Abstract
Invasive non-native fish species can profoundly disrupt ecosystems. In invasion ecology, using the functional similarity with native species to help predict demographic rates of non-native species and infer the ecological processes underlying it remains largely unexplored. Utilizing a comprehensive analysis of 2,903 species pairs across 153 sampling sites in rivers distributed in different continents, we evaluated interspecific synchrony patterns among populations of native and non-native fish species and explored their relationship with functional and phylogenetic dissimilarities using a linear mixed model. Our results indicate that non-native fish exhibit higher synchrony with native species that share similar ecological and morphological traits. This finding corroborates our hypothesis that co-occurring non-native and native species that are more functionally similar are more synchronized and emphasizes the importance of environmental filtering significantly shaping population dynamics between native communities and coexisting non-native species. We highlight the potential of widespread non-native species in increasing synchronous patterns and consequently decreasing community stability. By elucidating which type of dissimilarities (ecological, life history, morphological, and phylogenetic) can predict synchrony and which ecological mechanisms facilitate the coexistence of native and non-native species, this research underscores the ecological implications of invasion dynamics in the long term and helps to guide conservation efforts.
Similar content being viewed by others
Introduction
Non-native species are one of the major threats to biodiversity1. They can affect ecosystems in various ways, such as extirpating native species, altering community structure2,3,4, and disrupting important functions and services in invaded ecosystems5,6. Invasion ecology studies have commonly used functional similarity with native species to help predict different stages of invasion, i.e., the establishment and dispersion/impact of non-natives7,8,9,10. Various invasion hypotheses are indeed being tested within the framework of this trait-based approach11. However, using the functional similarity with native species to help predict demographic rates of non-native species and infer the ecological processes underlying it remains largely unexplored12. More specifically, little is known about the extent to which the temporal population dynamics of native and non-native species are similar when they coexist or how these similarities in dynamics relate to functional differences between species. Investigating these aspects could provide insights into the mechanisms that drive long-term coexistence within invaded communities, an essential aspect in invasion dynamics13,14. Also, analyzing long-term patterns at the population level is critical for understanding invasion dynamics and forecasting the potential impacts of non-native species15,16,17. Therefore, addressing these questions offers valuable insights into invasion ecology from the population level, a poorly explored perspective, which has been recognized as having the ability to capture the variability and complexity of invasion dynamics more effectively than species-level approaches18,19.
When assessing temporal population dynamics, the abundances of pairs of co-existing species can vary synchronously through time (e.g., the abundance of native species increases/decreases when the abundance of non-native species increases/decreases or vice-versa), show compensatory dynamics (e.g., the abundance of native species decreases when the abundance of non-native species increases or vice-versa), or vary independently of each other. This degree of interspecific synchrony can depend on similarities in their responses to variations in environmental conditions and species interactions. For example, synchronous dynamics can arise from species responding similarly to environmental variation20,21 or due to positive interactions between them22,23. On the other hand, compensatory dynamics between populations are thought to arise from competition24,25,26 but also from different responses to environmental variation27,28,29. Finally, idiosyncratic responses to the environment and neutral mechanisms (i.e., stochasticity) can ultimately generate independent dynamics between populations27,30,31. Since functionally similar species are thought to respond similarly to environmental changes (i.e., environmental filtering hypothesis) or to compete for available resources (i.e., limiting similarity principle32), the relationship between species functional similarity and interspecific synchrony patterns can help to draw inferences about the ecological mechanisms driving coexistence between native and non-native species. If functionally similar native and non-native species vary synchronously with each other, then it is likely that they respond similarly to environmental change22,33, backing up the environmental filtering hypothesis. In opposite, if functionally similar pairs of native and non-native species have compensatory dynamics, competitive interactions (the limiting similarity principle) may contribute more to determine their coexistence in the community22,34. Despite the valuable ecological insights provided by combining functional similarity and interspecific synchrony approaches, this method has yet to be tested, to the best of our knowledge, with a focus on non-native species.
In general, previous studies examining native and non-native species functional traits through a comparative approach have revealed a lack of consensus regarding the role of ecological processes driving invasion dynamics. For instance, some studies suggested that non-native species with traits closely aligned with those of the native community establish or spread more20,33,35,36,37. This is because their invasion success relies on possessing specific traits that enable survival in the environmental conditions of the recipient ecosystems. This pattern highlights the importance of the environmental filtering process in driving invasion dynamics8. Other studies showed a contrasting view, suggesting that species with dissimilar traits are more successful in the invasion process because they experience less biotic resistance exerted by competition and predation pressure from the native community9,38,39. The variability in outcomes regarding the mechanisms underlying the success of non-native invasions and their coexistence with natives in communities has been reported to depend on the specific traits considered in the study8,35.
Studies assessing species functional distance commonly use traits categorized into different types33,37,38,40,41. For example, life-history traits involve characteristics related to species growth, reproduction, and survival. Ecological traits relate to species’ interactions with their environment, including feeding habits and preferred food sources42,43. Morphological traits, on the other hand, encompass the observable physical or structural characteristics of the organism, e.g., body size and shape44. Finally, researchers have also used phylogenetic relatedness as a surrogate for functional similarities between species45,46, because this type of information (i.e., the evolutionary story) can be a surrogate for various phenotypic, genetic, and behavioral features of species47,48. All of these traits are assumed to capture resource partitioning within resident communities or non-native ecological preferences and tolerances to environmental conditions. However, it is crucial to compare and understand the contribution of these different trait types when assessing the relationship between functional similarity and synchrony level between native and non-native species.
