez et al., both studies explore the use of BCG-based vaccines in enhancing immune protection against SCV2. However, while the current study utilized traditional BCG combined with the SCV2 vaccine in Syrian hamsters to assess the impact on viral load, pulmonary pathology, and antibody titers, Ramírez et al. employed a recombinant BCG (rBCG) expressing the SCV2 nucleoprotein (N) along with additional recombinant SCV2 proteins (S and RBD-Omicron) in BALB/c mice. This rBCG-based approach by Ramírez et al. focused on generating a strong antigen-specific cellular response, particularly CD4 + and CD8 + T cells, and observed cytokine profiles with antiviral properties, as well as robust antibody titers against multiple SCV2 antigens. Both studies underscore the potential of BCG-based strategies for SCV2, though the present study highlights dose-dependent immune modulation and viral control with traditional SCV2 combinations, whereas Ramírez et al. emphasize the use of genetically engineered BCG for broader antigen-specific responses, suggesting potential applications for variant-specific immunity11.
When comparing the present study with that of Hilligan et al., both investigations examine the potential of BCG vaccination to confer protection against SCV2 infection, emphasizing BCG’s role in boosting innate immunity against unrelated respiratory pathogens. However, while the current study used intramuscular administration of BCG combined with the SCV2 vaccine in Syrian hamsters, Hilligan et al. focused on intravenous BCG administration in human-ACE2 transgenic mice and non-transgenic mice. Hilligan et al. demonstrated that intravenous BCG inoculation reduced viral loads, tissue pathology, and inflammatory markers following SCV2 infection, particularly for the α variant. In contrast, the present study revealed that intramuscular BCG/SCV2 vaccination effectively reduced lung pathology and viral load, with a focus on dose-dependent immune modulation and reduced inflammatory responses. While Hilligan et al. linked BCG’s protective effects to changes in the pulmonary cellular compartment impacting the innate immune response, the current study observed enhanced immunogenicity and protection through combined vaccination strategies52.
Another study by Hilligan et al. (2023) investigated the potential of BCG vaccination to offer non-specific protection against SCV2, sharing some commonalities with the present study, though the approaches and outcomes varied considerably. Hilligan et al. focused on the intravenous administration of BCG, demonstrating strong protection against SCV2 infection in small animal models, including hamsters and mice, with reduced viral loads and disease severity. They highlighted that conventional intradermal or subcutaneous BCG administration—common in clinical settings—did not confer similar protective effects, thus questioning its efficacy in preventing COVID-19. In contrast, the present study employed intramuscular BCG combined with an SCV2 vaccine in Syrian hamsters, finding that this combination effectively reduced lung pathology, viral spread, and inflammation, particularly in high-dose BCG groups48. While Hilligan et al. emphasize the superior protective effect of intravenous BCG, a route not clinically viable, the present study suggests that a combined BCG/SCV2 intramuscular approach may offer a more practical and effective alternative for enhancing immune responses and mitigating SCV2 pathogenesis in a clinically relevant manner.
A comparable study by White et al. took a similar focus but employed different methodologies and yielded varying results compared to the present study. White et al. explored aerosol delivery of BCG in rhesus macaques, hypothesizing that targeting the respiratory tract could enhance cross-protective effects against COV2. While they observed that aerosolized BCG vaccination primed innate immune responses, including increased cytokine and chemokine secretion and activation of unconventional T-cell populations, it did not reduce viral loads or disease severity following the SCV2 challenge. In contrast, the current study employed an intramuscular administration of BCG combined with the SCV2 vaccine in Syrian hamsters, leading to significantly reduced lung pathology, viral spread, and improved immunogenicity, especially in groups with higher BCG doses. While White et al. found no effect on viral clearance or early disease pathology with aerosolized BCG, the combined BCG/SCV2 approach in the present study demonstrated effective control of COV2 infection and reduced pulmonary damage, suggesting that the route and combination of vaccine administration play critical roles in achieving optimal cross-protection against COV249.
