Abstract
Lead (Pb) is an environmental toxicant reported to impair male reproductive system. Galbanic acid (GBA), a natural sesquiterpene coumarin compound renowned for its diverse biological activities, exhibits anti-apoptotic, anti-hypoxic, anti-proliferative, anti-hepatitis, anti-angiogenic, antibacterial, and anti-thrombotic effects. In this experimental study, we investigated the protective effects of GBA on lead acetate-induced testicular damage in rats. Thirty-two immature male rats were divided equally into four groups: control group, PbAc group (0.3% lead acetate in drinking water), GBA group (1 mg/kg daily) and PbAc + GBA group (0.3% lead acetate in drinking water + 1 mg/kg daily GBA). Our results indicated that the lead-administration significantly reduced the mean number of different cells at seminiferous tubules, increase the ROS and MDA levels and also cause histopathological changes in testis (p < 0.001). Galbanic acid inhibited the reduction in number of cells, generation of ROS and MDA and histophatological changes in PbAc + GBA group (p < 0.001). In addition, Bax/Bcl-2 ratio expression which was up-regulated in PbAc group, downregulated by co-administration of Galbanic acid and lead in in comparison with PbAc group (p < 0.001). In conclusion, PbAc administration adversely impacted on the testis; on the other hand, Galbanic acid co-administration with lead partially ameliorated these adverse effects.
Similar content being viewed by others
Introduction
Any deleterious or harmful effect on the reproductive system during development is called developmental reproductive toxicity. Many evidences show the fact that body organs are highly vulnerable to the effects of chemical agents in the process of development1. This sensitivity and vulnerability is of particular importance regarding the developmental stages of the reproductive system1.
Heavy metals which are liberated in the environment by natural sources and human activities, can alter the biological function and cause damages. Heavy metals alter several reproductive functions in both males and females2,3. Lead (Pb), one of the most common heavy metal, can lead to significant reproductive defects in humans4. According to previous investigations, Pb exposure disrupt sperm motility, spermatogenesis, and cause premature acrosome reaction. Pb also reduce zona-intact oocyte penetrating capability and plasma testosterone levels3,4.
The role of oxidative stress and its associated complications have been well described in Pb-induced reproductive toxicity5. A close relationship between impairment of male reproductive function and Pb-induced oxidative stress has been previously reported6. High levels of ROS can influence intracellular components, proteins, lipids, and nucleic acid, resulting in reproductive cells injury7. ROS induce lipid peroxidation and DNA fragmentation in spermatozoon and disrupting both their motility and the ability of these cells to support normal embryonic development8. At the level of the testes, inhibition of steroidogenesis in Leydig cells can occur due to high ROS levels9, moreover the capacity of the germinal epithelium to differentiate normal spermatozoa impaired by ROS10. Consequently, oxidative-stress induced damage in testicular tissue occurs, which can result in poor semen quality and infertility11,12.
To mitigate lead toxicity, significant research has focused on natural phenolic compounds due to their antioxidant properties13,14. Galbanic acid (GBA), a natural sesquiterpene coumarin, exhibits anti-apoptotic, anti-hypoxic, anti-proliferative, anti-hepatitis, anti-angiogenic, antibacterial, and anti-thrombotic effects15. GBA demonstreated free radical-scavenging activity in DPPH and ABTS tests and also enhanced the cellular redox state in the human dermal fibroblasts by up-regulating SOD, CAT, and GPx genes16. Ferula hermonis root extract which contain GBA improves sperm count and serum testosterone levels in cyclophosphamide-inuced testicular toxicity in male rats17. Hence, the potential protective capabilities of GBA have prompted us to unravel its protective role against Pb-induced testicular toxicity at both the biochemical and histopathological levels.
Materials and methods
Chemicals
Bovine Serum Albumin (BSA), Hank’s Balanced Salt Solution (HBSS), N-(2- hydroxyethyl) piperazine-N′ -(2-ethanesulfonic acid) (HEPES), Rotenone, D-mannitol, Sucrose, 2′,7′ -Dichlor-fluorescein diacetate (DCFD), Rhodamine123, Ellman’s reagent (5,5′ -dithiobis-(2-nitrobenzoic acid, 3-morpholinopropane-1-sulfonic acid (MOPS), 2-Amino-2-hydroxymethyl-propane-1,3-diol (TRIS), 4,5-dimethylthiazol2-yl)-2,5 diphenyltetrazolium bromide (MTT), Dimethyl sulfoxide (DMSO), Sodium succinate, Potassium chloride, Monopotassium phosphate and Magnesium chloride were purchased from Sigma (St. Louis, MOUSA). Galbanic acid (GBA) was generously provided by Dr. Zohreh Abolhassanzadeh.
