Abstract
Understanding the behaviour of malaria vectors could lead to improved designs of partially treated insecticidal nets and low-cost nets with less insecticide content. The behaviour of Anopheles arabiensis around cow baited bed nets with partial adhesive treatments were assessed in experimental huts. The study was conducted in Moshi Tanzania, using a Latin Square design with five arms: no bed-net, intact untreated bed-net, roof, sides and whole adhesive treated bed-nets. The data analyses were done using generalized linear mixed effects models with proportions of mosquitoes on the bed-net panels, and induced exiting as outcomes and trial arm as the predictor. There were significant reductions in the likelihood of An. arabiensis found on the side (adjusted odds ratio, AOR: 0.50; 95% CI 0.26–0.98; p value = 0.044) and roof (AOR: 0.18; 0.07–0.43; p value < 0.001) of adhesive treated bed-nets compared to the whole adhesive treated bed-net. The likelihood of An. arabiensis exiting was significantly higher in the intact untreated net trial arm compared to the no net trial arm (AOR: 2.12; 1.19–3.79; p value = 0.010). Host seeking An. arabiensis is not persistent and untreated bed-nets induced exiting, implying reduced efficacy of partially treated insecticidal nets.
Similar content being viewed by others
Introduction
Vector control using insecticide treated bed nets (ITNs) and indoor residual spraying (IRS) are the main malaria control strategy and contributed to approximately 78% of the decline in malaria cases between 2000 and 20151,2. ITN intervention exploits the host seeking behaviour of female mosquitoes by acting as a physical barrier between the host and the vector and the insecticide component could act by killing, sterilizing or repelling the vector that contacts the net3,4. IRS intervention exploits the resting behaviour of the malaria vector as it is likely to rest on indoor surfaces after taking a blood meal5. As such, IRS is used to treat potential malaria vector resting surfaces such as internal walls, eaves and ceilings of all housing including domestic animal shelters5.
Insecticide resistance has the potential to reduce the impact of ITNs and IRS in the fight against malaria6. With widespread resistance of malaria vectors to pyrethroids7, the main active ingredient (AI) in ITNs, there has been a drive to develop ITNs with AIs that have different modes of action8,9,10,11. Similarly, there are reports of wide spread resistance to insecticides (carbamates, organophosphates and organochlorines) used in IRS7 hence the drive to develop products with AIs that have different modes of actions12,13,14,15,16,17. Development of ITNs and IRS products with AIs that have different modes of action to the conventional insecticides used for malaria vector control, offers opportunities to manage insecticide resistance via rotation. Furthermore, an increased awareness to detrimental effects to non-target organisms due to increased use of insecticides and disposal practices and call to reduce costs, has catalysed innovation of tools with less insecticides for vector control such as selective spraying for IRS18 or weighing the use of durable untreated nets19 to reflect a shift to environmentally safe and cost-effective options; and could contribute to sustaining gains so far made in malaria control.
The malaria burden continuously declined between 2000 and 2015, but progress has stalled20. To accelerate progress in reducing the burden of malaria, deployment of both ITNs and IRS has been explored in randomized control trials (RCTs) and observational studies21. However, these studies have produced contradictory evidence21. For example, a RCT in Tanzania showed that deploying ITNs and IRS together reduced malaria prevalence compared to deploying the ITN only22. On the contrary, in Benin and Gambia deploying ITNs and IRS did not significantly reduce clinical malaria compared to deploying ITNs only22,23.
The World Health Organization (WHO) does not recommend co-deployment of ITNs and IRS for prevention and control of malaria in areas where there is ongoing malaria transmission, as evidenced from a systematic review20,24. However, co-deployment of ITNs and IRS may be considered for insecticide resistance prevention, mitigation or management20.
Understanding the behaviour of malaria vectors in the vicinity of control tools such as ITNs and IRS could allow optimal co-deployment of ITNs and IRS. A number of methods have been used to study the behaviour of mosquitoes around bed nets including the use of infra-red video tracking and adhesives among others25,26. Using adhesive on bed nets to study mosquito behaviour in experimental huts is ideal as it may represent a theoretical potent insecticide that immediately kills all mosquitoes that come in contact or highlights the key contact net panels where most mosquitoes contact first. This may enhance the understanding of the behaviour of host-seeking malaria vectors that come in contact with ITNs, and in houses where ITNs and IRS are co-deployed or selectively applied with the view of optimizing insecticide use and deployment of the interventions.