Moreover, when considering common and widespread non-native species, specific characteristics are reported to make them more successful in persisting in a new ecosystem49,50. For fishes, these species are reported as having (among other attributes) a larger body size than natives51,52 and/or exhibit diet plasticity (i.e., omnivores), or still consume a wide variety of food from specific trophic guilds (i.e., piscivores; Tonella et al.53). These characteristics are linked to lower chances of being predated and higher competitive advantage when considering more specialist native species54,55. Therefore, non-native fishes are expected to be less affected by local ecological processes, such as biotic resistance, due to competition for available resources and predation interactions56. On the other hand, non-native species tend to strongly respond to changes in environmental conditions57. In general, due to their opportunistic characteristics, these species tend to positively respond (e.g., higher growth and fecundity) to the environment when it offers suitable conditions58. Conversely, these species negatively respond to more stressful conditions (e.g., instability in hydrological conditions or severe climatic events)59,60. This happens when non-native species are not well adapted to the environmental conditions of the recipient habitat61,62. Despite previous studies showing the importance of environmental filtering driving non-native communities within ecosystems56, this relation between the environment and temporal dynamics of non-native species was poorly explored at the population level, and little has been done for comparing these dynamics with patterns observed in native species.
Therefore, considering these aspects, here we evaluated interspecific synchrony patterns among populations of native and non-native species within communities and explored their relationship with functional and phylogenetic similarities. For this, we used an extensive database containing temporal data on riverine fish communities, the RivFishTime63. In this dataset, some sampled points are inhabited by some widespread non-native fish species. We expect to answer whether the functional and phylogenetic relatedness of coexisting native and non-native fishes are good predictors of synchrony patterns in their population dynamics. In addition, we investigated if sets of traits (life-history, ecological, and morphological) or phylogenetic information can predict the similarity in their population dynamics, and if outcomes are consistent across the different trait types. Because successful non-native species tend to possess more generalists traits that reduce the competition by available resources55,64, we do not expect compensatory dynamics (i.e., negative covariation between populations) due to limiting similarity to be the main mechanism driving the coexistence of populations of non-native fish species with native ones. Therefore, we hypothesize that co-occurring non-native and native species that are more functionally similar are more synchronized, suggesting that environmental filtering is the primary mechanism driving non-native and native species coexistence.
Methods
Fish database
We obtained fish occurrence and abundance data from the ‘RivFishTIME’ database63. This is a global database of long-term riverine fish surveys from 46 regional and national monitoring programs and individual academic research efforts, encompassing the period between 1951 and 2019. It includes 11,386 time series of riverine fish community catch data, with abundance records, geographical location, and sampling methodology information for each time series. The spatial range of the database includes 19 countries, five biogeographical realms, and 402 hydrographical basins worldwide63.
Data selection
To ensure the quality and consistency of our data for statistical analysis, we filtered the time series depending on the sampling methods and the species sampled. Regarding the sampling methods, we considered the time series length (at least 10 years;57), sampling periodicity (annual), and sampling unit (abundance - number of individuals and CPUE). Concerning the sampled species, we selected species that occurred in at least 60% of the time series to exclude rare and infrequent species22. Additionally, for non-native species, we filtered out those occurring in less than ten sampling sites. We determined the species status (native and non-native) related to where the species was sampled using literature information42,65,66,67. Native species are those fish that naturally occur in a particular watershed, while non-native species are those that have been introduced to regions outside their native ranges46. According to these criteria, eight non-native species and 129 native species were selected for this study. The non-native fish species were Ameiurus melas, Ctenopharyngodon idella, Cyprinus carpio, Gambusia holbrooki, Hypophthalmichthys molitrix, Lepomis gibbosus, Pseudorasbora parva, and Xiphophorus hellerii. Finally, according to these criteria, 153 time series, i.e., sampling sites, were selected for the present study. The selected time series were sampled in countries from different geographic regions (continents), including Australia (Oceania), Canada and the United States (North America), France, Hungary, and Spain (Europe) (Figure S1; Table S1).
Functional and phylogenetic distances
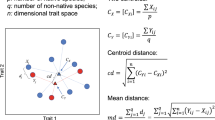
We collected 17 functional traits of each species from specific literature68,69, see Table S2. All selected traits are commonly used in studies about fish invasion ecology and are reported as representing niche similarity with native community or non-native ecological preferences and tolerances to environmental conditions33,37,38,40,41. They were classified into three trait groups: ecological, life history, and morphological (Table S2;42,70). We calculated the functional distance between each pair of native and non-native species co-occurring at the same sampling site. We obtained the functional distance for each trait group, resulting in three functional distance values for each species pair. For ecological and life-history traits, we used an adaptation of the Gower distance using the function gawdis from the “gawdis” package71. For the morphological traits (all continuous), we used the Euclidean distance to represent morphological distances. To obtain phylogenetic information on the fish species, we used the FishPhyloMaker function from the “FishPhyloMaker” package72. This function constructs a synthetic phylogenetic tree for a subset of species, based on the backbone phylogeny of ray-finned fishes from73 and rules for taxa insertion based on cladistic hierarchy and species taxonomic information72. After obtaining the phylogeny for our subset of species, we measured phylogenetic distances between each species pairs using the cophenetic function from the “ape” package74.