The dose-dependency observed in BCG’s efficacy is another noteworthy aspect of our study. Previous studies have shown inconsistent results with lower BCG doses, highlighting the importance of dose optimization in clinical settings. Group A, which received a higher BCG dose, displayed fewer pathological lesions and stronger antibody responses compared to Group F, which received a lower BCG dose. This result suggests that a higher dose of BCG enhances both the humoral and cellular immune responses, providing greater protection against SCV2. However, it also indicates the potential limitations of BCG at lower doses, which may explain inconsistent results in previous studies on BCG’s effectiveness against COVID-19. This finding reinforces the importance of optimizing BCG dosing in future combined vaccine regimens to achieve maximum efficacy. BCG is also recognized as an effective vaccine adjuvant, known to enhance the immunogenicity of protein subunit, DNA, and viral vector vaccines in pre-clinical studies. Consequently, this study included a strategy to evaluate BCG across various groups with distinct vaccination protocols and varying BCG doses37,53,54.
Studies consistently highlight the critical role of vaccine dose in determining immunogenicity and performance55,56,57. Lower doses have been shown to produce inconsistent outcomes, underscoring the importance of optimized and tailored dosing strategies in clinical applications. Notably, higher vaccine doses have been associated with a significant increase in BCG-specific T-cell responses, thereby enhancing protection against infections58. The findings of the present study align with those of Krmeská et al., who demonstrated the dose-dependent effects of BCG vaccination in a mouse skin infection model. Krmeská et al. observed that higher BCG doses promoted enhanced dendritic cell (DC) migration to draining lymph nodes (DLNs) and robust T cell priming, with responses peaking six days post-infection. Their results demonstrate the critical role of vaccine dose in immune activation, particularly through improved antigen presentation and DC transport to lymphoid tissues. Although the models and focus differ, both studies reinforce the importance of dose optimization for maximizing BCG’s immunogenic and protective effects59.
Building upon these findings, the present study offers important insight into the potential of a combined BCG and SCV2 vaccination strategy as a viable approach for mitigating severe SCV2 pathogenesis. While this study was conducted in a Syrian hamster model, which provides a translationally relevant platform due to its immunological similarity to humans in respiratory infections, further research involving longer follow-up periods, additional animal models, and eventual clinical studies will be required to validate and refine these findings for human application. Nevertheless, the current results lay a strong foundation and may serve as a cornerstone for advancing BCG-based adjuvant strategies in future vaccine development against SARS-CoV-2 and other emerging respiratory pathogens.
Conclusion
The current investigation findings support the potential of the combined BCG and SCV2 vaccination approach as an effective strategy against severe SCV2 pathogenesis. The observed reduction in viral load, lung pathology, antibody levels, and inflammatory markers in the BCG/SCV2 groups highlights the therapeutic potential of this strategy in preventing severe COVID-19 outcomes. These results have significant implications for refining COVID-19 vaccination strategies, particularly in exploring the role of BCG as an immunological adjuvant to enhance immune responses to SCV2. Further research is warranted to fully understand the mechanisms underlying the protective effects of the BCG/SCV2 regimen and to optimize its application as a preventive measure in the global fight against COVID-19.
Data availability
All relevant data generated, analyzed, and presented in this manuscript are available on request from the corresponding author.
Change history
17 December 2025
The original online version of this Article was revised: The Acknowledgements section in the original version of this Article was incomplete. It now reads: “The authors would like to express their sincere gratitude to the Department of Microbiology and Immunology, Faculty of Veterinary Medicine, University of Tehran and Center for International Scientific Studies and Collaboration (Grant No. 4020472) for their valuable support and resources throughout the study. Special thanks are extended to the Razi Vaccine and Serum Research Institute (RVSRI) in Iran for providing critical materials and technical assistance. The authors also acknowledge the contributions of the Scion Research Institute in New Zealand for their collaboration and guidance in ensuring the successful completion of this research."