Approval from animal’s ethics committee
This investigation was carried out at Shahrekord University of Medical Sciences and obtained approval from the university’s Animals Ethics Committee, with the reference number IR.SKUMS.AEC.1402.048. All experiments were performed in accordance with relevant guidelines and regulations. The authors confirm that all animal experiments in the present study have been performed in accordance with ARRIVE guidelines.
Laboratory animals and grouping
Approval for the present study was obtained from the Research Ethics Committee of Shahrekord University of Medical Sciences. Thirty-two Prepubertal male wistar rats, weighing between 200 and 240 g, were from the Pasteur Institute of Iran (Tehran, Iran). Rats were housed in a controlled environment, with the temperature maintained at 21 ± 2◦C. The rats were subjected to a 12-hour light-dark cycle and were provided ad libitum access to food and water. Following this, the animals were randomly allocated to four distinct groups, each comprising 8 rats. The first group (control) received deionized drinking water continuously for a period of 35 days. The second group (lead acetate) (PbAc) received deionized drinking water containing 0.3% PbAc continually for 35 days18. In the third group (lead acetate + Galbanic acid) (PbAc + GBA), the rats were provided with deionized drinking water containing 0.3% PbAc over the same 35-day duration, along with a 1 mg/kg dose of Galbanic acid (GBA) by gavage, administered every other day. The fourth group (GBA) received deionized drinking water unrestrictedly for 35 days, along with a 1 mg/kg dose of GBA by gavage administered every other day over the same 35-day period19.
Sperm collection
Rats were anesthetized through intraperitoneal injection of ketamine (90 mg/kg) and xylazine (10 mg/kg) after 5 weeks. Animals were sacrificed by cervical dislocation, their epididymides and testis were carefully removed, trimmed free of connective tissue and fat. The caudal epididymis was separated and immersed in 1 mL prewarmed Hams/f10 medium at 37 °C. The tissues were divided to several fragments. Sperm suspension was incubated at 37 °C for 10 min for spermatozoa flow out.
Acridine orange (AO) assay
The AO staining evaluates the susceptibility of sperm nuclear DNA to denature by acid which forms metachromatic shift of AO fluorescence from green for normal DNA or double-stranded DNA to red for denatured DNA or single-stranded DNA. The monomeric AO bound to normal DNA emits green fluoresce, while the aggregated AO on denatured or single-stranded DNA emits red fluorescence.
Smears were incubated with acid detergent solution pH ¼ 1.2 [0.08 N HCl, 0.15 M NaCl, 0.1% Triton- X 100], and stained with freshly prepared AO (0.19 mg/mL) in citrate phosphate buffer (80 mL citric acid 0.1Mþ 5mL NaH2PO4 0.3 M, pH ¼ 2.5) for 10 min. The slides were washed with distilled water and examined under a ZEISS mot plus (Germany) fluorescent microscope at the excitation wavelength of 450–490 nm. An average of 200 sperm was evaluated for each sample. The results were indicated as the mean percentage of sperm with DNA fragmentation in experimental groups20.
Acrosome staining using coomassie brilliant blue
Smears from semen samples were prepared. Sperm smears were air-dried onto glass slides, fixed with 5% paraformaldehyde in phosphate-buffered saline (PBS; pH 7.4) for 15 min, and washed once with PBS. Slides were stained with aqueous 0.25% Coomassie brilliant blue in 10% glacial acetic acid and 25% methanol for 5 min and they were washed. According to this method, acrosomal cap in sperm with intact acrosome appear blue, acrosome-reacted sperm or damaged acrosomes does not stain. Approximately, we have evaluated different microscopic fields with 80 to 100 sperm in each field21.
Testicular histomorphometry
The testis of the sacrificed animals were removed. After weighing them, Testis tissues were primarily fixed in Bouin’s fixation solution for 24 h, dehydrated in ethanol and embedded in paraffin. Sections of 5 mm were obtained, deparaffinized and stained with Hematoxylin and Eosin (H and E). The histomorphometry observations of the testis included the thickness of the seminiferous epithelial layer which was measured from the basement membrane to the lumen of tubules, outer diameter of seminiferous tubules as well as the number of tubules per field. The analysis of sections was carried out by light microscope (Olympus CH30, Japan). Also, the mean number of spermatogonia, spermatocytes (according to the cell morphology, size of cell nucleus and distance to the basement of seminiferous tubules) and Leydig cells was calculated in the captured photographs, using image J software (Version 1.4.3.67)( https://imagej.net/ij/)22.