Data analyses
In each site, interspecific synchrony was calculated between each native and non-native species pair. To do so, we first removed long-term trends in the abundance time series of each species75 by applying a linear regression between abundance and time and using the residuals of this model in the following steps. Then, we used Spearman’s rank correlation coefficient between the detrended abundance time series (residuals) of each species pair. After, to assess if the functional and phylogenetic distances between native and non-native species are good predictors of interspecific synchrony patterns, we performed a Linear Mixed Model (LMM) using the synchrony values between species pairs as the response variable. As predictor variables (fixed terms), we included the functional distances (i.e., ecological, life history, and morphological), the phylogenetic distances, and the number of sampling years of each time series.
The environmental context is a crucial aspect to take into account when assessing functional similarity patterns, especially when considering an invasion process76. Therefore, we also included the air temperature (°C) information for each location, a surrogate to water temperature, as a fixed covariable. Water temperature is related to resource availability and is an important environmental constraint for ectothermic organisms such as fish77,78. For this, we extracted the mean annual air temperature recorded at the height of 2 m above the Earth’s surface at each location from NASA’s POWER database (Prediction of Worldwide Energy Resource), utilizing the package “nasapower”79. In addition, the species pair ID, the non-native species ID, and the sampling site ID were included as random terms. The interactions between functional distances and temperature were not included in the final model as they were not statistically significant.
We ran the LMM using the lmer function from the “lme4” package80. Multicollinearity was assessed using the ‘check_collinearity’ function from the ‘performance’ package81, and VIF values < 1.5 indicated no significant issues. To evaluate the model’s assumptions, we employed the testResiduals function from the “DHARMa” package82. The assessment of model goodness-of-fit involved the computation of marginal (R²m) and conditional (R²c) coefficients of determination83. For this, we used the r.squaredGLMM function from the “MuMIn” package84 and the tab_model function from the “sjPlot package”85. All statistical analyses were conducted in the R environment86.
Results
In total, we analyzed 2,903 pairs of species distributed in 153 sampling sites, with 290 unique pairs of species encompassing eight non-native and 129 native species. Time series length ranged from 10 to 31 years. The number of native and non-native species analyzed per sampling site ranged, respectively, from one to 37 and from one to three (Table S1).
Among the non-native species (Fig. 1), Lepomis gibbosus presented the highest mean synchrony with co-occurring native species (mean ρ = 0.104 ± 0.31), followed by Cyprinus carpio (mean ρ = 0.090 ± 0.26). Lepomis gibbosus also showed the highest synchrony value (maximum ρ = 0.979). On the other hand, Gambusia holbrooki showed the strongest compensatory dynamics (minimum ρ = -0.738, Table S3). In Europe, the non-native species Lepomis gibbosus presented the highest synchrony values with native species (Table 1) but also high compensatory dynamics (ρ< -0.70) with the native species Perca fluviatilis (Fig. 2). In North America, Cyprinus carpio was greatly synchronous with native species of the genus Ictiobus (Fig. 2) and Aplodinotus grunniens (Table 1). In Oceania, Xiphophorus hellerii was highly synchronized with Gobiomorphus australis (Table 1).
Morphological and ecological distances between species were significantly related to interspecific synchrony (Table 2). Synchrony between pairs of native and non-native species decreased as morphological and ecological distances increased (Fig. 3). Life history and phylogenetic distances did not explain significant variation in pairwise synchrony between native and non-native species.
Relationships between interspecific synchrony (pairwise Spearman’s correlations) and functional distances between species pairs (native x non-native species). (a) Morphological distances (traits related to body morphology - see Table S2) and (b) Ecological distances (trophic level and type of habitat), n = 2903.
Discussion
We evaluated the interspecific synchrony patterns among native and widespread non-native fishes, exploring their relationship with functional and phylogenetic similarities. Pairs of native and non-native species showed a range of dynamics, from highly synchronous (i.e., both species abundances increasing and decreasing at the same time) to compensatory dynamics (i.e., increases in abundance of one species while the other decreases in abundance, sensu31. We found that the similarity between native and non-native fish species can predict their level of synchrony. Specifically, we showed that non-native species tend to be more synchronized with native ones with more similar ecological and morphological characteristics. On the other hand, similarities in life-history traits and phylogenetic relatedness were not related to synchrony in abundance between species. Finally, the observed relationship between interspecific synchrony and trait similarity may suggest that similar responses to environmental variation drive the similarity in population dynamics of the studied native and non-native species20,22,87.