Behavioural studies focussing on host seeking for a blood meal suggest that host seeking Anopheles gambiae and A arabiensis make multiple brief contacts on the roof compared with side panels of ITNs25,26,27,28. This has opened possibilities for designing nets to optimize insecticide use while controlling resistance. If host seeking malaria vectors are persistent, those that first contact the roof panel could also move on to contact with the side panel of a partially treated insecticidal net and vice versa; resulting in mortality equivalent to a whole treated ITN. A previous study showed that nets partially treated with a pyrethroid on the roof or side were as efficacious as fully treated nets against An. arabiensis29. In contrast, another study showed that the efficacy of a dual AI (pyrethroid and chlorfenapyr) roof treated net against An. arabiensis had reduced efficacy compared to a whole dual AI treated net in cow baited experimental huts30. Such partially treated insecticidal nets could be co-deployed with IRS to manage resistance. There is need to investigate the behaviour of malaria vectors around ITNs in experimental huts to inform strategies on co-deployment of IRS and ITNs or development of selective treated ITNs. In this study the behaviour of An. arabiensis around cow baited partially treated adhesive bed nets in experimental huts was investigated. It included persistence, panel of a bed net which more mosquitoes contact, and associations between induced exophily, and blood feeding.
Results
A total of 836 female An. arabiensis were collected over 25 trap nights in all trial arms. Of these, 214 (25.6%; 95% CI 22.7–28.7%) were from the no net; 121 (14.5%; 95% CI 12.1–17.0%) from the untreated, 140 (16.7%; 95% CI 14.3–19.4%) from the roof panel with adhesive, 144 (17.4%; 95% CI 14.7–20.0%) from the side panel with adhesive and 217 (26.0%; 95% CI: 23.0–29.1%) from whole adhesive treated net trial arms.
In the trial arms with adhesive coated nets, the highest proportion of An. arabiensis caught on the nets was recorded in the whole adhesive treated net arm compared to the side panel and roof panel adhesive treated net arms (Fig. 1). There was no persistence shown as there were significant reductions (p value > 0.05) in the likelihood of An. arabiensis being caught on either the side panel or roof panel adhesive treated nets compared to the whole adhesive treated net (Table 1).
The predicted mean numbers of An. arabiensis that were caught on the roof and side panels of the whole adhesive treated net were 0.64 (95% CI 0.29–0.99) and 1.32 (95% CI 0.77–1.87) respectively. These results showed that a significant majority of An. arabiensis first contacted the bed net on the side panel compared to the roof panel of the whole adhesive treated net (Table 2).
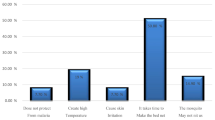
A total of 428 An. arabiensis exited the experimental hut of which 94, 73, 78, 76, and 107 were in the no net, untreated net, side adhesive treated net, roof adhesive treated net, and whole adhesive treated net trial arms respectively. The highest percentage of induced exophily was shown in the untreated net trial arm (Fig. 2). The likelihood of An. arabiensis exiting was significantly higher in the untreated net trial arm compared the no net trial arm (AOR: 2.12; 95% CI 1.19–3.79; p value = 0.010; Z = 2.57). There was no significant difference in the likelihood of exiting between the no net trial arm and the other trial arms (Table 3). Further analysis in the no net trial arm showed that blood feeding success significantly reduced the likelihood of An. arabiensis exiting the huts (AOR: 0.10; 95% CI 0.02–0.41; p value = 0.001; Z = − 3.22).
The association between blood fed status and exiting in the No net trial arm is summarized in Table 4 below.
