Abstract
Inferior vena cava thrombosis (IVCT) is a special form of venous thromboembolism. Lower extremity deep vein thrombosis (LEDVT) is associated with an increased incidence of pulmonary embolism (PE), but the association between concomitant IVCT and PE in patients with LEDVT has not been reported. We conducted a retrospective analysis of clinical data from patients confirmed with LEDVT at the First Affiliated Hospital of Xi’an Jiaotong University. LASSO regression was used to screen important variables, and multivariable logistic regression was used to identify independently associated factors. Of the 2929 patients, 40.9% had PE and 12.8% had IVCT. Multivariable logistic regression suggested that IVCT was associated with an increased odds of PE (OR 1.42, 95% CI 1.13–1.79). Subgroup analysis showed that IVCT was associated with increased odds of PE in patients with left LEDVT (OR 2.00, 95% CI 1.50–2.67), right LEDVT (OR 2.05, 95% CI 1.20–3.50), distal LEDVT (OR 5.15, 95% CI 1.31–20.22), and proximal LEDVT (OR 1.48, 95% CI 1.19–1.86). Concomitant IVCT significantly increased the prevalence of PE in patients with LEDVT. There was no difference in PE severity or risk stratification between patients with and without IVCT. These findings could contribute to further improve the understanding of IVCT among clinicians, and optimize monitoring and management strategies of patients with LEDVT.
Similar content being viewed by others
Introduction
Venous thromboembolism (VTE) is the third most common vascular disease after myocardial infarction and stroke, affecting nearly 10 million people worldwide each year and causing a huge global disease burden1. VTE comprises deep vein thrombosis (DVT) and pulmonary embolism (PE), both of which share the same predisposing factors. It is well known that PE usually occurs rapidly and has a high mortality. An international registry study showed that the 7-day all-cause mortality rate was 2.6% and the 30-day all-cause mortality rate was 5.9% for PE2. In Europe, PE accounted for 8–13 deaths per 1 000 women and 2–7 deaths per 1 000 men among people aged 15–55 years3. Studying the risk factors for PE and evaluating their potential impacts on PE is critical for preventing PE occurrence. Various risk factors for PE have been reported, including surgery, bone fractures, immobilisation, infection, cancer, autoimmune diseases, pregnancy, heritability, and family history4. Therefore, it is important to identify other relevant risk factors for PE.
Emboli that cause PE can originate from the inferior vena cava pathway, superior vena cava pathway, or the right heart chamber. Pulmonary emboli most commonly arise from a lower extremity deep vein thrombosis (LEDVT)5. Inferior vena cava thrombosis (IVCT) is a special form of VTE associated with significant mortality6. However, IVCT remains under-recognized7,8. IVCT most commonly derives from proximal extension of an LEDVT, whereas isolated IVCT is rare and is usually associated with inferior vena cava outflow obstruction9. The incidence of IVCT is 4–15% in patients with confirmed DVT8,9. Previous studies have also found that 30% of untreated patients with IVCT will develop PE6. However, the association between concomitant IVCT and PE in patients with LEDVT has not yet been reported.
This study aimed to investigate the association between concomitant IVCT and PE in patients with LEDVT. A subgroup analysis was performed based on the location characteristics of LEDVT. Moreover, PE severity and risk stratification of early death were compared between patients with and without IVCT.
Methods
Patients and study design
We retrospectively collected data from 3211 consecutive inpatients diagnosed with VTE at the First Affiliated Hospital of Xi’an Jiaotong University from June 2008 to June 2018. The inclusion criteria were as follows: (1) confirmed diagnosis of LEDVT, and (2) completion of CT angiography or angiography of the pulmonary artery. The exclusion criteria were as follows: (1) indwelling inferior vena cava filter before admission, (2) previous unhealed LEDVT, refers to patients with previous LEDVT who underwent follow up imaging to confirm that the thrombus was not been fully resolved and patients with previous LEDVT who did not undergo follow up imaging, (3) age < 18 years, and (4) incomplete baseline information. This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Review Committee of the First Affiliated Hospital of Xi’an Jiaotong University. Informed consent was waived by the Ethics Review Committee of the First Affiliated Hospital of Xi’an Jiaotong University due to the retrospective study design.