We found that non-native fish populations exhibit greater synchrony with native species that share similar functional traits. This finding suggests that, in general, non-native fish respond to changes in environmental conditions similarly to native species that are morphologically and ecologically similar. Consequently, environmental filtering appears to be the primary mechanism driving the temporal dynamics of non-native species and long-term coexistence with native communities. This aligns with the recent study by46, which demonstrated the significance of environmental filtering in shaping spatial co-occurrence patterns between functionally similar native and non-native riverine fish species. Thus, our hypothesis that co-occurring native and non-native species that are more functionally similar are more temporally synchronized was supported. In contrast, compensatory dynamics (i.e., negative covariation between populations) arising from the limiting similarity process do not seem to rule the coexistence of native and non-native fish populations with similar traits. This is likely because successful non-native species often exhibit more generalist preferences and higher trait plasticity in invaded environments. These features may allow them to adapt more readily to resource availability and avoid competitive interactions with similar native species, or, in some cases, outcompete them, sometimes leading to their extirpation55,64. This finding underscores the importance of considering the role of non-native species in community dynamics when assessing levels of synchrony. Finally, our results suggest that non-native species that are functionally like native communities may enhance synchronous patterns within communities, potentially reducing ecosystem stability in the face of future disturbances. Future research should explore how non-native species affect the stability of communities over time (e.g57,88).
Ecological and morphological functional distances have proven to be effective in predicting synchrony in population fluctuations between native and non-native fishes. This finding is consistent with prior research assessing the relationship between interspecific synchrony and similarities in ecological or morphological traits of terrestrial87,89 and aquatic groups20,90,91. For example33, related levels of interspecific synchrony of phytoplankton with ecological traits such as motility and silica use. For freshwater fish, ecological traits such as diet and habitat preference are crucial for assessing the ecological mechanisms driving communities and populations, directly influencing how species utilize available resources and respond to changes in abiotic conditions92,93. Additionally90, found that body size, a morphological trait, predicted synchrony among fish species (including non-native ones) experiencing severe drought in a tropical reservoir. The significant contribution of morphological distance explaining the variance in similarities of population dynamics reinforces their role as a good surrogate of fish features (e.g., ecological or reproductive) directly related to species fitness94. Such a finding highlights functional trait similarity with native communities as a valuable approach to understanding population dynamics when assessing the invasion process. On the other hand, the lack of congruence in results for the other trait distances (i.e., life history and phylogenetic) emphasizes the necessity for careful trait selection when comparing population dynamics between native and non-native species, indicating the need for considering different traits to ensure robust comparisons.
Regarding patterns of each non-native species, Lepomis gibbosus (pumpkinseed) and Cyprinus carpio (common carp) presented synchronous dynamics with a variety of native species in the invaded area. As these species are generalists and have high abundance and a wide distribution in the non-native area, they likely dwell in diverse environmental conditions and habitat types95,96. Thus, their responses to environmental variation probably coincide with the responses of many native species in the invaded areas, generating synchrony between their populations. On the other hand, Gambusia holbrooki (eastern mosquitofish) presented a high frequency of compensatory dynamics with native species in the invaded areas. Like the other non-native species in this study, this small-sized fish with a fast life-cycle also dwells in a variety of environmental conditions97, but presented a contrasting population dynamics with native species. Therefore, a likely explanation for this pattern is negative interactions. This includes competition and aggressiveness, as this species has high competitive potential and niche overlap with native species and shows fin-nipping behavior97,98,99. Additionally, predation on the eggs and larvae of native species by this non-native fish may further elucidate the contrasting patterns observed in their populations100. Finally, similar species can still respond differently to environmental changes if there is temporal differentiation in their niches14. This could lead to a pattern of compensatory dynamics in their abundance in the absence of negative interactions.
Regarding patterns in different geographic regions, some non-native species, such as L. gibbosus in Europe, presented both highly synchronous and strong compensatory dynamics with native species, depending on the sampling site (e.g., water body and/or country). The similarity in ecological and morphological traits can partially explain this variation in dynamics. For example, L. gibbosus and Rhodeus amarus (ρ = 0.91) are ecologically similar, as both species prefer to inhabit vegetated areas in still or slow-flowing waters69,101. On the other hand, L. gibbosus had compensatory dynamics with Perca fluviatilis (ρ = -0.73), as they differ largely in habitat preferences and trophic level (mainly because of the larger prey size consumed by P. fluviatilis). However, it is important to note that the pair Lepomis gibbosus/Perca fluviatilis also presented high synchrony in some locations (see Table 1), therefore it is likely that local abiotic and biotic conditions can also influence their population dynamics. Further research could investigate the drivers of spatial variation in interspecific synchrony between pairs of native and non-native species.
It is well known that the environmental context is an important aspect to consider when analyzing patterns in invasion ecology102 and when using a trait similarity approach with native species12. Selected sites were located in geographic regions placed in temperate zones (even in Australia), where temporal data is more readily available. Therefore, future studies should consider extrapolating these questions to different environments, such as tropical habitats and those with varying levels of disturbance, to understand better how these conditions might influence the importance of selected traits and the ecological mechanisms driving invasion dynamics. Also, it is crucial to replicate or adapt this study for different ecosystems (e.g., terrestrial and other aquatic environments). Another limitation of our study is that we were not able to determine the exact invasion stage of the non-native species analyzed (e.g., recently introduced or naturalized and spread) at each site despite all locations being sampled over an extended period. However, our results provide a general understanding of the main patterns involved in the long-term coexistence of native and non-native species in freshwater ecosystems.