Determination of reactive oxygen radicals (ROS)
ROS production in the testicular tissues was quantitated using the DCFDA (2, 7-dichlorodihydrofluorescein diacetate) assay, a method based on the ROS-dependent oxidation of DCFDA to 2, 7-dichlorofluorescin (DCF). Briefly, after thawing the frozen testicular tissue and homogenizing it in lysis buffer (0.5 ml), the homogenate was centrifuged at 14,000 rpm for 30 min at 4 °C, and the pellet was resuspended. DCFDA solution (final concentration 25 µM) was added to the suspension, and the mixture was incubated in the dark for 30 min at 37 °C, during which nonfluorescent DCFDA was oxidized to the highly fluorescent DCF in the presence of ROS. Subsequent assessment of the ROS levels is conducted via fluorimetric analysis based on the unit of fluorescence intensity, with excitation and emission wavelengths set at 470 nm and 540 nm, respectively23.
Determination of lipid peroxidation
Lipid peroxidation in testis was measured by the thio-barbituric acid (TBA) method that determines aldehyde formed by degradation of hydroperoxide, including ma-londialdehyde (MDA). In this procedure, two molecules of thiobarbituric acid (TBA) react with one molecule of MDA under acidic conditions, yielding a pink-colored product with absorption in the range of 532–535 nm; the intensity of absorption at this wavelength is directly proportional to the formation of the TBA-MDA complex. Specifically, 200 µl of thiobarbituric acid reagent is added to 100 µl of the homogenised tissue sample. Subsequently, the microtubes are heated at 90 °C for 60 min to facilitate the formation of the MDA-TBA bond. To extract the formed MDA-TBA complex within the butanol phase, the microtubes are cooled on ice, and 300 micro-liters of butanol is added to each microtube. Lastly, the absorbance of the clear supernatant was determined at 535 nm and MDA concentration calculated using 1.56 × 105 mol− 1 cm− 1 as molar absorbance coefficient. MDA results were expressed as nmol per gram of wet tissue24.
Evaluation of lead concentration
Samples of the left testis were weighed and dried at 70 ° C until reaching constant dry weight. The dried samples were placed in Erlenmeyer flasks with 1.5 mL of concentrated nitric acid (HNO3) and 0.5 mL of perchloric acid (HClO4, 70%) and transferred to the hot plate. The temperature was gradually raised to 90 °C so that the digestion of the material was complete. The samples were diluted in deionized water and filtered. The concentration of lead within the testis tissue samples was ascertained using atomic absorption spectroscopy, specifically employing the Varian model Spectr AA24025.
Quantitative Real-Time polymerase chain reaction (qRT-PCR)
The genes expression of Bax and BcL-2 in the testis were measured using Real-Time PCR. After collecting the testis tissue, total RNA was extracted using RNX-plus. The RNA was then reverse transcribed into cDNA using a PrimeScript RT reagent kit (Takara Bio, Inc., Otsu, Japan). Gene-specific primers and a fluorescent probe for Bax and BcL-2 were designed and optimized. Real-time PCR was done on the cDNA samples using a light cycler instrument (Roche Diagnostics, Mannheim, Germany) (Takara Bio). The results were analyzed using the 2 − ΔΔCT method to calculate the relative gene expression levels of Bax and BcL-2 in the testis. The housekeeping gene B2M was used as a reference gene to normalize the gene expression levels. The primer sequences are demonstrated in Table 1.
Statistical analysis
The statistical analysis was conducted using GraphPad Prism 5 (GraphPad software, La Jolla, CA). (https://www.graphpad.com/support/prism-5-updates/). The data underwent analysis utilizing one-way ANOVA and Tukey post hoc test. The results are expressed as Mean ± SEM, and statistical significance was defined as P < 0.05.
Results
Acridine orange (AO) assay
The mean percentage of DNA fragmentation significantly increased in the lead treated group in comparison with the other groups (P < 0.001). The mean percentage of sperm with DNA fragmentation significantly decreased in GBA + PbAc group as compared to PbAc group (P < 0.001), although there was a significant difference in comparison with control group (p < 0.001). The mean percentage of sperm DNA fragmentation significantly decreased after oral administration of GBA compared with control group (P < 0.05) (Fig. 1). The images obtained through a fluorescent microscope from the AO staining shows that Bright green sperm were considered to have normal DNA, while red or orange sperm were of fragmented DNA (Fig. 2).
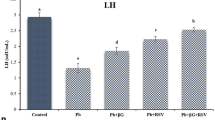
Comparison of the mean percentage of global DNA methylation. The percentage of DNA fragmentation in the group receiving PbAc has increased significantly compared to the control group (P < 0.001). In PbAc + GBA group, the mean percentage of the percentage of DNA fragmentation increased significantly compared with PbAc group (P < 0.001). (Control: Deionized drinking water, PbAc: Lead acetate, PbAc + GBA: Lead acetate + Galbanic acid, GBA: Galbanic acid, *: Significant difference with Control group; #: Significant difference with PbAc group, ***,### P > 0.001)
Sperm acrosomal integrity in experimental groups
Sperm acrosome integrity was assessed by Coomassie Brilliant Blue staining. The mean percentage of sperm with intact acrosome remarkably decreased in PbAc group as compared to the other groups (P < 0.001). The mean percentage of sperm acrosome integrity significantly increased after treatment with GBA along with PbAc (P < 0.001). But, yet indicated a significant difference compared to the control group (P < 0.001). As shown in Fig. 3, the mean percentage of sperm acrosome integrity in GBA group showed no significant difference in comparison with control group.
