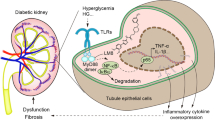
Abstract
This study examined the impact of inflammation on hypoxic renal tubular epithelial cell (RTEC) injury in a hyperglycemic environment, emphasizing the regulatory role of miR-125b and the mechanisms by which diabetes influences acute kidney injury. A hypoxia/reoxygenation (H/R) model was established in mouse RTECs. Mouse RAW264.7 macrophages were pre-treated under seven conditions: high glucose (HG), normal glucose (NG), HG + miR-125b inhibition (HG + miR-125b inhibitor), HG control (HG + vector), mannitol control (NG + mannitol), and M1/M2 macrophage positive controls. Each group was co-cultured with hypoxic/reoxygenated RTECs for 24 h. The optimal H/R model was achieved with 4 h of hypoxia followed by 24 h of reoxygenation. Macrophages pre-treated with HG and co-cultured with H/R RTECs showed significantly increased apoptosis, reactive oxygen species (ROS) fluorescence intensity, and epithelial injury/oxidative stress markers (lactate dehydrogenase [LDH] and malondialdehyde [MDA]), along with decreased antioxidant superoxide dismutase (SOD) levels. IL-1β levels significantly increased, while IL-10 levels decreased. All renal tubular injury markers increased significantly (P < 0.01 or P < 0.05). However, miR-125b inhibition reduced apoptosis, ROS, LDH, MDA, and renal injury marker levels while increasing SOD and IL-10 levels (P < 0.01 or P < 0.05). Thus, hyperglycemia-induced macrophage polarization toward the M1 phenotype exacerbates hypoxic RTEC damage, which can be partially mitigated by miR-125b inhibition.
Similar content being viewed by others
Introduction
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disease. The World Health Organization has identified T2DM as a serious global health threat1. The International Diabetes Federation estimates that the prevalence of diabetes will be 11.3% in 2030 and 12.2% in 20452. Acute kidney injury (AKI) has also become an important health problem worldwide in recent years. The incidence of AKI has increased annually (especially among hospitalized patients), affecting 13–18% of hospitalized patients and up to 40% of critically ill patients3,4. Many previous studies have shown that T2DM is an independent risk factor for AKI and that AKI is a powerful predictor of long-term renal complications and all-cause mortality in patients with T2DM5,6. More severe AKI or poor recovery from it has been associated with a higher risk of complications7. However, research on the relationship between the pathogenic mechanisms of AKI and DM is currently limited. In recent years, the microinflammatory state caused by glycolipid metabolism-related disorders and other factors has been shown to be an important cause of organ damage in T2DM8. Moreover, patients with diabetes have a weakened immune function and are prone to various infections. We previously reported that infection is one of the main causes of AKI in hospitalized patients9. Our research on the impact of diabetes on the recurrence and prognosis of acute renal injury in older men showed that, among hospitalized patients with AKI, those with T2DM were more likely to have recurrent AKI. Etiological analysis showed a significantly higher proportion of patients with T2DM with recurrent AKI due to infection than that of patients without T2DM10, suggesting that hyperglycemia, infection, and inflammation may play important roles in AKI occurrence.
We previously demonstrated that changes in the renal tubulointerstitial area can be observed early, accompanied by inflammatory cell infiltration, represented by macrophages (mainly of the M1 type) in a rat model of T2DM11. Macrophages can be categorized as pro-inflammatory (M1) or anti-inflammatory (M2) based on their polarization effects12. We speculated that a shift toward a pro-inflammatory phenotype and infiltration into renal tissue may lead to the early micro-inflammatory environment that characterizes diabetic kidneys. In this microenvironment, hypoxic–ischemic changes can induce renal tubular damage, leading to AKI. In recent years, a study reported that AKI is also an inflammatory response and that macrophages play an important role in the renal damage caused by this response and in the progression of AKI to chronic kidney disease (CKD)13. M1 macrophages are highly expressed within the first 24 h after the occurrence of ischemic AKI and significantly decreased after 3 days14. In the early stage of AKI, macrophages are mainly of the M1 type, mediating renal inflammation and causing local tissue damage, whereas at the later stage, M2 macrophages play a major role in inhibiting renal inflammatory response and promoting tissue repair15. Thus, macrophages play an important role in the inflammatory state of diabetes and in the occurrence and development of AKI caused by diabetes. The aim of this study was to identify targets to improve the micro-inflammatory environment of T2DM by regulating the direction of macrophage polarization.
Can macrophage polarization be reversed through targeted regulation of some factors to reduce the occurrence of AKI? MicroRNAs (miRNAs) are small non-coding RNAs that play important roles in the post-transcriptional regulation of genes. Among these, miR-125b may be an effective target for regulating macrophage polarization. Upregulation of miR-125b expression promotes macrophage activation and enhances macrophage-mediated inflammatory responses16. Moreover, miR-125b is closely related to microvascular disease in diabetes17. Studies suggest that miR-125b is significantly increased during renal ischemia–reperfusion (IR) injury, making it a potential novel marker that can be used to diagnose and treat renal IR injury18.