Abbreviations
- BCG:
-
Bacille Calmette-Guérin
- SCV2:
-
SARS-CoV-2
- COVID-19:
-
Coronavirus Disease 2019
- ELISA:
-
Enzyme-Linked Immunosorbent Assay
- qRT-PCR:
-
Quantitative Reverse Transcriptase Polymerase Chain Reaction
- TCID50 :
-
Tissue Culture Infectious Dose 50%
- RBD:
-
Receptor Binding Domain
- ACE2:
-
Angiotensin-Converting Enzyme 2
- Th1:
-
T Helper Type 1
- Th17:
-
T Helper Type 17
- HSCs:
-
Hematopoietic Stem Cells
- MPPs:
-
Multipotent Progenitors
- MAIT:
-
Mucosa-Associated Invariant T Cells
- NK:
-
Natural Killer
- CPE:
-
Cytopathic Effect
References
Gao, J. et al. Immunomics in one health: Understanding the human, animal, and environmental aspects of COVID-19. Front. Immunol. 15 https://doi.org/10.3389/fimmu.2024.1450380 (2024).
Parkins Michael, D. et al. Wastewater-based surveillance as a tool for public health action: SARS-CoV-2 and beyond. Clin. Microbiol. Rev. 37, e00103–00122. https://doi.org/10.1128/cmr.00103-22 (2023).
Chang-Rabley, E., van Zelm, M. C., Ricotta, E. E. & Edwards, E. S. J. An Overview of the Strategies to Boost SARS-CoV-2-Specific Immunity in People with Inborn Errors of Immunity. Vaccines 12 (2024).
Wei, J. et al. Antibody responses to SARS-CoV-2 vaccines in 45,965 adults from the general population of the united Kingdom. Nat. Microbiol. 6, 1140–1149. https://doi.org/10.1038/s41564-021-00947-3 (2021).
Simon, D. et al. SARS-CoV-2 vaccination responses in untreated, conventionally treated and anticytokine-treated patients with immune-mediated inflammatory diseases. Ann. Rheum. Dis. 80, 1312. https://doi.org/10.1136/annrheumdis-2021-220461 (2021).
Aggarwal, M. et al. What is the role of primary care in the COVID-19 vaccine roll-out and the barriers and facilitators to an equitable vaccine roll-out? A rapid scoping review of nine jurisdictions. BMJ Open. 13, e065306. https://doi.org/10.1136/bmjopen-2022-065306 (2023).
Counoupas, C. et al. A single dose, BCG-adjuvanted COVID-19 vaccine provides sterilising immunity against SARS-CoV-2 infection. Npj Vaccines. 6, 143. https://doi.org/10.1038/s41541-021-00406-4 (2021).
Guo, Z. et al. COVID-19: from immune response to clinical intervention. Precision Clin. Med. 7, pbae015. https://doi.org/10.1093/pcmedi/pbae015 (2024).
Gonzalez-Perez, M., Sanchez-Tarjuelo, R., Shor, B., Nistal-Villan, E. & Ochando, J. The BCG vaccine for COVID-19: first verdict and future directions. Front. Immunol. 12 https://doi.org/10.3389/fimmu.2021.632478 (2021).
Larson, E. C. et al. Intravenous Bacille Calmette–Guérin vaccination protects Simian immunodeficiency virus-infected macaques from tuberculosis. Nat. Microbiol. 8, 2080–2092. https://doi.org/10.1038/s41564-023-01503-x (2023).
Ramírez, M. A. et al. Co-administration of Recombinant BCG and SARS-CoV-2 proteins leads to robust antiviral immunity. Vaccine 42, 126203. https://doi.org/10.1016/j.vaccine.2024.126203 (2024).
Khandelia, P., Yadav, S. & Singh, P. An overview of the BCG vaccine and its future scope. Indian J. Tuberculosis. 70, S14–S23. https://doi.org/10.1016/j.ijtb.2023.05.012 (2023).
Irvine, E. B. et al. Robust IgM responses following intravenous vaccination with Bacille Calmette–Guérin associate with prevention of Mycobacterium tuberculosis infection in macaques. Nat. Immunol. 22, 1515–1523. https://doi.org/10.1038/s41590-021-01066-1 (2021).
Koster, K. J., Webb, H. L. & Cirillo, J. D. COVID-19 and beyond: exploring public health benefits from Non-Specific effects of BCG vaccination. Microorganisms 9 (2021).