Discussion
This study assessed persistence, point of first contact on bed nets, and associations between induced exophily, and blood feeding of An. arabiensis in East African style experimental huts. These are aspects of mosquito behaviour that could be exploited for vector control and inform rational strategies for management of insecticide resistance; including hybrid nets, selective treatment of ITNs and co-deployment of ITNs and IRS. The results in this study showed that An. arabiensis is not persistent on cow baited bed nets, i.e. mosquitoes that first have contact with the roof panel are unlikely to go on and contact the side panel. Additionally, the results showed that the number of An. arabiensis mosquitoes contacting the side panels were twice those contacting the roof panel, but less than whole adhesive net. The implication is that bed nets with insecticides restricted to either the roof or side panel may not be as efficacious as a whole insecticide treated bed nets. This assertion is supported by a previous study by Mbewe et al. (2022) and a systematic review by Lissenden et al. (2025), which reported a significantly lower mortality of An. arabiensis in a trial arm with insecticide treated roof bed net panels compared to whole insecticide treated bed net30,31. Furthermore, a meta-analysis of studies conducted at nine facilities showed that bed nets with insecticides restricted to either the roof or side panels were not equivalent or superior to whole insecticide treated bed nets in terms of mortality and blood feeding inhibition31. Therefore, studies on different designs of bed nets with restricted applied insecticides are recommended. Such designs could include bed nets with strip(s) of insecticide treated netting running across from one side panel to another through the roof panel.
It is possible that the lack of persistence could be unique to An. arabiensis, the bait (cow) and experimental hut design used in this study. More so that other studies have implicated odour plumes from the host, indoor air currents and cross-draughts to influence An. gambiae activity at either the roof or side panels of untreated bed nets32,33. However, a meta-analysis of nine studies that used more than one Anopheles species, human and animal baits, and a variety of experimental hut designs showed that partially treated insecticidal nets were less efficacious in terms of mortality compared to whole treated insecticidal nets31. Therefore, the results observed in this study could imply that the lack of persistence observe by An. arabiensis applies to other Anopheles mosquitoes.
Exiting of An. arabiensis from the huts was highest in the untreated net compared to the no net and adhesive treated trial arms. It was further observed that the likelihood of exiting was significantly higher in the untreated net arm compared to the no net arm. The observation suggests that the untreated net induced exiting. On the other hand, the likelihood of exiting in the adhesive treated net arms was not significantly different to the no net arm. This could have been due to the adhesive treated nets’ ability to retain the mosquitoes. Furthermore, mosquitoes that were blood fed in the no net arm were more likely to rest indoors than blood unfed. It is possible that the observed higher exiting in the untreated net arm was due to unsuccessful blood feeding attempt by host seeking An. arabiensis, attributed to the net barrier effect. With host seeking mosquitoes unlikely to rest indoors in the presence of bed net, the implications are that, where ITNs (more so with excito repellent insecticides like pyrethroids) are deployed and usage is high, IRS may not have an additive killing effect. This assertion seems to be supported by a meta-analysis that reported no additive impact of IRS on malaria incidence in communities using pyrethroid-like ITNs34.
The current study’s observations seem to reinforce the WHO guideline of not recommending co-deployment of ITNs and IRS for prevention and control of malaria in children and adults in areas with ongoing transmission20. Furthermore, the induced exiting of An. arabiensis in the presence of a bed net raises a number of questions. Where next do the exiting blood unfed mosquitoes make an attempt to take a blood meal? Could these blood unfed exiting mosquitoes be responsible for outdoor malaria transmission? More studies are required to answer these questions and understand the malaria transmission dynamics especially that the core vector control interventions ITNs and IRS target indoor resting and biting mosquitoes. Such studies could help in developing further guidelines and strategies for malaria vector control.
An aspect that has not been looked at in this study is whether a torn net would still induce An. arabiensis to exit the huts. With a higher chance of blood feeding on a host under a torn bed net, it is possible that mosquitoes could rest indoors; and if indoor resting surfaces are sprayed with insecticides, perhaps there could be an additive impact on mosquito mortality. In such a case, co-deployment of ITNs and IRS could be synergetic to impact malaria transmission, especially that overtime, ITNs get damaged and develop holes35,36,37,38. Therefore, there is need to investigate whether IRS could have an additive effect on mosquito mortality in the presence of a torn net.
Conclusions
This study found that more An. arabiensis contacted the side than the roof panel of a bed net; and those that contact the roof panel do not go on to contact the side panel and vice versa. Therefore, studies on different designs of bed nets that do not only restrict treatments to particular panels to reduce loading of insecticides are recommended. Additionally, host seeking mosquitoes are unlikely to rest indoors in the presence of an intact bed net. Therefore, co-deploying of intact bed nets and IRS may not be appropriate, as the latter requires mosquitoes to rest indoors to be effective. However, further studies on co-deployment of damaged bed nets and IRS are recommended.