However, at present, only few studies, both domestically and internationally, have reported on the regulation of macrophage polarity by miR-125b. An miR-125b-induced change in the polarization state of macrophages stimulated by high sugar levels may modify the microinflammatory environment in diabetic kidneys and reduce AKI occurrence. Therefore, we co-cultured mouse RAW264.7 macrophages and renal tubular epithelial cells (RTECs) in vitro to explore the impact of inflammation on hypoxic RTEC injury under high sugar conditions and investigate the role of miR-125b in evaluating the mechanism by which diabetes affects AKI occurrence.
Methods
Cell culture and experimental groups
RAW264.7 cells were cultured in RPMI-1640 medium containing 10% FBS at 37 °C in a 5% CO2 incubator and passaged. Healthy growing RAW264.7 cells were plated at a density of 2 × 105 cells per well in a six-well cell culture plate and cultured overnight. The cells were treated with different experimental interventions according to the experimental group: (1) high glucose (HG): high-glucose medium (30 mmol/L glucose); (2) normal control (NG): normal medium (5.6 mmol/L glucose); (3) HG + miR-125b inhibitor (HG + miR-125b inhibitor); (4) HG control (HG + vector): HG medium (30 mmol/L glucose) + empty vector; (5) mannitol control (NG + mannitol): normal medium + mannitol (24.4 mmol/L); (6) M1 macrophage positive control (NG + LPS/IFN-γ): normal medium + LPS (100 ng/mL)/IFN-γ (20 ng/ml); (7) M2 macrophage positive control (NG + IL-4): normal medium + IL-4 (20 ng/mL). The M1 and M2 positive control groups were cultured for 24 h, whereas the rest were cultured for 72 h (Fig. 1).
Materials
The mouse RAW264.7 monocyte-macrophage cell line was purchased from the Chinese Academy of Sciences Cell Bank (Beijing, China). Mouse RTECs were obtained from the Shanghai Hu Zhen Biotechnology Company (Shanghai, China). The cells were cultured in 0.25% trypsin, fetal bovine serum (FBS), 1% penicillin–streptomycin, glucose-free Roswell Park Memorial Institute (RPMI)-1640 medium, and Dulbecco’s Modified Eagle Medium (DMEM) purchased from Gibco (Carlsbad, CA, USA). Interleukin-4 (IL-4), lipopolysaccharide (LPS), and interferon-γ (IFN-γ) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Lipofectamine 2000 and TRIzol reagent were purchased from Invitrogen (Carlsbad, CA, USA). Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) kits were obtained from TOYOBO (Osaka, Japan). Cell Counting Kit-8 (CCK-8) assay and cellular reactive oxygen species (ROS) detection kits were acquired from Shanghai Bi Yun Tian Biotechnology Company (Shanghai, China). Annexin V–fluorescein isothiocyanate/propidium iodide (FITC/PI) kits were obtained from Sigma-Aldrich (St. Louis, MO, USA). Lactate dehydrogenase (LDH) (microplate method), malondialdehyde (MDA) (thiobarbituric acid [TBA] method), and superoxide dismutase (SOD) (water-soluble tetrazolium 1 [WST-1] method) assay kits were acquired from Nanjing Jian Cheng Technology Company (Nanjing, China). Mouse liver fatty acid binding protein (L-FABP), neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1), hypoxia-inducible factor-1α (HIF-1α), monocyte chemoattractant protein 1 (MCP-1), and intercellular cell adhesion molecule-1 (ICAM-1) enzyme-linked immunosorbent assay (ELISA) kits were purchased from BYK (Bad Homburg County, Hesse, Germany). Mouse interleukin (IL)-10, IL-1β, IL-6, and IL-12 ELISA kits were obtained from Sigma-Aldrich (St. Louis, MO, USA). NGAL and KIM-1 antibodies were purchased from Affinity (Melbourne, Australia). L-FABP and ICAM-1 antibodies were obtained from Proteintech (Chicago, IL, USA). CO2 incubators (MCO-15AC) were purchased from SANYO (Osaka, Japan). Inverted microscopes (OLYMPUS IX51) were acquired from Beijing Pure Science Instrument Co., Ltd (Beijing, China). Transwell chambers were obtained from Corning (Corning, NY, USA). Low-speed centrifuges were purchased from Eppendorf (Hamburg, Germany). Microplate readers (MULTISKAN MK3) were acquired from Thermo Fisher Scientific (Waltham, MA, USA). Flow cytometers (CytoFLEX) were purchased from BECKMAN (La Brea, CA, USA).
Methods
miR-125b Inhibition
miRNA inhibitors are chemically modified molecules that specifically target a particular miRNA and can enter the cell via packaging in transfection reagents. In this study, we used Lipofectamine 2000 to transfect the miR-125b inhibitor into the RAW264.7 cells to inhibit miR-125b expression. The inhibitor was synthesized by Hanheng Biotechnology Co., Ltd. (sequence: UCACAAGUUAGGGUCUCAGGA), and transfection was performed according to the manufacturer’s instructions. The efficiency of miR-125b inhibition was assessed in our previous study19 (Supplementary Fig. 1). Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) was used to evaluate this inhibition efficiency, with U6 as an internal reference. miR-125b expression level was calculated using the 2 − ΔΔCt method.