Talat Iqbal, N. & Hussain, R. Non-specific immunity of BCG vaccine: A perspective of BCG immunotherapy. Trials Vaccinol. 3, 143–149. https://doi.org/10.1016/j.trivac.2014.08.002 (2014).
Fu, W. et al. Reconcile the debate over protective effects of BCG vaccine against COVID-19. Sci. Rep. 11, 8356. https://doi.org/10.1038/s41598-021-87731-9 (2021).
Giamarellos-Bourboulis, E. J. et al. Activate: randomized clinical trial of BCG vaccination against infection in the elderly. Cell 183, 315–323e319. https://doi.org/10.1016/j.cell.2020.08.051 (2020).
Chen, J. et al. BCG-induced trained immunity: history, mechanisms and potential applications. J. Translational Med. 21, 106. https://doi.org/10.1186/s12967-023-03944-8 (2023).
Covián, C. et al. BCG-Induced Cross-Protection and development of trained immunity: implication for vaccine design. Front. Immunol. 10 https://doi.org/10.3389/fimmu.2019.02806 (2019).
Wang, X. et al. Vaccine-induced protection against SARS-CoV-2 requires IFN-γ-driven cellular immune response. Nat. Commun. 14, 3440. https://doi.org/10.1038/s41467-023-39096-y (2023).
Borges, K. C. M. et al. BCG vaccination, and COVID-19: are they connected?? Mini Rev. Med. Chem. 22, 1631–1647. https://doi.org/10.2174/1389557522666220104152634 (2022). Tuberculosis.
Upton, C. M. et al. Safety and efficacy of BCG re-vaccination in relation to COVID-19 morbidity in healthcare workers: A double-blind, randomised, controlled, phase 3 trial. eClinicalMedicine 48 https://doi.org/10.1016/j.eclinm.2022.101414 (2022).
Akkiz, H. in PreprintsPreprints, (2024).
O’Neill, L. A. J. & Netea, M. G. BCG-induced trained immunity: can it offer protection against COVID-19? Nat. Rev. Immunol. 20, 335–337. https://doi.org/10.1038/s41577-020-0337-y (2020).
Miao, J., Chard, L. S., Wang, Z. & Wang, Y. Syrian Hamster as an animal model for the study on infectious diseases. Front. Immunol. 10, 2329. https://doi.org/10.3389/fimmu.2019.02329 (2019).
Castellan, M. et al. Host response of Syrian Hamster to SARS-CoV-2 infection including differences with humans and between sexes. Viruses 15 (2023).
Schirm, S. et al. A biomathematical model of SARS-CoV-2 in Syrian hamsters. Sci. Rep. 14, 30541. https://doi.org/10.1038/s41598-024-80498-9 (2024).
McGrath, J. C. & Lilley, E. Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br. J. Pharmacol. 172, 3189–3193. https://doi.org/10.1111/bph.12955 (2015).
Bayne, K. & Turner, P. V. Animal welfare standards and international collaborations. ILAR J. 60, 86–94. https://doi.org/10.1093/ilar/ily024 (2019).
Bewley, K. R. et al. Quantification of SARS-CoV-2 neutralizing antibody by wild-type plaque reduction neutralization, microneutralization and pseudotyped virus neutralization assays. Nat. Protoc. 16, 3114–3140. https://doi.org/10.1038/s41596-021-00536-y (2021).
Zapata-Cardona, M. I. et al. Comparison among plaque assay, tissue culture infectious dose (TCID(50)) and real-time RT-PCR for SARS-CoV-2 variants quantification. Iran. J. Microbiol. 14, 291–299. https://doi.org/10.18502/ijm.v14i3.9758 (2022).
Chan, J. F. et al. Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel Real-Time reverse Transcription-PCR assay validated in vitro and with clinical specimens. J. Clin. Microbiol. 58 https://doi.org/10.1128/jcm.00310-20 (2020).
Chan, J. F. et al. Development and evaluation of novel Real-Time reverse Transcription-PCR assays with locked nucleic acid probes targeting leader sequences of Human-Pathogenic coronaviruses. J. Clin. Microbiol. 53, 2722–2726. https://doi.org/10.1128/jcm.01224-15 (2015).