Data collection
Clinical data such as age, sex, heart rate, blood pressure, comorbidities (chronic heart failure, paralytic stroke, autoimmune disease, and nephrotic syndrome), risk factors for VTE (malignant tumor, immobilization, fracture of lower limb, leg varicosities, pregnancy or postpartum, infection, blood transfusion, smoking), D-dimer value, the imaging information of LEDVT characteristics (LEDVT limb and LEDVT type), presence of IVCT, and presence of PE were collected from the electronic medical record system of the First Affiliated Hospital of Xi’an Jiaotong University.
LEDVT was primarily diagnosed using Doppler ultrasonography, and venography or computed tomography (CT) venography was used for further clarification in cases in which Doppler ultrasonography was inconclusive. IVCT was diagnosed using Doppler ultrasonography, CT angiography or IVC angiography. PE required confirmation using either CT pulmonary angiography or pulmonary angiography. Plasma D-dimer levels were measured within 24 h of admission. Age-adjusted D-dimer (AADD) > 0.5 µg/mL was deemed abnormal for patients aged < 50 years, and an age-adjusted threshold (age × 0.01 µg/ml) was used for patients aged > 50 years10. The Pulmonary Embolism Severity Index (PESI) class and risk stratification of early death in patients with PE were assessed based on the European Society of Cardiology guidelines for PE11.
Statistical analysis
Continuous variables are presented as means ± standard deviations or medians (interquartile range, IQR) and were compared using independent samples Student’s t-test or the Mann-Whitney U test. Categorical variables were compared using the chi-squared test or Fisher’s exact test. LASSO regression was used to screen important variables, and multivariable logistic regression was used to identify independently associated factors. Binary logistic regression was used to evaluate the effect of IVCT on the probability of PE in subgroup analysis. A two-sided P-value < 0.05 was considered statistically significant. Statistical analyses were performed using R software (version 4.2.1; R Development Core Team) and SPSS version 26.0 (IBM Corp., Armonk, NY, USA).
Results
A total of 3047 patients were diagnosed with LEDVT during the study period. After exclusion of 118 patients, 2929 patients with LEDVT were included in the analysis. The patient inclusion flowchart is summarized in Fig. 1.
Baseline and clinical characteristics of patients
Among 2929 LEDVT patients, 1198 (40.9%) patients with PE were categorized as the PE group, and 1731 (59.1%) patients without PE were categorized as the non-PE group. Table 1 compares the demographic and clinical characteristics of the patients in the two groups. Patients with PE were older than patients without PE. The proportion of male patients with PE was higher than that of male patients without PE. IVCT was noted in 12.8% (374/2929) of the patients. Among patients with IVCT, 3.7% were diagnosed by Doppler ultrasonography, 4.5% by CT angiography, and 91.7% by IVC angiography. The proportion of IVCT in patients with PE was higher than that in patients without PE (16.0% vs. 10.5%). Furthermore, patients with PE were more likely to have higher heart rate and D-dimer level, bilateral LEDVT, proximal LEDVT, chronic heart disease, nephrotic syndrome, or infection than those without PE. There were no significant differences in other clinical characteristics between the two groups.
Risk factor analysis for PE in LEDVT patients
First, we used LASSO regression to select variables associated with the occurrence of PE in patients with LEDVT. The variation characteristics of the coefficients of these variables are shown in Fig. 2a. The 10-fold cross-validation method was applied to the iterative analysis, and a model with excellent performance but a minimum number of variables was obtained when λ was 0.02025312 (Log (λ) = -3.899446) (Fig. 2b). After LASSO regression, eight factors were selected: age, LEDVT limb, IVCT, LEDVT type, infection, heart rate, chronic heart failure, and AADD (Fig. 2c).
Variables selection associated with PE using LASSO regression. (a) LASSO coefficient profiles of the 20 variables. A coefficient profile plot was produced against the log (λ) sequence. (b) The process of selecting the optimal value of parameter λ in LASSO using the 10-fold cross-validation method. The value between the two dotted vertical lines is the range of standard deviations of log(λ). The right dotted vertical line indicates the least number of variables required when the cross-validation error is minimized. Eight variables were selected when λ was 0.02025312 (Log (λ) = -3.899446). (c) Bar chart of 8 important variables based on coefficients. PE pulmonary embolism, LEDVT lower extremity deep vein thrombosis, IVCT inferior vena cava thrombosis, AADD age-adjusted D-dimer.