Establishment of a hypoxia/reoxygenation (H/R) model in mouse RTECs
This experiment included a blank control group and an H/R treatment group. H/R treatment group: RTECs were digested, passaged in cell culture flasks, and seeded into six-well plates at a density of 2 × 10⁵ cells per well. The oxygen concentration in a tri-gas incubator was adjusted to 1% O₂, 94% N₂, and 5% CO₂. The six-well plates were then placed in the incubator for hypoxic culture for 4, 12, and 24 h. After hypoxia, the plates were removed, the culture medium was discarded, and the cells were washed with phosphate-buffered saline (PBS) before being placed in a CO₂ incubator with 21% O₂ for reoxygenation. Cells were collected at 0, 4, 12, and 24 h. Blank control group: Cells were continuously cultured under normoxic conditions (21% O₂, 5% CO₂, 37℃), with all other procedures identical to those in the H/R group. After culturing both groups, cell viability and apoptosis rates were assessed, and appropriate time points were selected to establish the H/R model for mouse RTECs.
Detection of hypoxia injury indicators
CCK-8 to detect cell viability
At different time points in the H/R culture, the RTECs and supernatant were collected. Next, 10 µL of CCK-8 was added to each well, and the cells were cultured at 37 °C for 3 h. The absorbance at OD450 was then measured using a microplate reader (Thermo MULTISKAN MK3). The time point with the lowest cell viability was selected to detect apoptosis in mouse RTECs through flow cytometry.
Flow cytometry to detect cell apoptosis
The cells were digested with trypsin without ethylenediaminetetraacetic acid (EDTA) and centrifuged at 1000 rpm for 5 min. The cells were then resuspended once with pre-cooled 1× PBS at 4 °C, centrifuged at 1000 rpm for 5 min, and washed. Next, 300 µL of PBS was added to resuspend the cells, and 5 µL each of Annexin V–FITC and PI were added. The cell suspension was mixed well and incubated at 37 °C in the dark for 10 min. Fluorescence was then detected via flow cytometry.
Detection of oxidative stress indicators
After cell centrifugation, the levels of MDA, SOD, and LDH in the supernatant were measured according to the manufacturer’s instructions.
MDA determination
According to manufacturer’s instructions, supernatant samples were mixed evenly, incubated at 95 °C for 40 min, cooled with running water, and centrifuged at 4000 rpm for 10 min. The supernatant was removed, and the absorbance value in each tube was measured at 532 nm using a spectrophotometer.
SOD assay. Supernatant samples were mixed evenly and incubated at 37 °C for 20 min. The results of ELISA were determined by measuring absorbance at 450 nm. Each sample was run in triplicate.
LDH determination
Supernatant samples were mixed evenly and incubated at room temperature for 5 min. Absorbance at 450 nm was determined using a reader for ELISA. Each sample was run in triplicate.
\(\:LDH\:activity\:\left(\frac{U}{L}\right)=\frac{measured\:OD\:value\:-\:control\:OD\:value}{standard\:OD\:value\:-\:blank\:OD\:value}*\:standard\:concentration\:\left(2\frac{mmol}{L}\right)*\:1000\)L
ELISA to detect levels of IL-10, IL-1β, and renal tubular injury markers in cell supernatants
After cell centrifugation, the levels of IL-10, IL-1β, MCP-1, KIM-1, ICAM-1, NGAL, L-FABP, and HIF-1α in the cell supernatants were detected following the manufacturer’s instructions in each case, with three replicates performed per sample.
Western blotting to detect intracellular protein levels of renal tubular injury markers
Total protein was extracted from each experimental group, and the concentrations were determined. The samples were then subjected to electrophoresis and transferred and blocked with defatted milk at room temperature. Primary antibodies for NGAL (1:1000), L-FABP (1:1000), ICAM-1 (1:1000), KIM-1 (1:1000), and GAPDH (1:5000) were diluted in blocking solution and incubated with the membranes overnight at 4 °C. The membranes were then washed 5–6 times with tris-buffered saline with Tween® 20 Detergent (TBST) for 5 min each and then incubated with HRP-conjugated AffiniPure goat anti-rabbit IgG (H + L) (1:5000; Boster Biological Technology, Wuhan, China. Catalog#BA1054) at room temperature for 1 h. The TBST washes were then repeated, and the membranes were developed with enhanced chemiluminescence (ECL) reagent (Applygen, China). The molecular weight and net optical density of the target bands were measured using an imaging equipment (Carestream Health, China) and analyzed using ImageJ software.
Statistical analysis
All experiments were conducted independently and repeated three times. Statistical analysis was performed using IBM SPSS Statistics for Windows, version 26.0. The levels of miR-125b in each experimental group; those of IL-10, IL-1β, MCP-1, KIM-1, ICAM-1, NGAL, L-FABP, and HIF-1α in the cell supernatant; and the expression levels of ICAM-1, KIM-1, NGAL, and L-FABP protein in the cells all showed normal distributions and are represented as means ± standard deviations. Comparisons between and among groups were performed using t-tests and one-way analysis of variance (ANOVA), respectively. Post hoc pairwise comparisons between groups were performed using the least significant difference (LSD) t-test. Differences with P < 0.05 were considered statistically significant.