Methods
Study site
The study was conducted at the Kilimanjaro Christian Medical University College Pan African Malaria Vector Research Consortium (KCMUco-PAMVERC) Pasua field site (S03° 22.764′, E37° 20.793′)39 in Lower Moshi, Tanzania from June to October 2022. The site has seven East African style experimental huts40 next to the Lower Moshi rice irrigation scheme. The rice plant periods: June to September and November to January coincide with the peak density of the major Anopheline species, An. arabiensis in the area29,39. This strain of An. arabiensis is partly zoophilic, feeding on cattle and humans41,42; and exhibits moderate pyrethroid resistance driven by overexpression of P450 mixed function oxidases43,44.
Study design
A behavioural experiment was conducted in the experimental huts (Fig. 3) focusing on the behaviour of An. arabiensis in huts with intact cow baited glue treated bed nets. The experiment used wild free flying mosquitoes that entered the huts. Due to the partly zoophilic behaviour of the local An. arabiensis, young cows were used as bait instead of human volunteers41,42. Wooden enclosures (cow frames) of dimensions 140 cm width, 120 cm height and 180 cm length were made to hold the cows within the huts (Fig. 3). The cows were put in the huts at 18:00 until mosquito collections at 06:00 the following morning. The untreated bed nets (Safi net: A to Z Textile Mills, Arusha Tanzania) used in the experiment were locally sourced and prepared at the KCMUCo-PAMVERC Whole net store in Moshi.
Net preparation and trial arms: The net panels were coated with Tangle-Trap® sticky coating brush adhesive (The Tanglefoot Company, Grand Rapids USA). The present experiment hut trial had five arms where four arms had nets while in one arm there was no net. Four nets were assigned unique codes 178B–179B and 181B–182B. Therefore, the treatment arms were as follows:
-
i.
178B: Roof coated with adhesive, Sides coated with adhesive
-
ii.
179B: Roof without adhesive, Sides coated with adhesive
-
iii.
181B: Roof coated with adhesive, Sides without adhesive
-
iv.
182B: Roof without adhesive, Sides without adhesive
-
v.
No net
Net preparations
Net 178B was prepared by cutting the stitching of all the panels using scissors to end up with five panels comprising of one roof panel, two short-side panels and two long-side panels. Each net panel was placed on a horizontal surface and adhesive coats applied. Both sides of each net panel were covered with non-stick baking paper and roll up. The ends of the cylinders that formed were fastened with rubber bands and label as 178B-R for the roof panel, 178B-SS1 for the first short-side panel, 178B-SS2 for the second short-side panel, 178B-LS1 for the first-long side panel and 178B-LS2 for the second-long side panel. Net 179B was prepared similarly to net 178B except for coating the roof panel with adhesive. These panels were labelled as 179B-SS1 for the first short-side panel, 179B-SS2 for the second short-side panel, 179B-LS1 for the first long-side panel and 179B-LS2 for the second long-side panel. The detached roof panel was rolled up and placed in a sealable plastic bag labelled 179B-R. Net 181B was prepared by cutting the stitching that joins the roof panel to the side panels of the net. The roof panel of the net was placed on a horizontal surface and coats of the adhesive applied on it. Both sides of the entire panel were covered with non-stick baking paper and rolled up. Both ends of the cylinder that formed were fastened with rubber bands and labelled 181B-R. Then the line of stitching on the detached side netting of 181B was cut and then rolled up. The netting was put in a sealable plastic bag and labelled 181B. The control net 182B was prepared by cutting the stitching that joins the roof panel to the side panels. Thereafter, one line of stitching was cut down on the detached side netting. The roof and side panels were placed in separate sealable plastic bags labelled 182B-R and 182B-S respectively. All nets prepared, were transferred to Pasua field station where, each roof and side panel were stapled to a wooden roof and side panel frame of the cow frames respectively. Using a permanent marker, each frame was labelled with the treatment arm code.