Multivariate logistic regression showed that IVCT (OR 1.42; 95% CI 1.13–1.79, P = 0.003), age (OR 1.02; 95% CI 1.01–1.02, P < 0.001), right LEDVT (OR 1.21; 95% CI 1.01–1.45, P = 0.043), bilateral LEDVT (OR 1.87; 95% CI 1.49–2.35, P < 0.001), proximal LEDVT (OR 1.53; 95% CI 1.22–1.92, P < 0.001), chronic heart failure (OR 2.55; 95% CI 1.74–3.80, P < 0.001), infection (OR 2.00; 95% CI 1.48–2.73, P < 0.001), heart rate > 100 bpm (OR 1.97; 95% CI 1.53–2.55, P < 0.001), or positive AADD (OR 3.00; 95% CI 2.29–3.97, P < 0.001) increased the probability of PE in patients with LEDVT (Fig. 3).
Association between PE and IVCT in different subgroup
By dividing the study population into subgroups based on LEDVT limb and LEDVT type, we further compared the probability of PE in patients with LEDVT with or without IVCT. The results showed that IVCT was associated with increased odds of PE in patients with left LEDVT (OR 2.00; 95% CI 1.50–2.67, P < 0.001), patients with right LEDVT (OR 2.05; 95% CI 1.20–3.50, P = 0.008), patients with distal LEDVT (OR 5.15; 95% CI 1.31–20.22, P = 0.019), and patients with proximal LEDVT (OR 1.48; 95% CI 1.19–1.86, P = 0.001). In contrast, IVCT was associated with decreased odds of PE in patients with bilateral LEDVT (OR 0.61; 95% CI 0.38–0.95, P = 0.030) (Table 2; Fig. 4).
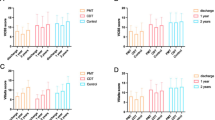
Association between PE severity, risk stratification, and IVCT
The severity of PE was assessed using the PESI score. In classes I, II, III, IV, and V of PESI score, the proportions of patients with IVCT were 37.5%, 39.1%, 14.6%, 4.7%, and 4.2%, respectively, compared with 32.6%, 41.9%, 15.9%, 4.9%, and 4.7% of patients without IVCT, respectively. There were no statistically significant differences between the groups (P = 0.780) (Table 3; Fig. 5a). We also assessed risk stratification for early death in patients with PE. The proportions of patients with IVCT at low, intermediate-low, intermediate-high, and high risk were 60.9%, 7.8%, 18.8%, and 12.5%, respectively. The proportions of patients without IVCT at low, intermediate-low, intermediate-high, and high risk were 58.9%, 11.2%, 17.2%, and 12.6%, respectively. There were no statistically significant differences between the groups (P = 0.552) (Table 3; Fig. 5b).
Discussion
This retrospective observational study investigated the effect of IVCT on the prevalence of PE in patients with LEDVT. The prevalence of PE was higher in patients with LEDVT with concomitant IVCT. However, there was no statistically significant in PE severity or risk stratification between patients with and without IVCT.
In the present study, 12.8% of LEDVT cases were complicated by IVCT. As previously reported, the incidence of IVCT is 4–15% in patients with LEDVT8,9. Our results fall between the results of previous studies. Some previous studies on LEDVT showed that LEDVT can trigger an increased risk of IVCT with extension of the thrombus burden6,12. Our study included patients hospitalised with LEDVT, most of whom had proximal or extensive thrombosis. Thus, we obtained a prevalence of IVCT at the high level of the range.