Results
Establishment of the mouse RTEC H/R model
RTEC viability
The CCK-8 assay results indicated significantly decreased RTEC viability with increasing duration of hypoxia and reoxygenation. Notably, after 4 h of hypoxia followed by 24 h of reoxygenation (H4h/R24h), cell viability was significantly reduced (P < 0.01; Fig. 2).
RTEC apoptosis rates
Flow cytometry showed that, compared with that in the blank control group, the early apoptosis rate significantly increased in RTECs subjected to the H4h/R24h conditions (1.65% vs. 24.4%, P < 0.01; Fig. 3). RTECs are primarily characterized by apoptosis during the early stages of AKI. With AKI progression, necrosis occurs, leading to a rapid deterioration in renal function. We found that cell viability was the lowest at H4h/R24h (P < 0.01) and that the early apoptosis rate was significantly higher than that in the control group (P < 0.01). This is consistent with the early pathological and physiological changes in renal tubular injury in AKI and represents the optimal times for H/R.
Detection of indicators after co-culture of macrophages with H/R RTECs
Apoptosis rates
The results of flow cytometry performed after 24 h of co-culture of pre-treated macrophages and H/R RTECs showed significantly increased overall apoptosis rates of RTECs in the M1 macrophage-positive control and HG co-culture groups compared with those in the NG group (11.5% vs. 2% and 7.1% vs. 2%, respectively P < 0.01). After inhibiting the expression of miR-125b in macrophages, the apoptosis rate of RTECs in the HG + miR-125b inhibitor group (3.6%) was significantly lower than that in the HG group (P < 0.01; Fig. 4).
Detection of oxidative stress indicators (ROS, LDH, MDA, and SOD)
The results of flow cytometry after 24 h of co-culture of pre-treated macrophages with H/R RTECs results showed significantly increased (P < 0.01) and decreased (P < 0.05) mean fluorescence intensity of ROS in RTECs from the M1 macrophage-positive and M2 macrophage-positive control co-culture groups, respectively. The mean fluorescence intensity of ROS in the RTECs from the HG co-culture group was significantly higher than that from the NG group (P < 0.01). After inhibiting miR-125b expression in macrophages, the mean fluorescence intensity of ROS in the RTECs of the HG + miR-125b inhibitor group was significantly lower than that in the HG group (P < 0.01; Fig. 5).
Flow cytometry detection of ROS fluorescence intensity in each cell group. (a) NG group; (b) NG + mannitol group; (c) NG + M1group; (d) NG + M2 group; (e) HG group; (f) HG + vector group; (g) HG + miR-125b inhibitor group; (h) histogram. *Compared with the NG and HG groups, P < 0.05. **Compared with the NG and HG groups, P < 0.01. HG, high glucose; NG, normal glucose; ROS, reactive oxygen species.
The SOD levels decreased significantly (P < 0.01) in the cell supernatant from the M1 macrophage-positive control co-culture group, whereas the LDH and MDA levels increased significantly (P < 0.01). In the M2 macrophage-positive control co-culture group, the SOD levels increased significantly, while the LDH and MDA levels decreased significantly (P < 0.01). The SOD level in the cell supernatant of the HG co-culture group decreased significantly compared with that in the NG group (P < 0.01), while the LDH and MDA levels increased significantly (P < 0.05 and P < 0.01, respectively). After inhibiting miR-125b expression in the macrophages, the SOD level in the cell supernatant from the HG + miR-125b inhibitor group increased significantly compared with that in the HG group (P < 0.01), while the LDH and MDA levels decreased significantly (P < 0.05 and P < 0.01, respectively; Fig. 6).
Levels of LDH, MDA, and SOD in the supernatant. (a) LDH level; (b) MDA level; (c) SOD level. *Compared with the NG and HG groups, P < 0.05. **Compared with the NG and HG groups, P < 0.01. HG, high glucose, LDH, lactate dehydrogenase; MDA, malondialdehyde; NG, normal glucose; SOD, superoxide dismutase.
Levels of IL-10 and IL-1β and renal tubular injury marker levels in cell supernatants
ELISA performed after 24 h of co-culture with pre-treated macrophages and H/R RTECs revealed significantly increased IL-1β levels and significantly decreased IL-10 levels in the cell supernatant from the M1 macrophage-positive control co-culture group (all P < 0.01). The levels of renal tubular injury markers (KIM-1, ICAM-1, NGAL, L-FABP, MCP-1, and HIF-1α) all increased significantly (P < 0.05 and P < 0.01, respectively). In the M2 macrophage-positive control co-culture group, the IL-1β level in the cell supernatant showed a decreasing trend (P > 0.05), while the IL-10 level increased significantly (P < 0.01). The MCP-1, KIM-1, NGAL, and L-FABP levels showed decreasing trends (P > 0.05), while the ICAM-1 and HIF-1α levels decreased significantly (P < 0.01). Compared with the NG group, cell supernatant from the HG co-culture group showed a significant increase and decrease in IL-1β and IL-10 levels, respectively (P < 0.05 and P < 0.01, respectively), as well as significant increase in MCP-1, HIF-1α, KIM-1, ICAM-1, NGAL, and L-FABP levels (P < 0.05 and P < 0.01, respectively). After inhibiting miR-125b expression in macrophages, the MCP-1, HIF-1α, ICAM-1, KIM-1, NGAL, and L-FABP levels in the RTECs in the HG + miR-125b inhibitor group all decreased significantly compared with those in the HG group (all P < 0.05), while the IL-1β and IL-10 levels decreased (P < 0.05) and increased (P < 0.01) significantly (Fig. 7).