Hut trial
Five experimental huts were used to compare five trial arms in a 5 × 5 Latin square design. The trial arms were randomly allocated to huts and rotated every sixth day to account for possible location bias of the huts.
Cow frames, blankets and other items used in mosquito collection were also rotated on the 6th day along with the treatment arms to reduce the chance of contamination of these items. The 6th day was also reserved for cleaning and airing the huts to reduce any possible contamination between the treatments. Cows were rotated daily between the huts. This hut trial ran for 25 nights to complete one full Latin square rotation. The number of mosquitoes was recorded according to the location of collection in the experimental hut, i.e. on the net, resting in the room, exit trap and verandah. Two nested surveys were conducted in the adhesive whole treated net and no net trial arms of the hut trial. In the adhesive whole treated net trial arm, data on the point of first contact on the net were collected, while data on associations between induced exophily and blood feeding were collected in the no net arm. In the whole adhesive treated trial arm, mosquito collection on the net was recorded according to the panel i.e. side and roof.
Data analysis
The behaviour of An. arabiensis in each trial arm was compared to the control in terms of the outcomes: persistence, point of first contact on the bed net and induced exophily. Persistence is the percent increase in the number of mosquitoes caught on the side panel and roof panel only adhesive treated nets relative to the whole adhesive treated net. Point of first contact on the net is the number of mosquitoes entering the hut found stuck on the roof relative to those found on the side panels of the whole adhesive treated bed net or vice versa in the morning. Induced exophily is percentage of the mosquitoes collected from the exit and verandah traps of treated huts relative to the percentage caught in exit and verandah traps of the control hut.
Stata SE version 16.1 (StataCorp LCC, College Station, TX, USA) to perform statistical analyses. Generalised linear mixed effects models (GLMM) with either a log or logit link function were used to analyse each outcome variable: induced exophily (proportions) and point of first contact on the net (counts); with predicators trial arms, and blood feeding; and random effects due to cows, huts and Latin Square rotation of the trial arm. All graphs were created in Microsoft Excel Office 2019 (Microsoft Corporation, Redmond, WA, USA). Means predicted from the GLMM with the log link are reported. Statistical significance was considered at α < 0.05.
Ethics declarations
Informed consent and permission were sought from owners before the cows were used as baits in the trial.
The trial was conducted in conformity with London School of Hygiene and Tropical Medicine’s Animal Welfare and Ethical Review Board ethical rules for studies with animal under reference 2020-02. Ethical approval to conduct the study was granted by Tanzania’s National Institute for Medical Research reference NIMR/HQ/R.8a/Vol.IX/1656. All methods were performed in accordance with the guidelines and regulations of Tanzania’s National Institute for Medical Research.
Data availability
The data generated and analysed during the current study are available from the corresponding author upon reasonable request.
References
World Health Organization. Global Plan for Insecticide Resistance Management in Malaria Vectors. https://apps.who.int/iris/handle/10665/44846 (2012).
Bhatt, S. et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526, 207–211 (2015).
Bermejo, A. & Veeken, H. Insecticide-impregnated bed nets for malaria control: A review of the field trials. Bull. World Health Organ. 70, 293–296 (1992).
Azizi, S. et al. Evaluation of durability as a function of fabric strength and residual bio-efficacy for the olyset plus and interceptor G2 LLINs after 3 years of field use in Tanzania. Trop. Med. Infect. Dis. 8, 379 (2023).
WHO. Indoor Residual Spraying, An Operational Manual for Indoor Residual Spraying (IRS) for Malaria Transmission Control and Elimination. (WHO, Geneva, 2015).
WHO. Global Technical Strategy for Malaria 2016–2030, 2021 Update. (2012).
WHO. World Malaria Report 2022. (Geneva, 2022).
Abílio, A. P. et al. Bio-efficacy of new long-lasting insecticide-treated bed nets against Anopheles funestus and Anopheles gambiae from central and northern Mozambique. Malar. J. 14, 352 (2015).
Ngufor, C. et al. Olyset duo® (a pyriproxyfen and permethrin mixture net): An experimental hut trial against pyrethroid resistant Anopheles gambiae and Culex quinquefasciatus in Southern Benin. PLoS ONE 9, e93603 (2014).