Bullen, C. K., Davis, S. L. & Looney, M. M. in SARS-CoV-2: Methods and Protocols (eds Justin Jang Hann Chu, Bintou Ahmadou Ahidjo, & Chee Keng Mok) 131–146Springer US, (2022).
Lei, C., Yang, J., Hu, J. & Sun, X. On the calculation of TCID50 for quantitation of virus infectivity. Virol. Sin. 36, 141–144. https://doi.org/10.1007/s12250-020-00230-5 (2021).
Basak, P., Sachdeva, N. & Dayal, D. Can BCG vaccine protect against COVID-19 via trained immunity and tolerogenesis? Bioessays 43 (e2000200). https://doi.org/10.1002/bies.202000200 (2021).
Singh, A. K. et al. Dynamic single-cell RNA sequencing reveals BCG vaccination curtails SARS-CoV-2 induced disease severity and lung inflammation. bioRxiv (2022). https://doi.org/10.1101/2022.03.15.484018
Moorlag, S., Arts, R. J. W., van Crevel, R. & Netea, M. G. Non-specific effects of BCG vaccine on viral infections. Clin. Microbiol. Infect. 25, 1473–1478. https://doi.org/10.1016/j.cmi.2019.04.020 (2019).
Singh, A. K., Netea, M. G. & Bishai, W. R. BCG turns 100: its nontraditional uses against viruses, cancer, and Immunologic diseases. J. Clin. Invest. 131 https://doi.org/10.1172/jci148291 (2021).
Kleinnijenhuis, J. et al. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J. Innate Immun. 6, 152–158. https://doi.org/10.1159/000355628 (2014).
Chua, W. J. et al. Polyclonal mucosa-associated invariant T cells have unique innate functions in bacterial infection. Infect. Immun. 80, 3256–3267. https://doi.org/10.1128/iai.00279-12 (2012).
Steigler, P. et al. BCG vaccination drives accumulation and effector function of innate lymphoid cells in murine lungs. Immunol. Cell. Biol. 96, 379–389. https://doi.org/10.1111/imcb.12007 (2018).
Cirovic, B. et al. BCG vaccination in humans elicits trained immunity via the hematopoietic progenitor compartment. Cell. Host Microbe. 28, 322–334e325. https://doi.org/10.1016/j.chom.2020.05.014 (2020).
Kaufmann, E. et al. BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis. Cell 172, 176–190e119. https://doi.org/10.1016/j.cell.2017.12.031 (2018).
Na, H. N. & Nam, J. H. Proof-of-concept for a virus-induced obesity vaccine; vaccination against the obesity agent adenovirus 36. Int. J. Obes. (Lond). 38, 1470–1474. https://doi.org/10.1038/ijo.2014.41 (2014).
Covián, C. et al. BCG-Induced Cross-Protection and development of trained immunity: implication for vaccine design. Front. Immunol. 10, 2806. https://doi.org/10.3389/fimmu.2019.02806 (2019).
Namdar, N., Fasaei, N., Shariati, B., Joghataei, P., Arpanaei, A. & S. M. & Mesoporous silica nanoparticles co-loaded with lysozyme and Vancomycin for synergistic antimicrobial action. Sci. Rep. 14, 29242. https://doi.org/10.1038/s41598-024-78922-1 (2024).
Hilligan, K. L., Namasivayam, S. & Sher, A. BCG mediated protection of the lung against experimental SARS-CoV-2 infection. Front. Immunol. 14, 1232764. https://doi.org/10.3389/fimmu.2023.1232764 (2023).
White, A. D. et al. Influence of aerosol delivered BCG vaccination on immunological and disease parameters following SARS-CoV-2 challenge in Rhesus macaques. Front. Immunol. 12, 801799. https://doi.org/10.3389/fimmu.2021.801799 (2021).
Singh, A. K. et al. Intravenous BCG vaccination reduces SARS-CoV-2 severity and promotes extensive reprogramming of lung immune cells. iScience 26, 107733. https://doi.org/10.1016/j.isci.2023.107733 (2023).