Levels of IL-10, IL-1β, and renal tubular injury markers in each experimental group, measured by ELISA. (a) IL-10 levels; (b) IL-1β levels; (c) KIM-1 and IL-10 levels; (d) ICAM-1 levels; (e) NGAL levels; (f) L-FABP levels; (g) HIF-1α levels; (h) MCP-1 levels. *Compared with the NG and HG groups, P < 0.05. **Compared with the NG and HG groups, P < 0.01. HG, high glucose; HIF-1α, hypoxia-inducible factor-1 alpha; ICAM-1, intercellular cell adhesion molecule-1; IL, interleukin; KIM-1, kidney injury molecule-1; L-FABP, liver fatty acid binding protein; MCP-1, monocyte chemoattractant protein 1; NG, normal glucose; NGAL, neutrophil gelatinase-associated lipocalin.
Protein levels of renal tubular injury markers
The western blotting results after 24 h of co-culture of pre-treated macrophages with H/R RTECs showed that the protein expression levels of KIM-1, ICAM-1, NGAL, and L-FABP in the RTECs were significantly increased in the M1 macrophage-positive control co-culture group compared with those in the NG group (all P < 0.01). In the M2 macrophage-positive control group, the protein level of KIM-1 was significantly decreased (P < 0.01), while those of ICAM-1, NGAL, and L-FABP showed decreasing trends (P > 0.05). The HG co-culture group showed significantly increased protein expression levels of KIM-1, ICAM-1, NGAL, and L-FABP (all P < 0.01). After inhibiting expression of miR-125b in macrophages, the protein expression levels of KIM-1, ICAM-1, NGAL, and L-FABP decreased significantly in the HG + miR-125b inhibitor group compared with those in the HG group (P < 0.05 and P < 0.01, respectively; Figs. 8 and 9).
Western blotting analysis of protein expression of renal tubular injury markers in each cell group by electrophoresis. Lane 1: NG group; Lane 2: NG + mannitol group; Lane 3: NG + LPS + IFN-γ group; Lane 4: NG + IL-4 group; Lane 5: HG group; Lane 6: HG + vector group; Lane 7: HG + miR-125b inhibitor group. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HG, high glucose; ICAM-1, intercellular cell adhesion molecule-1; IFN-γ, interferon-gamma; KIM-1, kidney injury molecule-1; IL-4, interleukin 4; L-FABP, mouse liver fatty acid binding protein; LPS, lipopolysaccharide; miR-125b, microRNA-125b; NG, normal glucose.
Western blot analysis of protein expression levels of renal tubular injury markers in each cell group. (a) ICAM-1 protein content; (b) NGAL protein content; (c) L-FABP protein content; (d) KIM-1 protein content. *Compared with the NG and HG groups, P < 0.05. **Compared with the NG and HG groups, P < 0.01. HG, high glucose; ICAM-1, intercellular cell adhesion molecule-1; IFN-γ, interferon-gamma; KIM-1, kidney injury molecule-1; IL-4, interleukin 4; L-FABP, mouse liver fatty acid binding protein; LPS, lipopolysaccharide; miR-125b, microRNA-125b; NG, normal glucose.
Discussion
T2DM is a global public health concern. According to 2019 survey data from the International T2DM Alliance, approximately 463 million people worldwide have T2DM, corresponding to a prevalence of 9.3%. By 2030, the number of affected individuals worldwide is expected to reach 578 million20. T2DM is a persistent disease with a poor prognosis that often leads to complications in multiple organs. Recent studies have reported the high incidence of AKI in patients with T2DM and that these patients are more likely to develop AKI than those without T2DM. In their retrospective study, Hapca et al.21 reported two- and five-fold increased risks of developing AKI among patients with T2DM with and without CKD, respectively. Our recent study also revealed that patients with T2DM are more likely to have recurrent AKI than patients without T2DM and that T2DM is a risk factor for recurrent AKI10. Without timely intervention after AKI development, patients with T2DM can experience deteriorated kidney function, progression to CKD and ESRD, premature entry into dialysis, or even death. We previously reported the poor prognosis of patients with T2DM and multiple recurrent AKI within 2 years10. Thakar et al.22 identified AKI attacks as an independent risk factor for T2DM progression to CKD stage 4. The more AKI attacks that occur, the higher the risk of progression to CKD stage 4. Therefore, clinical management should focus on AKI caused by T2DM.