N’Guessan, R. et al. Mosquito nets treated with a mixture of chlorfenapyr and alphacypermethrin control pyrethroid resistant Anopheles gambiae and Culex quinquefasciatus mosquitoes in West Africa. PLoS ONE 9, 1–6 (2014).
Tungu, P. K., Michael, E., Sudi, W., Kisinza, W. W. & Rowland, M. Efficacy of interceptor G2, a long—lasting insecticide mixture net treated with chlorfenapyr and alpha—cypermethrin against Anopheles funestus: Experimental hut trials in north—eastern Tanzania. Malar. J. 20, 1–15. https://doi.org/10.1186/s12936-021-03716-z (2021).
Tungu, P. K. et al. Large-scale (Phase III) evaluation of broflanilide 50WP (VECTRONTM T500) for indoor residual spraying for malaria vector control in Northeast Tanzania: Study protocol for a two-arm, non-inferiority, cluster-randomised community trial. BMC Infect. Dis. 22, 171 (2022).
Govoetchan, R. et al. VECTRONTM T500, a new broflanilide insecticide for indoor residual spraying, provides prolonged control of pyrethroid-resistant malaria vectors. Malar. J. 21, 324 (2022).
Mbewe, N. J. et al. A non-inferiority and GLP-compliant study of broflanilide IRS (VECTRONTM T500), a novel meta-diamide insecticide against Anopheles arabiensis. Front. Trop. Dis. 4, 1126869 (2023).
Ngufor, C. et al. Indoor spraying with chlorfenapyr (a pyrrole insecticide) provides residual control of pyrethroid-resistant malaria vectors in southern Benin. Malar. J. 19, 249 (2020).
Oxborough, R. M. et al. Evaluation of indoor residual spraying with the pyrrole insecticide chlorfenapyr against pyrethroid-susceptible Anopheles arabiensis and pyrethroid-resistant Culex quinquefasciatus mosquitoes. Trans. R. Soc. Trop. Med. Hyg. 104, 639–645 (2010).
Ngufor, C., Fongnikin, A., Rowland, M. & N’Guessan, R. Indoor residual spraying with a mixture of clothianidin (a neonicotinoid insecticide) and deltamethrin provides improved control and long residual activity against pyrethroid resistant Anopheles gambiae sl in Southern Benin. PLoS ONE 12, e0189575 (2017).
Irish, S. R. et al. A review of selective indoor residual spraying for malaria control. Malar. J. 23, 252 (2024).
Okumu, F. The fabric of life: What if mosquito nets were durable and widely available but insecticide-free?. Malar. J. 19, 260 (2020).
World Health Organization. WHO Guidelines for Malaria (World Health Organization, 2023).
Protopopoff, N. et al. Effectiveness of a long-lasting piperonyl butoxide-treated insecticidal net and indoor residual spray interventions, separately and together, against malaria transmitted by pyrethroid-resistant mosquitoes: A cluster, randomised controlled, two-by-two fact. Lancet 391, 1577–1588 (2018).
Collins, P. Y., Insel, T. R., Chockalingam, A., Daar, A. & Maddox, Y. T. Grand challenges in global mental health: Integration in research, policy, and practice. PLoS Med. 10(4), e1001434. https://doi.org/10.1371/journal.pmed (2013).
Corbel, V. et al. Combination of malaria vector control interventions in pyrethroid resistance area in Benin: A cluster randomised controlled trial. Lancet Infect. Dis. 12, 617–626 (2012).
Choi, L., Pryce, J. & Garner, P. Indoor residual spraying for preventing malaria in communities using insecticide-treated nets. Cochrane Database of Systematic Reviews vol. 2019 Preprint at https://doi.org/10.1002/14651858.CD012688.pub2 (2019).
Parker, J. E. A. et al. Infrared video tracking of Anopheles gambiae at insecticide-treated bed nets reveals rapid decisive impact after brief localised net contact. Sci. Rep. 5, 13392 (2015).
Lynd, A. & Mccall, P. J. Clustering of host-seeking activity of Anopheles gambiae mosquitoes at the top surface of a human-baited bed net. Malar. J. 12, 267 (2013).
Parker, J. E. A. et al. Host-seeking activity of a Tanzanian population of Anopheles arabiensis at an insecticide treated bed net. Malar. J. 16, 1–14 (2017).