Ravichandran, S., Vijayakumar, K., P, M. S. & G, V. V. & Vaccination can prevent severe pulmonary disease in COVID-19 positive patients: A Case-Control study. Cureus 15, e45638. https://doi.org/10.7759/cureus.45638 (2023).
Hilligan, K. L. et al. Intravenous administration of BCG protects mice against lethal SARS-CoV-2 challenge. J. Exp. Med. 219 https://doi.org/10.1084/jem.20211862 (2022).
Wu, Y. et al. Heterologous boost following Mycobacterium bovis BCG reduces the late persistent, rather than the early stage of intranasal tuberculosis challenge infection. Front. Immunol. 9, 2439. https://doi.org/10.3389/fimmu.2018.02439 (2018).
Ma, J. et al. Mycobacterium tuberculosis multistage antigens confer comprehensive protection against pre- and post-exposure infections by driving Th1-type T cell immunity. Oncotarget 7, 63804–63815. https://doi.org/10.18632/oncotarget.11542 (2016).
Umer, M., Shabbir, F. & Siddiqui, A. A. Efficacy of high and low dose of BCG and their complications in superficial transtional cell carcinoma of urinary bladder. Pakistan J. Med. Health Sci. 16, 91–91. https://doi.org/10.53350/pjmhs2216591 (2022).
Esmaeili, H., Ghorani, M., Joghataei, S. M., Villanueva-Saz, S. & Lacasta, D. Live attenuated goatpox vaccination in pregnant Murcia-Granada goats: dosage implications and outcomes. BMC Vet. Res. 20, 544. https://doi.org/10.1186/s12917-024-04395-z (2024).
Naranbhai, V. et al. Comparative immunogenicity and effectiveness of mRNA-1273, BNT162b2, and Ad26.COV2.S COVID-19 vaccines. J. Infect. Dis. 225, 1141–1150. https://doi.org/10.1093/infdis/jiab593 (2022).
Khatri, B. et al. Efficacy and immunogenicity of different BCG doses in BALB/c and CB6F1 mice when challenged with H37Rv or Beijing HN878. Sci. Rep. 11, 23308. https://doi.org/10.1038/s41598-021-02442-5 (2021).
Krmeská, V., Shen, L., Nylén, S., Wowk, P. F. & Rothfuchs, A. G. BCG infection dose guides dendritic cell migration and T cell priming in the draining lymph node. Scand. J. Immunol. 99, e13342. https://doi.org/10.1111/sji.13342 (2024).
Acknowledgements
The authors would like to express their sincere gratitude to the Department of Microbiology and Immunology, Faculty of Veterinary Medicine, University of Tehran and Center for International Scientific Studies and Collaboration (Grant No. 4020472) for their valuable support and resources throughout the study. Special thanks are extended to the Razi Vaccine and Serum Research Institute (RVSRI) in Iran for providing critical materials and technical assistance. The authors also acknowledge the contributions of the Scion Research Institute in New Zealand for their collaboration and guidance in ensuring the successful completion of this research.
Funding
This research received no specific grant from any funding agency.
Author information
Authors and Affiliations
Contributions
B.N.F. and M.T. conceptualized and designed the study. Methodology was developed by A.A., M.T., and B.N.F. Project administration was led by B.N.F. and M.T., with supervision provided by B.N.F., M.T., M.D., and N.M. Data collection was performed by A.A., M.D., N.M., and M.L. Data interpretation was conducted by A.A., M.D., N.M., and M.E., while S.M.J., B.N.F., and A.Ar. analyzed the data. M.E. carried out pathological evaluations. All authors participated in the research process and investigation. S.M.J. wrote the manuscript, drafted the initial text, and integrated feedback from all co-authors to produce the final version. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ashtari, A., Nayeri Fasaei, B., Taghizadeh, M. et al. Evaluation of combined BCG and SARS-CoV-2 vaccination for immune enhancement and lung protection in Syrian hamsters. Sci Rep 15, 18908 (2025). https://doi.org/10.1038/s41598-025-04246-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-04246-3