Conclusion
In conclusion, we highlight that functional traits have proven to be valuable tools for inferring the temporal population dynamics of common non-native fish species and their synchrony with native communities. Also, we identified the primary mechanistic explanation (environmental filtering) that helps to drive the long-term coexistence between native and non-native fish species. This knowledge is essential for advancing our understanding of invasion ecology and informing conservation efforts. Firstly, we showed that trait similarity with native communities can help to understand the invasion process and the ecological mechanisms underlying temporal dynamics within populations in invaded ecosystems, using an approach that relies on interspecific synchrony - a relatively unexplored method. Secondly, it enhances our ability to forecast the temporal dynamics of widespread non-native species populations. This aspect can be a powerful strategy for policymakers and management decision-makers in developing effective prevention and management strategies for ecosystems threatened by widespread non-native species, one of the main concerns in ecology.
Data availability
The data used in this study are publicly available and were originally published in Comte et al. (2021). The dataset can be accessed at https://idata.idiv.de/ddm/Data/ShowData/1873?version=12.
References
Simberloff, D. Non-native species DO threaten the natural environment! J. Agric. Environ. Ethics. 18, 595–607. https://doi.org/10.1007/s10806-005-2851-0 (2005).
Doherty, T. S., Glen, A. S., Nimmo, D. G., Ritchie, E. G. & Dickman, C. R. Invasive predators and global biodiversity loss. Proc. Natl. Acad. Sci. 113, 11261–11265 (2016).
Gallardo, B., Clavero, M., Sánchez, M. I. & Vilà, M. Global ecological impacts of invasive species in aquatic ecosystems. Glob. Change Biol. 22, 151–163 (2016).
McNeely, J. Invasive species: a costly catastrophe for native biodiversity. Land Use Water Resour. Res. 1 (2001).
Gallardo, B. et al. InvasiBES: Understanding and managing the impacts of invasive alien species on Biodiversity and Ecosystem Services. NeoBiota. 50, 109–122, (2019).
Pejchar, L. & Mooney, H. A. Invasive species, ecosystem services and human well-being. Trends Ecol. Evol. 24, 497–504 (2009).
Catford, J. A., Jansson, R. & Nilsson, C. Reducing redundancy in invasion ecology by integrating hypotheses into a single theoretical framework. Divers. Distrib. 15, 22–40 (2009).
Hulme, P. E. & Bernard-Verdier, M. Comparing traits of native and alien plants: can we do better? Funct. Ecol. 32, 117–125 (2018).
Mowery, M. A., Vink, C., Mason, A. C. & Andrade, M. C. B. Behavioural, morphological, and life history shifts during invasive spread. Biol. Invasions. 23, 3497–3511. https://doi.org/10.1007/s10530-021-02593-6 (2021).
Rodrigues, A. C., Cucherousset, J., Cunha, E. R., dos Santos, N. C. L. & Gomes, L. C. Functional dissimilarity correlates to the co-occurrence patterns of native and non-native species. Biol. Invasions. 1–13. https://doi.org/10.1007/s10530-024-03321-6 (2024).
Enders, M. et al. A conceptual map of invasion biology: integrating hypotheses into a consensus network. Glob. Ecol. Biogeogr. 29, 978–991 (2020).
Gallien, L. & Carboni, M. The community ecology of invasive species: where are we and what’s next? Ecography 40, 335–352 (2017).
Carrara, F., Giometto, A., Seymour, M., Rinaldo, A. & Altermatt, F. Experimental evidence for strong stabilizing forces at high functional diversity of aquatic microbial communities. Ecology 96, 1340–1350 (2015).
Mori, A. S., Furukawa, T. & Sasaki, T. Response diversity determines the resilience of ecosystems to environmental change. Biol. Rev. 88, 349–364 (2013).
Carlsson, N. O., Jeschke, J. M., Holmqvist, N. & Kindberg, J. Long-term data on invaders: when the fox is away, the mink will play. Biol. Invasions. 12, 633–641 (2010).
Strayer, D. L., Eviner, V. T., Jeschke, J. M. & Pace, M. L. Understanding the long-term effects of species invasions. Trends Ecol. Evol. 21, 645–651 (2006).
Strayer, D. L., Solomon, C. T., Findlay, S. E. G. & Rosi, E. J. Long-term research reveals multiple relationships between the abundance and impacts of a non‐native species. Limnol. Oceanogr. 64 https://doi.org/10.1002/lno.11029 (2019).
Haubrock, P. J. et al. Biological invasions are a population‐level rather than a species‐level phenomenon. Glob. Change Biol. 30, e17312. https://doi.org/10.1111/gcb.17312 (2024).
Sousa, R., Nogueira, J. G. & Padilha, J. Moving from the species to the population level in biological invasions. Glob. Change Biol. 30, e17396 (2024).
Granzotti, R. V., Cassemiro, F. A., Agostinho, A. A. & Bini, L. M. Drivers of interspecific synchrony and diversity–stability relationships in floodplain fish communities. J. Anim. Ecol. (2024).
Houlahan, J. E. et al. Compensatory dynamics are rare in natural ecological communities. Proc. Natl. Acad. Sci. 104, 3273–3277. https://doi.org/10.1073/pnas.0603798104 (2007).
Mönkkönen, M., Devictor, V., Forsman, J. T., Lehikoinen, A. & Elo, M. Linking species interactions with phylogenetic and functional distance in European bird assemblages at broad spatial scales. Glob. Ecol. Biogeogr. 26, 952–962. https://doi.org/10.1111/geb.12605 (2017).