Sutcliffe, J. F. & Yin, S. Behavioural responses of females of two anopheline mosquito species to human-occupied, insecticide-treated and untreated bed nets. Malar. J. 13, 1–19 (2014).
Oxborough, R. M. et al. Mosquitoes and bednets: Testing the spatial positioning of insecticide on nets and the rationale behind combination insecticide treatments. Ann. Trop. Med. Parasitol. 102, 717–727 (2008).
Mbewe, N. J. et al. Efficacy of bednets with dual insecticide-treated netting (Interceptor® G2) on side and roof panels against Anopheles arabiensis in north-eastern Tanzania. Parasit Vectors 15, 326 (2022).
Lissenden, N. et al. Meta-analysis on the entomological effects of differentially treated ITNs in a multi-site experimental hut study in sub-Saharan Africa. Malar. J. 24, 34 (2025).
Guillet, P. et al. Combined pyrethroid and carbamate ‘two-in-one’ treated mosquito nets: field efficacy against pyrethroid-resistant Anopheles gambiae and Culex quinquefasciatus. Med. Vet. Entomol. 15, 105–112 (2001).
Sutcliffe, J. F. & Yin, S. Effects of indoor air movement and ambient temperature on mosquito (Anopheles gambiae) behaviour around bed nets: implications for malaria prevention initiatives. Malar. J. 20, 427 (2021).
Choi, L., Pryce, J. & Garner, P. Indoor residual spraying for preventing malaria in communities using insecticide-treated nets. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD012688.pub2 (2019).
Kilian, A. et al. Evidence for a useful life of more than three years for a polyester-based long-lasting insecticidal mosquito net in Western Uganda. Malar. J. 10, 1–13 (2011).
Batisso, E. et al. A stitch in time: A cross-sectional survey looking at long lasting insecticide-treated bed net ownership, utilization and attrition in SNNPR, Ethiopia. Malar. J. 11, 1–7 (2012).
Abílio, A. P. et al. Monitoring the durability of the long-lasting insecticidal nets MAGNet and Royal Sentry in three ecological zones of Mozambique. Malar. J. 19, 209 (2020).
Bhatt, R. M., Sharma, S. N., Uragayala, S., Dash, A. P. & Kamaraju, R. Effectiveness and durability of Interceptor long-lasting insecticidal nets in a malaria endemic area of central India. Malar. J. 11, 1–8 (2012).
Azizi, S. et al. The First GLP Compliant Study in Africa for the Evaluation of LLIN’s; Efficacy of SafeNet® and SafeNet NF®. Preprint at https://doi.org/10.21203/rs.3.rs-1006810/v1 (2021).
WHO. Guidelines for Testing Mosquito Adulticides for Indoor Residual Spraying and Treatment of Mosquito Nets. (WHO, Geneva, Switzerland, 2006).
Mahande, A., Mosha, F., Mahande, J. & Kweka, E. Feeding and resting behaviour of malaria vector, Anopheles arabiensis with reference to zooprophylaxis. Malar. J. 6, 100 (2007).
Kweka, E. J. & Mahande, A. M. Comparative evaluation of four mosquitoes sampling methods in rice irrigation schemes of lower Moshi, northern Tanzania. Malar. J. 8, 149 (2009).
Matowo, J. et al. Biochemical basis of permethrin resistance in Anopheles arabiensis from Lower Moshi, north-eastern Tanzania. Malar. J. 9, 193 (2010).
Matowo, J. et al. Dynamics of insecticide resistance and the frequency of kdr mutation in the primary malaria vector Anopheles arabiensis in rural villages of Lower Moshi, North Eastern Tanzania. J. Parasitol. Vector Biol. 6, 31–41 (2014).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
NJM, MJK, SA, WRM, FWM and JM conceived and designed the study. NJM, KE, BM, MFS and NMP contributed to field work and data collection. NJM analysed the data and drafted the first version of the manuscript, which was revised by all co-authors. All co-authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mbewe, N.J., Azizi, S., Rowland, M.W. et al. Assessments of Anopheles arabiensis behaviour around bed nets using partial adhesive treatments. Sci Rep 15, 20966 (2025). https://doi.org/10.1038/s41598-025-06510-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-06510-y