Many studies have demonstrated that LEDVT is associated with an increased incidence of PE; however, the association between concomitant IVCT and PE in patients with LEDVT has not been well elucidated. A previous study showed that symptomatic PE was more frequently observed in patients with IVCT when compared with sex- and age-matched patients with LEDVT, but the difference was not significant (27% vs. 12%, P = 0.064)13. Another previous study also showed that patients with IVCT had a higher PE risk than patients without IVCT, but the difference was also not statistically significant (62.5% vs. 54.2%, P = 0.329)14. These results may be influenced by the small sample size of the studies and the presence of some confounding variables. In the present study, the prevalence of PE was significantly higher in patients with LEDVT with concomitant IVCT than in patients with LEDVT alone (51.3 vs. 39.4%, P < 0.001), and multivariate logistic regression showed that IVCT was associated with an increased odds of PE in patients with LEDVT (OR, 1.42; 95%CI, 1.13–1.79, P = 0.003). The probability of PE is known to be higher in patients with proximal LEDVT than in patients with distal LEDVT15,16. Similarly, our study showed that patients with proximal LEDVT had a higher odds of PE than those with distal LEDVT, which is consistent with the results of previous studies17,18. The above evidence suggests that the location of the thrombus is associated with PE, which may be related to different clot burden and embolic potential. IVCT is located in the inferior vena cava and is closer to the lungs. It may have a larger clot burden or higher embolic potential than LEDVT. Hence, our study suggested that LEDVT combined with IVCT was associated with a higher risk of PE compared to LEDVT alone, which is consistent with its anatomical predisposition for embolization. Regarding the association between the LEDVT limb and PE, our study found that the risk of PE was in the order bilateral LEDVT > right LEDVT > left LEDVT. Li et al. and Shi et al. reported that patients with right LEDVT have a higher risk of PE than those with left LEDVT19,20. This phenomenon may be due to anatomical factors such as frequent compression of the left common iliac vein, which may lead to DVT, but at the same time may prevent thrombi from escaping from the lower extremity veins to lungs19,20,21. A study by Zhang et al. also drew the same conclusion. They provided a different explanation, suggesting that it may be related to more comorbidities in patients with right LEDVT22. However, the proportion of comorbidities did not differ between the right and left LEDVT groups in our study. Therefore, we believe that their explanation requires further exploration. Previous DVT studies have paid little attention to bilateral LEDVT. A previous retrospective case-control study showed that patients with bilateral LEDVT exhibited a 2.4-fold higher risk of PE than those with unilateral LEDVT23. In the present study, we also found patients with bilateral LEDVT had a significantly higher prevalence of PE compared to those with unilateral LEDVT (53.9% vs. 38.8%, p < 0.001). We believe that patients with bilateral LEDVT tend to have greater thrombotic extent and heavier thrombus burden, resulting in a higher risk of PE. In addition, we found that age, chronic heart failure, infection, heart rate > 100 bpm, and positive AADD results were more likely to have PE in patients with LEDVT. Of these, age, heart failure, infection, and AADD are widely recognised risk factors for VTE2,24. However, the association between these factors and PE in patients with LEDVT has not yet been elucidated. Therefore, further studies are warranted. A heart rate > 100 bpm is one of the items of the Wells score. The Wells score has been used to distinguish patients with suspected PE from those with DVT, and our study confirmed its predictive role for PE. Although the Odds ratio of IVCT was not the highest compared with some other factors in our study, it still has important evaluation value because it represents a directly observable thrombus burden originating from the central venous system.
To further clarify the relationship between concomitant IVCT and PE at different LEDVT sites, the study population was divided into subgroups. The results showed that in the left, right, proximal, and distal LEDVT subgroups, the probability of PE was higher in patients with IVCT than in those without IVCT. Unexpectedly, however, these results were reversed in patients with bilateral LEDVT. IVCT has been reported to result in fatal PE; the patient in the report died on the way to the emergency room25. Furthermore, the mortality rate of IVCT is 2-fold higher than that of LEDVT alone7. In view of the above, we speculate that some patients with bilateral LEDVT and concomitant IVCT who have a very heavy thrombus burden may have fatal or severe PE, leading to death before admission or abandonment of treatment in the emergency department. This subset of patients could not be included in the present study; thus, the probability of PE is underestimated in patients with bilateral LEDVT and IVCT. We believe that the probability of PE is higher in patients with bilateral LEDVT and IVCT than in those without IVCT. Further studies are required to clarify this issue. Interestingly, patients with distal LEDVT and IVCT had a 5-fold higher odds of PE than those without IVCT. The increased odds in distal LEDVT was higher than that in proximal LEDVT. Therefore, clinicians should pay more attention to patients with distal LEDVT to identify the potential risk of IVCT and reduce the missed diagnosis of PE.