Shoemaker, L. G. et al. The long and the short of it: mechanisms of synchronous and compensatory dynamics across temporal scales. Ecology. 103, e3650 (2022).
Gonzalez, A. & Loreau, M. The causes and consequences of compensatory dynamics in ecological communities. Annu. Rev. Ecol. Evol. Syst. 40, 393–414. https://doi.org/10.1146/annurev.ecolsys.39.110707.173349 (2009).
Lepš, J., Májeková, M., Vítová, A., Doležal, J. & de Bello, F. Stabilizing effects in temporal fluctuations: management, traits, and species richness in high-diversity communities. Ecology 99, 360–371 (2018).
Thibaut, L. M., Connolly, S. R. & Sweatman, H. P. Diversity and stability of herbivorous fishes on coral reefs. Ecology 93, 891–901. https://doi.org/10.1890/11-1753.1 (2012).
Ives, A. R., Gross, K. & Klug, J. L. Stability and variability in competitive communities. Science 286, 542–544 (1999).
Klug, J. L., Fischer, J. M., Ives, A. R. & Dennis, B. Compensatory dynamics in planktonic community responses to pH perturbations. Ecology. 81, 387–398 (2000).
Morante-Filho, J. C. et al. Compensatory dynamics maintain bird phylogenetic diversity in fragmented tropical landscapes. J. Appl. Ecol. 55, 256–266 (2018).
Brown, B. L., Downing, A. L. & Leibold, M. A. Compensatory dynamics stabilize aggregate community properties in response to multiple types of perturbations. Ecology 97, 2021–2033 (2016).
Micheli, F. et al. The dual nature of community variability. Oikos. 161–169, (1999).
Case, E. J., Harrison, S. & Cornell, H. V. Do high-impact invaders have the strongest negative effects on abundant and functionally similar resident species? Funct. Ecol. 30, 1447–1453 (2016).
Rocha, M. R., Gaedke, U. & Vasseur, D. A. Functionally similar species have similar dynamics. J. Ecol. 99, 1453–1459. https://doi.org/10.1111/j.1365-2745.2011.01893.x (2011).
Ranta, E. et al. Detecting compensatory dynamics in competitive communities under environmental forcing. Oikos 117, 1907–1911 (2008).
Divíšek, J. et al. Similarity of introduced plant species to native ones facilitates naturalization, but differences enhance invasion success. Nat. Commun. 9, 4631 (2018).
Ricciardi, A., Hoopes, M. F., Marchetti, M. P. & Lockwood, J. L. Progress toward understanding the ecological impacts of nonnative species. Ecol. Monogr. 83, 263–282 (2013).
Rocha, B. S. & Cianciaruso, M. V. Water temperature and lake size explain Darwin’s conundrum for fish establishment in boreal lakes. Hydrobiologia. 1–10 (2020).
Azzurro, E. et al. External morphology explains the success of biological invasions. Ecol. Lett. 17, 1455–1463 (2014).
Henriksson, A., Yu, J., Wardle, D. A. & Englund, G. Biotic resistance in freshwater fish communities: species richness, saturation or species identity? Oikos 124, 1058–1064 (2015).
Ricklefs, R. E. & Miles, D. B. Ecological and evolutionary inferences from morphology: an ecological perspective. Ecol. Morphology: Integr. Organismal Biology. 1, 13–41 (1994).
Tedesco, P. & Hugueny, B. Life history strategies affect climate based Spatial synchrony in population dynamics of West African freshwater fishes. Oikos 115, 117–127 (2006).
Frimpong, E. A. & Angermeier, P. L. Trait-based approaches in the analysis of stream fish communities. Community ecology of stream fishes: concepts, approaches, and techniques. Symposium. vol. 73, 109–136, (American Fisheries Society, 2010).
Winemiller, K. O. Ecomorphological diversification in lowland freshwater fish assemblages from five biotic regions. Ecol. Monogr. 61, 343–365 (1991).
Adite, A. & Winemiller, K. O. Trophic ecology and ecomorphology of fish assemblages in coastal lakes of Benin, West Africa. Ecoscience 4, 6–23 (1997).
Xu, M. et al. Exotic fishes that are phylogenetically close but functionally distant to native fishes are more likely to establish. Glob. Change Biol. 28, 5683–5694 (2022).
Xu, M. et al. Global freshwater fish invasion linked to the presence of closely related species. Nat. Commun. 15, 1411 (2024).
Cano-Barbacil, C., Radinger, J., Grenouillet, G. & García‐Berthou, E. Phylogenetic signal and evolutionary relationships among traits of inland fishes along elevational and longitudinal gradients. Freshw. Biol. 67, 912–925 (2022).
Mouquet, N. et al. Ecophylogenetics: advances and perspectives. Biol. Rev. 87, 769–785 (2012).
Capellini, I., Baker, J., Allen, W. L., Street, S. E. & Venditti, C. The role of life history traits in mammalian invasion success. Ecol. Lett. 18, 1099–1107 (2015).
McKinney, M. L. & Lockwood, J. L. Biotic homogenization: a few winners replacing many losers in the next mass extinction. Trends Ecol. Evol. 14, 450–453 (1999).
Blanchet, S. et al. Non-native species disrupt the worldwide patterns of freshwater fish body size: implications for Bergmann’s rule. Ecol. Lett. 13, 421–431 (2010).