Recent research has established that diabetes is not merely a metabolic disorder but a disease mediated by inflammation23. In individuals with diabetes, adipocytes can recruit macrophages to secrete a large number of pro-inflammatory cytokines, including cytokines/chemokines, matrix metalloproteinases, and growth factors, thereby forming a systemic inflammatory environment24,25,26. This systemic inflammatory environment exacerbates renal injury in patients with type 2 diabetes. A previous study has reported the involvement of multiple signaling pathways, such as the NF-κB, JAK/STAT, nuclear factor erythroid 2-related factor 2 (Nrf2), and Rho kinase pathways, in the production of pro-inflammatory factors27. We previously demonstrated that the first lesion appeared in the renal tubulointerstitial area, mainly manifesting as RTEC degeneration and inflammatory cell infiltration in the renal tubular interstitium, in a T2DM rat model induced by a high-fat, high-sugar diet and a low dose of streptozotocin. Subsequently, we observed increased glomeruli volume, mesangium proliferation, focal interstitial fibrosis, and vascular lesions. Among these effects, inflammatory cell infiltration, renal tubular degeneration, and necrosis appeared almost simultaneously. In inflammatory cell infiltration, the number of CD68-positive cells increased significantly compared with that in the control group, and the expression of Toll-like receptor 4 also increased significantly, indicating that T2DM micro-inflammation may primarily manifest as renal tubulointerstitial damage and that monocyte/macrophage infiltration may be important pathological and physiological changes in the early renal tubulointerstitial lesions of T2DM11. Similarly, Calle et al. reported that macrophage infiltration occurs almost simultaneously with kidney damage in in type 2 diabetes28, and the persistent infiltration of pro-inflammatory M1 macrophages leads to a decline in renal function. Based on these observations, we speculate that the proinflammatory microenvironment makes patients with T2DM more susceptible to AKI, with more severe damage and a poorer prognosis. Reversing the micro-inflammatory environment of the kidney in the early stages of T2DM may improve renal tubular tolerance to hypoxic damage in patients with T2DM, thereby reducing AKI incidence and improving the prognosis in these patients. The results of the present study demonstrated that downregulation of miR-125b expression may partially reverse the M1 polarization of macrophages induced by high-sugar stimulation. In this study, we simulated AKI-induced renal tubular injury by co-culturing RTECs with macrophages using the H/R RTEC model and mimicked the high sugar-induced inflammatory microenvironment to evaluate the impact of macrophage polarization changes induced by high sugar on hypoxic injury to RTECs and the regulatory role of miR-125b.
Our results demonstrated that under high sugar stimulation, the levels of inflammatory factors in RTECs increased, indicating that high sugar levels promote M1 polarization in macrophages, consistent with our previous findings19. Moreover, the levels of renal tubular injury markers also increased, indicating that renal tubular injury is aggravated by inflammation. Skrypnyk et al. reported that the IL-6 levels in the plasma of mice increased 2 h after the occurrence of AKI29. Similarly, Yang et al. examined AKI in patients with aneurysmal subarachnoid hemorrhage and reported that the high-sensitivity C-reactive protein (hs-CRP) levels were significantly higher in patients who developed AKI than in those who did not30. Increased macrophage infiltration and M1 macrophage polarization have been reported in a rat model for T2DM with periodontal lesions31. In vitro experiments have demonstrated that chronic high sugar levels can induce macrophages to transform into the M1 type and mediate a sustained chronic inflammatory response, which may be related to the inhibition of AKT protein phosphorylation32. In an experimental mouse model of T2DM, macrophage infiltration occurred in the early stage of T2DM and was related to kidney damage28. Macrophages play an important role in kidney damage caused by inflammatory responses and in the progression of AKI to CKD33. After the kidney has been subjected to IR injury, inflammatory cells play a key role in AKI development and prognosis. In the early stages of AKI, macrophages are mainly of the M1 type, promoting inflammation, whereas in the later stages of AKI, they are mainly of the M2 type, promoting tissue repair15. Long-term inflammatory stimulation or improper repair can lead to irreversible damage to kidney structure and function, which is one of the main reasons for AKI progression to CKD34. Animal models of AKI have demonstrated that direct or indirect control of the intensity of the inflammatory response can significantly reduce the degree of kidney damage35,36. Therefore, regulation of the direction of macrophage polarization is critical for reducing renal tubular injury, delaying kidney function deterioration, and protecting kidney function. Additionally, in the establishment of the H/R model, we found that viability was the lowest at H4h/R24h, possibly due to cells gradually adapting to prolonged hypoxia and activating protective mechanisms, resulting in relatively higher cell activity after reoxygenation. Additionally, prolonged hypoxia may also lead to removal of severely damaged cells, leaving behind healthier cells that exhibit increased activity upon reoxygenation.