In the present study, we evaluated the association between IVCT and PE severity and risk stratification. The PESI class and risk stratification for PE represent the risk of death within the first 30 days11. There was no difference in the severity and risk stratification of PE between patients with and without IVCT, indicating that the presence of IVCT may not additionally worsen the severity of PE. Although a previous study suggested that IVCT was a risk factor for silent PE26 it is known that the presence or absence of symptoms of PE does not fully reflect the severity. Currently, few studies have investigated the prognosis of patients with LEDVT and concomitant IVCT. Previously, a prospective observational study compared the outcomes of IVCT and LEDVT and concluded that the 24-month all-cause mortality rate was higher in patients with IVCT than in those with LEDVT27. However, in-hospital and short-term outcomes were not reported.
The present study has several limitations. First, this was a single-centre retrospective observational study, and selection bias may have been unavoidable. Second, some risk factors for VTE, such as thrombophilia, hormone replacement therapy, and oral contraceptive use, were not analysed in this study because of the inability to obtain accurate information. Third, we did not consider medication use. Some patients used anticoagulants before admission, which may have influenced the D-dimer levels and prevented thrombus extension and subsequent PE. Fourth, we lacked data on the time from LEDVT diagnosis to the occurrence of PE and the time from LEDVT diagnosis to the appearance of IVCT, so we did not evaluate the association between IVCT and PE occurrence longitudinally. Despite these limitations, to our knowledge, our study is the first to investigate the effect of concomitant IVCT on PE in patients with LEDVT. Large-scale prospective multicentre studies are needed to verify these results.
In conclusion, concomitant IVCT was associated with higher prevalence of PE in patients with LEDVT. However, there was no difference in PE severity or risk stratification between patients with and without IVCT. Our findings could contribute to further improve the understanding of IVCT among clinicians, and suggest that, when managing patients with LEDVT, it is important to focus on identifying IVCT and adjust the monitoring and management strategies based on the presence or absence of IVCT.
Data availability
Our data is available from the corresponding authors on reasonable request.
References
Khan, F., Tritschler, T., Kahn, S. R. & Rodger, M. A. Venous thromboembolism. Lancet 398, 64–77 (2021).
Jiménez, D. et al. Trends in the management and outcomes of acute pulmonary embolism: analysis from the RIETE registry. J. Am. Coll. Cardiol. 67, 162–170 (2016).
Barco, S. et al. Trends in mortality related to pulmonary embolism in the European region, 2000-15: analysis of vital registration data from the WHO mortality database. Lancet Respir Med. 8, 277–287 (2020).
Lutsey, P. L. & Zakai, N. A. Epidemiology and prevention of venous thromboembolism. Nat. Rev. Cardiol. 20, 248–262 (2023).
Kahn, S. R. & de Wit, K. Pulmonary embolism. N Engl. J. Med. 387, 45–57 (2022).
Alkhouli, M., Morad, M., Narins, C. R., Raza, F. & Bashir, R. Inferior Vena Cava thrombosis. JACC Cardiovasc. Interv. 9, 629–643 (2016).
Lin, H. Y., Lin, C. Y. & Shen, M. C. Review Article inferior Vena Cava thrombosis: a case series of patients observed in Taiwan and literature review. Thromb. J. 19, 43 (2021).
McAree, B. J. et al. Inferior Vena Cava thrombosis: a review of current practice. Vasc Med. 18, 32–43 (2013).
Shi, W. & Dowell, J. D. Etiology and treatment of acute inferior Vena Cava thrombosis. Thromb. Res. 149, 9–16 (2017).
Kakkos, S. K. et al. (eds) ‘s Choice - European Society for Vascular Surgery (ESVS) 2021 Clinical Practice Guidelines on the Management of Venous Thrombosis. Eur J Vasc Endovasc Surg. 61, 9–82 (2021).
Konstantinides, S. V. et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European respiratory society (ERS). Eur. Heart J. 41, 543–603 (2020).
Avgerinos, E. D. et al. Impact of inferior Vena Cava thrombus extension on thrombolysis for acute iliofemoral thrombosis. J. Vasc Surg. Venous Lymphat Disord. 4, 385–391 (2016).
Kraft, C. et al. Patients with inferior Vena Cava thrombosis frequently present with lower back pain and bilateral lower-extremity deep vein thrombosis. Vasa 42, 275–283 (2013).