Su, G., Villéger, S. & Brosse, S. Morphological sorting of introduced freshwater fish species within and between donor realms. Glob. Ecol. Biogeogr. 29, 803–813 (2020).
Tonella, L. H. et al. Importance of feeding strategies on the long-term success of fish invasions. Hydrobiologia 817, 239–252 (2018).
Hayden, B. et al. Interactions between invading benthivorous fish and native whitefish in subarctic lakes. Freshw. Biol. 58, 1234–1250. https://doi.org/10.1111/fwb.12123 (2013).
McKnight, E., García-Berthou, E., Srean, P. & Rius, M. Global meta‐analysis of native and nonindigenous trophic traits in aquatic ecosystems. Glob. Change Biol. 23, 1861–1870 (2017).
Muniz, C. M., García-Berthou, E., Ganassin, M. J. M., Agostinho, A. A. & Gomes, L. C. Alien fish in Neotropical reservoirs: assessing multiple hypotheses in invasion biology. Ecol. Ind. 121, 107034 (2021).
Erős, T. et al. Effects of nonnative species on the stability of riverine fish communities. Ecography 43, 1156–1166 (2020).
Sorte, C. J. et al. Poised to prosper? A cross-system comparison of climate change effects on native and non‐native species performance. Ecol. Lett. 16, 261–270 (2013).
Gido, K. B., Propst, D. L., Olden, J. D. & Bestgen, K. R. Multidecadal responses of native and introduced fishes to natural and altered flow regimes in the American Southwest. Can. J. Fish. Aquat. Sci. 70, 554–564 (2013).
Light, T. & Moyle, P. 19 Assembly Rules and Novel Assemblages in Aquatic Ecosystems. (2015).
Eby, L. A., Fagan, W. F. & Minckley, W. L. Variability and dynamics of a desert stream community. Ecol. Appl. 13, 1566–1579 (2003).
Marchetti, M. P. & Moyle, P. B. Effects of flow regime on fish assemblages in a regulated California stream. Ecol. Appl. 11, 530–539 (2001).
Comte, L. et al. RivFishTIME: A global database of fish time‐series to study global change ecology in riverine systems. Glob. Ecol. Biogeogr. 30, 38–50 (2021).
Sakai, A. K. et al. The population biology of invasive species. Annu. Rev. Ecol. Syst. 32, 305–332 (2001).
Fricke, R. Eschmeyer’s catalog of fishes: Genera/species by family/subfamily. Electronic version accessed. 25, 2021 (2021).
Lintermans, M. Human-assisted dispersal of alien freshwater fish in Australia. N. Z. J. Mar. Freshwat. Res. 38, 481–501. https://doi.org/10.1080/00288330.2004.9517255 (2004).
Trochine, C. et al. Non-native fish occurrence and biomass in 1943 Western Palearctic lakes and reservoirs and their abiotic and biotic correlates. Ecosystems. 1–15 (2017).
Brosse, S. et al. FISHMORPH: A global database on morphological traits of freshwater fishes. Glob. Ecol. Biogeogr. 30, 2330–2336. https://doi.org/10.1111/geb.13395 (2021).
Froese, R. & Pauly, D. FishBase. (2010).
Blanck, A. & Lamouroux, N. Large-scale intraspecific variation in life‐history traits of European freshwater fish. J. Biogeogr. 34, 862–875 (2007).
de Bello, F., Botta-Dukat, Z., Leps, J., Fibich, P. & Fibich, M. P. Package ‘gawdis’. Multi-Trait Dissimilarity with More Uniform Contributions (2021).
Nakamura, G., Richter, A. & Soares, B. E. FishPhyloMaker: an R package to generate phylogenies for ray-finned fishes. Ecol. Inf. 66, 101481 (2021).
Rabosky, D. L. et al. An inverse latitudinal gradient in speciation rate for marine fishes. Nature 559, 392–395 (2018).
Paradis, E. & Schliep, K. Ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35, 526–528 (2019).
Lepš, J., Götzenberger, L., Valencia, E. & de Bello, F. Accounting for long-term directional trends on year‐to‐year synchrony in species fluctuations. Ecography. 42, 1728–1741 https://doi.org/10.1111/ecog.04528 (2019).
Catford, J. A., Wilson, J. R., Pyšek, P., Hulme, P. E. & Duncan, R. P. Addressing context dependence in ecology. Trends Ecol. Evol. 37, 158–170 (2022).
Pregler, K. C., Lu, X., Valentine, G. P., Kim, S. & Kanno, Y. Temperature variation generates interspecific synchrony but spatial asynchrony in survival for freshwater fish communities. Ecol. Evol. 13, e10700. https://doi.org/10.1002/ece3.10700 (2023).
Weber, M. J., Brown, M. L., Wahl, D. H. & Shoup, D. E. Metabolic theory explains latitudinal variation in common carp populations and predicts responses to climate change. Ecosphere 6, 1–16 (2015).
Sparks, A. Nasapower: A NASA POWER global meteorology, surface solar energy and climatology data client for R. J. Open. Source Softw. 3, 1035. https://doi.org/10.21105/joss.01035 (2018).
Bates, D. et al. Package ‘lme4’. CRAN. (R Foundation for Statistical Computing, 2012).