High sugar stimulation can cause severe inflammation, oxidative stress, and interstitial fibrosis in the kidney proximal tubules. miRNAs are involved in regulating these processes37 and can also promote RTEC apoptosis induced by high sugar levels38. In HG-induced HK-2 cells, miR-125b can promote ROS production by inhibiting angiotensin-converting enzyme 2 expression, promoting the injury and apoptosis of HK-2 cells39. miR-125b overexpression protects against myocardial IR injury by reducing the apoptosis of myocardial cells induced by it and the activity of caspase-3/7 and caspase-840. A recent study41 reported downregulated serum miR-125b levels, which were associated with higher mortality and readmission rates in patients with heart failure and AKI. Güçlü et al.18 observed elevated miR-125b levels in the mouse kidney ischemia–reperfusion model and proposed miR-125b as a marker of AKI occurrence.
In conclusion, in the present study, after downregulating miR-125b expression, the levels of inflammatory factors in RTECs decreased, as did those of renal tubular injury markers and the apoptosis and oxidative stress rates, indicating that miR-125b can reverse the inflammatory changes caused by macrophages during hypoxic renal tubular injury, reduce kidney damage, and play a protective role in the kidney. These findings provide new directions for future studies aimed at reducing kidney damage during AKI and delaying the deterioration of kidney function. However, further research is needed on the specific inflammatory pathways involving macrophages during hypoxic renal tubular injury and on the mechanism of action of miR-125b.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Ye, J. et al. The global, regional and National burden of type 2 diabetes mellitus in the past, present and future: a systematic analysis of the global burden of disease study 2019. Front. Endocrinol. (Lausanne). 14, 1192629 (2023).
Sun, H. et al. IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 183, 109119 (2022).
Ftouh, S., Lewington, A. & Acute Kidney Injury Guideline Development Group convened by the National Clinical Guidelines Centre and commissioned by the National Institute for Health and Care Excellence. Association with the Royal college of physicians’ clinic. Prevention, detection and management of acute kidney injury: concise guideline. Clin. Med. (Lond). 14, 61–65 (2014).
Nisula, S. et al. Incidence, risk factors and 90-day mortality of patients with acute kidney injury in Finnish intensive care units: the FINNAKI study. Intensive Care Med. 39, 420–428 (2013).
Waikar, S. S., Liu, K. D. & Chertow, G. M. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin. J. Am. Soc. Nephrol. 3, 844–861 (2008).
James, M. T. et al. A meta-analysis of the association of estimated GFR, albuminuria, diabetes mellitus, and hypertension with acute kidney injury. Am. J. Kidney Dis. 66, 602–612 (2015).
Jiang, G. et al. Clinical predictors and long-term impact of acute kidney injury on progression of diabetic kidney disease in Chinese patients with type 2 diabetes. Diabetes 71, 520–529 (2022).
Aly, R. H. et al. Patterns of toll-like receptor expressions and inflammatory cytokine levels and their implications in the progress of insulin resistance and diabetic nephropathy in type 2 diabetic patients. Front. Physiol. 11, 609223 (2020).
Wen, J. et al. Hospital-acquired acute kidney injury in Chinese very elderly persons. J. Nephrol. 26, 572–579 (2013).
Shen, X. et al. Impact of diabetes on the recurrence and prognosis of acute kidney injury in older male patients: A 10-year retrospective cohort study. Diabetes Ther. 13, 1907–1920 (2022).
Liu, Y. et al. Protective effect of Shenkang injection against renal ischemia-reperfusion injury via inflammation Inhibition in type 2 diabetic rats. Int. J. Clin. Exp. Med. 11, 10446–10457 (2018).
Juhas, U., Ryba-Stanisławowska, M., Szargiej, P. & Myśliwska, J. Different pathways of macrophage activation and polarization. Postepy Hig Med. Dosw (Online). 69, 496–502 (2015).
Kumar, S. Cellular and molecular pathways of renal repair after acute kidney injury. Kidney Int. 93, 27–40 (2018).
Lee, S. et al. Distinct macrophage phenotypes contribute to kidney injury and repair. J. Am. Soc. Nephrol. 22, 317–326 (2011).
Mu, Y. F. et al. Macrophage-driven inflammation in acute kidney injury: therapeutic opportunities and challenges. Transl Res. 278, 1–9 (2025).
Chaudhuri, A. A. et al. Microrna-125b potentiates macrophage activation. J. Immunol. 187, 5062–5068 (2011).
Villeneuve, L. M. et al. Enhanced levels of microRNA-125b in vascular smooth muscle cells of diabetic db/db mice lead to increased inflammatory gene expression by targeting the histone methyltransferase Suv39h1. Diabetes 59, 2904–2915 (2010).
Güçlü, A. et al. MicroRNA-125b as a new potential biomarker on diagnosis of renal ischemia-reperfusion injury. J. Surg. Res. 207, 241–248 (2017).
Shen, X. et al. Regulatory effect of high glucose on polarization of RAW264.7 macrophages via miR-125b in mice. J. Jilin Univ. (Med Ed). 48, 847–857 (2022).
Tomic, D. et al. Lifetime risk, life expectancy, and years of life lost to type 2 diabetes in 23 high-income jurisdictions: a multinational, population-based study. Lancet Diabetes Endocrinol. 10, 795–803 (2022).
Hapca, S. et al. The relationship between AKI and CKD in patients with type 2 diabetes: an observational cohort study. J. Am. Soc. Nephrol. 32, 138–150 (2021).