Gong, M. et al. Risk factors and a predictive model for nonfilter-associated inferior Vena Cava thrombosis in patients with lower extremity deep vein thrombosis. Front. Cardiovasc. Med. 9, 1083152 (2022).
Yamaki, T. et al. Factors predicting development of post-thrombotic syndrome in patients with a first episode of deep vein thrombosis: preliminary report. Eur. J. Vasc Endovasc Surg. 41, 126–133 (2011).
Konstantinides, S. V. et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur. Heart J. 35, 3033–3069 (2014).
Boc, A., Vene, N., Stalc, M., Košmelj, K. & Mavri, A. Unprovoked proximal venous thrombosis is associated with an increased risk of asymptomatic pulmonary embolism. Thromb. Res. 133, 1011–1015 (2014).
Kucher, N., Tapson, V. F. & Goldhaber, S. Z. Risk factors associated with symptomatic pulmonary embolism in a large cohort of deep vein thrombosis patients. Thromb. Haemost. 93, 494–498 (2005).
Li, F. et al. Risk factors associated with the occurrence of silent pulmonary embolism in patients with deep venous thrombosis of the lower limb. Phlebology 29, 442–446 (2014).
Shi, Y. et al. Impact of common Iliac vein compression on the incidence of pulmonary embolism in patients with acute deep vein thrombosis. Eur. J. Vasc Endovasc Surg. 65, 887–894 (2023).
Chen, F., Huang, J. G., Liu, X. & Zhou, W. Left Iliac vein involvement is a protective factor against symptomatic pulmonary embolism in lower left extremity deep vein thrombosis. J. Vasc Surg. Venous Lymphat Disord. 10, 1272–1278 (2022).
Zhang, C. et al. Relationship between the site of thrombosis and the prevalence of pulmonary embolism in acute lower extremity deep venous thrombosis. J. Vasc Surg. Venous Lymphat Disord. 8, 725–733 (2020).
Zhang, J. et al. Anatomic distribution of lower extremity deep venous thrombosis is associated with an increased risk of pulmonary embolism: A 10-year retrospective analysis. Front. Cardiovasc. Med. 10, 1154875 (2023).
Freund, Y., Cohen-Aubart, F. & Bloom, B. Acute pulmonary embolism: A review. JAMA 328, 1336–1345 (2022).
Hanterdsith, B. Fatal pulmonary thromboembolism due to inferior Vena Cava thrombosis. Ann. Vasc Dis. 4, 121–123 (2011).
Shi, Y. et al. Silent pulmonary embolism in deep vein thrombosis: relationship and risk factors. Clin. Appl. Thromb. Hemost. 28, 10760296221131034 (2022).
Cohen, O. et al. Management strategies and clinical outcomes in patients with inferior Vena Cava thrombosis: data from GARFIELD-VTE. J. Thromb. Haemost. 20, 366–374 (2022).
Acknowledgements
We thank to Xinye Yao and Bowen Fu (Xi’an Jiaotong University) for the data collection. We also thank Nurse Manager Zhou Hongyan, Nurse Manager Yin Wang and Yumeng Han (Department of Peripheral Vascular Diseases, The First Affiliated Hospital of Xi’an Jiaotong University) for patient care and data collection.
Funding
This study was supported by the Key Industry Innovation Chain Project of Shaanxi province, China (No. 2022ZDLSF02-02) and the Clinical Research Award of The First Affiliated Hospital of Xi’an Jiaotong University, China (No. XJTU1AF-CRF-2018-021).
Author information
Authors and Affiliations
Contributions
Y.Z., Q.Y. and H.T. designed the study. Y.M., Y.L. and M.K. collected the data. Y.Z., Q.M., J.Z. and Q.Y. analysed and interpreted the data. Y.Z. drafted the manuscript. Y.M., Y.L., M.K., Q.M., J.Z., J.Y., H.T. and Q.Y. revised the manuscript. All authors have read and approved this manuscript for submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, Y., Meng, Y., Li, Y. et al. Impact of inferior vena cava thrombosis on the prevalence of pulmonary embolism in patients with lower extremity deep vein thrombosis. Sci Rep 15, 19748 (2025). https://doi.org/10.1038/s41598-025-04377-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-04377-7