Lüdecke, D. et al. Performance: Assessment of Regression Models Performance. CRAN: Contributed Packages (2019).
Hartig, F. DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models. R package version 0.4. 6. (2022).
Nakagawa, S. & Schielzeth, H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 4, 133–142 (2013).
Barton, K. MuMIn: Multi-model Inference. R package version 1.9. 13. (R Foundation for Statistical Computing, 2013).
Lüdecke, D. sjPlot: data visualization for statistics in social science.–R package ver. 2.8. 14. (2023).
R Core Team. A Language and Environment for Statistical Computing. https://www.R-project.org (R Foundation for Statistical Computing, 2021).
van Klink, R., Lepš, J., Vermeulen, R. & de Bello, F. Functional differences stabilize beetle communities by weakening interspecific temporal synchrony. Ecology. 100, e02748. https://doi.org/10.1002/ecy.2748 (2019).
Davidson, J. L. & Shoemaker, L. G. Resistance and resilience to invasion is stronger in synchronous than compensatory communities. Ecology 104, e4162. https://doi.org/10.1002/ecy.4162 (2023).
Lepš, J., Májeková, M., Vítová, A., Doležal, J. & De Bello, F. Stabilizing effects in temporal fluctuations: management, traits, and species richness in high-diversity communities. Ecology 99, 360–371. https://doi.org/10.1002/ecy.2065 (2018).
Rocha, B. S., García-Berthou, E., Novaes, J. L. C., Bini, L. M. & Cianciaruso, M. V. Interspecific synchrony is related to body-length similarity in a fish community under prolonged drought conditions. Sci. Total Environ. 781, 146721 (2021).
Rocha, M. R., Vasseur, D. A. & Gaedke, U. Seasonal variations alter the impact of functional traits on plankton dynamics. PLoS ONE. 7, e51257 (2012).
Da Silva, V. E. et al. Functional traits of fish species: adjusting resolution to accurately express resource partitioning. Front. Mar. Sci. 6, 303 (2019).
Peters, R. H. The Ecological Implications of Body Size. 2, (1986).
Côte, J., Kuczynski, L. & Grenouillet, G. Morphology reflects differently the various facets of species traits in stream fish. Freshw. Biol. 67, 1203–1213. https://doi.org/10.1111/fwb.13911 (2022).
Koehn, J. D. Carp (Cyprinus carpio) as a powerful invader in Australian waterways. Freshw. Biol. 49, 882–894 (2004).
Ribeiro, F. & Collares-Pereira, M. J. Life‐history variability of non‐native centrarchids in regulated river systems of the lower river Guadiana drainage (south‐west Iberian Peninsula). J. Fish Biol. 76, 522–537 (2010).
Kurtul, I. et al. Exploring invasiveness and versatility of used microhabitats of the globally invasive Gambusia holbrooki. Sci. Total Environ. 925, 171718 (2024).
Arthington, A. H. Ecological and genetic impacts of introduced and translocated freshwater fishes in Australia. Can. J. Fish. Aquat. Sci. 48, 33–43 (1991).
Beatty, S. J. et al. What factors influence fin-nipping damage by the invasive Gambusia holbrooki (Poeciliidae) on native fishes in riverine systems? Freshw. Biol. 67, 325–337 (2022).
Andreoli Bize, J. & Fernandez, L. A. Invasion alert: new record of the exotic Gambusia holbrooki Girard, 1859 in the Puna Austral region, Northwestern of Argentina. (2019).
Laughlin, D. R. & Werner, E. E. Resource partitioning in two coexisting sunfish: pumpkinseed (Lepomis gibbosus) and Northern longear sunfish (Lepomis Megalotis peltastes). Can. J. Fish. Aquat. Sci. 37, 1411–1420 (1980).
Wang, J. et al. Analysing spatio-temporal patterns of non‐native fish in a biodiversity hotspot across decades. Divers. Distrib. 29, 1492–1507 (2023).
Acknowledgements
We want to thank FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) for granting the research scholarship to BSR (proc. Nº 2024/00555-7). ACR was funded through the ForestFisher project through the 2020–2021 Biodiversa and Water JPI joint call for research projects, under the BiodivRestore ERA-NET Cofund (GA n°101003777), with the EU and the funding organizations ANR, FCT, FAPEAM, DFG and FUNDECT. ACR was also supported by the CRBE Laboratory through the LABEX TULIP and CEBA (ANR-10-599 LABX-41, ANR-10-LABX-25-01). RVG is funded by CNPq (proc. Nº 380640/2022-8). This work was developed in the context of the National Institutes for Science and Technology (INCT) in Ecology, Evolution and Biodiversity Conservation (EECBio), supported by MCTI/CNPq (proc. 465610/2014-5) and FAPEG (Fundação de Amparo à Pesquisa do Estado de Goiás).
Author information
Authors and Affiliations
Contributions
All authors (B.S.R., A.C.R., and R.V.G.) contributed equally to this manuscript. They were involved in the conceptualization and design of the study, data analysis, and writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Rocha¹, B.S., Rodrigues, A.C. & Granzotti, R.V. Widespread freshwater non-native fishes exhibit synchronized population dynamics with functionally similar natives. Sci Rep 15, 19753 (2025). https://doi.org/10.1038/s41598-025-04587-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-04587-z