Thakar, C. V., Christianson, A., Himmelfarb, J. & Leonard, A. C. Acute kidney injury episodes and chronic kidney disease risk in diabetes mellitus. Clin. J. Am. Soc. Nephrol. 6, 2567–2572 (2011).
Sinha, S. K., Carpio, M. B. & Nicholas, S. B. Fiery connections: macrophage-mediated inflammation, the journey from obesity to type 2 diabetes mellitus and diabetic kidney disease. Biomedicines 12, 2209 (2024).
Youssef, N., Noureldein, M. H., Riachi, M. E., Haddad, A. & Eid, A. A. Macrophage polarization and signaling in diabetic kidney disease: a catalyst for disease progression. Am. J. Physiol. Ren. Physiol. 326, F301–F312 (2024).
Engel, J. E. & Chade, A. R. Macrophage polarization in chronic kidney disease: A balancing act between renal recovery and decline? Am. J. Physiol. Ren. Physiol. 317, F1409–F1413 (2019).
Matoba, K. et al. Unraveling the role of inflammation in the pathogenesis of diabetic kidney disease. Int. J. Mol. Sci. 20, 3393 (2019).
Donate-Correa, J. et al. Inflammatory cytokines in diabetic kidney disease: pathophysiologic and therapeutic implications. Front. Med. (Lausanne). 7, 628289 (2020).
Calle, P. & Hotter, G. Macrophage phenotype and fibrosis in diabetic nephropathy. Int. J. Mol. Sci. 21, 2806 (2020).
Skrypnyk, N. I. et al. IL-6-mediated hepatocyte production is the primary source of plasma and urine neutrophil gelatinase-associated Lipocalin during acute kidney injury. Kidney Int. 97, 966–979 (2020).
Yang, B. H., He, Q., Ding, C. Y., Kang, D. Z. & Tang, Q. X. High-sensitivity C-reactive protein as a predictive factor of acute kidney injury following aneurysmal subarachnoid hemorrhage: a prospective observational study. Acta Neurochir. (Wien). 161, 1783–1791 (2019).
Zhang, B., Yang, Y., Yi, J., Zhao, Z. & Ye, R. Hyperglycemia modulates M1/M2 macrophage polarization via reactive oxygen species overproduction in ligature-induced periodontitis. J. Periodont Res. 56, 991–1005 (2021).
Luo, W., Ai, L., Li, X., Wang, B. & Zhou, Y. [Chronic high glucose inhibits AKT phosphorylation and promotes M1 polarization of mouse RAW264.7 macrophages]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 35, 910–917 (2019).
Tian, Z. W. et al. Macrophages in acute kidney injury. Int. J. Geriatr. 41, 335–338 (2020).
Yu, S. M. W. & Bonventre, J. V. Acute kidney injury and progression of diabetic kidney disease. Adv. Chronic Kidney Dis. 25, 166–180 (2018).
Sutton, T. A. et al. p53 is renoprotective after ischemic kidney injury by reducing inflammation. J. Am. Soc. Nephrol. 23, 696–705 (2012).
Ahn, J. M. et al. Hypoxia-inducible factor activation protects the kidney from gentamicin-induced acute injury. PLOS One. 7, e48952 (2012).
Delić, D. et al. Urinary Exosomal MiRNA signature in type II diabetic nephropathy patients. PLOS One. 11, e0150154 (2016).
Yang, B. Y., Zhao, M. & Wang, M. Research advances of relationship between MicroRNA and renal tubular injury in diabetic nephropathy. J. Clin. Nephrol. 22, 161–165 (2022).
Huang, Y. F., Zhang, Y., Liu, C. X., Huang, J. & Ding, G. H. microRNA-125b contributes to high glucose-induced reactive oxygen species generation and apoptosis in HK-2 renal tubular epithelial cells by targeting angiotensin-converting enzyme 2. Eur. Rev. Med. Pharmacol. Sci. 20, 4055–4062 (2016).
Wang, X. et al. MicroRNA-125b protects against myocardial ischaemia/reperfusion injury via targeting p53-mediated apoptotic signalling and TRAF6. Cardiovasc. Res. 102, 385–395 (2014).
Xue, Q. et al. lncRNA ROR and miR-125b predict the prognosis in heart failure combined acute renal failure. Dis. Markers 6853939 (2022). (2022).
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (82170684 awarded to Qingli Cheng and Youth program 81600655 awarded to Guang Yang).
Author information
Authors and Affiliations
Contributions
QC: conceptualization, writing – review & editing, project administration. GY: methodology, writing – review & editing. XS: writing – original draft, supervision, investigation, methodology. YL: formal analysis, investigation, supervision. JZ: data curation, formal analysis, software. All authors contributed to the study conception and design, interpreted the data, and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shen, X., Liu, Y., Zhao, J. et al. Effects of inflammation on hypoxic renal tubular epithelial cell injury under high-glucose conditions and the regulatory role of miR-125b. Sci Rep 15, 21248 (2025). https://doi.org/10.1038/s41598-025-05701-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-05701-x