(A): Comparison of the mean percentage of acrosome integrity: The mean percentage of sperm acrosome integrity significantly decreased in PbAc group as compared to control group (P < 0.001). After treatment with GBA, The mean percentage of sperm acrosome integrity significantly increased in PbAc + GBA group compared with PbAc group (P < 0.001). (Control: Deionized drinking water, PbAc: Lead acetate, PbAc + GBA: Lead acetate + Galbanic acid, GBA: Galbanic acid, *: Significant difference with Control group; #: Significant difference with PbAc group, ***,### P > 0.001). (B): Coomassie blue staining for evaluation of sperm acrosomal integrity. Left arrow displays sperm with normal acrosomal integrity, while right arrow is related to sperm with damaged acrosome. Scale bar: 100 μm.
Testicular histomorphometric evaluation in experimental groups
Morphological changes of seminiferous tubules such as disorganization, distortion, and atrophy, were observed in testicular tissue of animals in PbAc group (Fig. 4). The mean number of spermatogonia, primary spermatocytes and leydig cells significantly reduced in PbAc group in comparison with the control group (P < 0.001). The mean number of spermatogonia (P < 0.001), primary spermatocytes (P < 0.001) and leydig cells (P < 0.05) significantly increased after oral administration of GBA along with PbAc as compared with the PbAc group (Table 2). Although a remarkable improvement can be seen after GBA treatment, there is a significant difference between GBA + PbAc and control group. There is no significant different in the mean number of spermatogonia, primary spermatocytes and leydig cells in GBA group in comparison with control group.
Light microscopic images showed the sections of the testis stained by H&E in the experimental groups (×400 magnification). (A) Control group: normal arrangement of germinal epithelium in seminiferous tubules. (B) Lead acetate group: Atrophy, detachment of basement membrane (right black arrow) and vacuolation (left black arrow) of seminiferous tubules as well as degeneration of spermatogenic cells can be seen in microscopic images of seminiferous tubules. Number of spermatogenic cells and diameter of tubules decreased. (C) GBA + PbAc: Recovery and improvement in the histological structure of seminiferous tubules were seen in this group. (D) GBA group: Normal appearance of germinal epithelium and spermatogenic cells were observed. SG: spermatogonia, PS: primary spermatocyte, LC: Leydig cell (indicated by white arrows).
The mean thickness of the germinal epithelium significantly decreased in PbAc group compared with the control group (P < 0.05). After GBA treatment, the mean thickness of seminiferous tubules increased in GBA + PbAc group compared with PbAc group (P < 0.05). Besides, the mean number of seminiferous tubules in PbAc group significantly decreased as compared with the control group (P < 0.001), and following GBA treatment, the mean number of seminiferous tubules was significantly increased compared with the PbAc group (P < 0.01). (Table 3)
ROS generation
A significant increase in the ROS content was observed in the testis tissue of rat in the PbAc group compared with the control (P < 0.001), as shown in Fig. 5. However, after treatment with GBA, in GBA + PbAc group, significant decrease in ROS generation was observed (P < 0.001) (Fig. 5).
ROS generation in the testis tissue. Receiving lead acetate significantly (P < 0.001) increased ROS generation in comparison to the control group. In GBA + PbAc group decreased percentage of ROS generation (P < 0.001). (Control: Deionized drinking water, PbAc: Lead acetate, PbAc + GBA: Lead acetate + Galbanic acid, GBA: Galbanic acid, *: Significant difference with Control group; #: Significant difference with PbAc group, * P < 0.05, ***,### P < 0.001).
MDA level
As demonstrated in Fig. 6, a significant increase in testicular malondialdehyde (MDA) levels was detected in PbAc treated group as compared to the control group (P < 0.001). The group co-treated with PbAc plus GBA showed a significant reduction in testicular MDA levels as compared to PbAc treated group (P < 0.001), as shown in Fig. 6.
Lipid peroxidation in testis tissue. The epididymal malondialdehyde (MDA) level in PbAc group significantly increased in comparison to the control group (P < 0.001). PbAc + GBA group had a significant decrease in the MDA level as compared to the PbAc group (P < 0.001). Control: Deionized drinking water, PbAc: Lead acetate, PbAc + GBA: Lead acetate + Galbanic acid, GBA: Galbanic acid, *: Significant difference with Control group; #: Significant difference with PbAc group, ** P < 0.01; ***,### P > 0.001).
Lead concentration in testis tissue
According to the lead measurement results, the lead concentration significantly increased (P < 0.001) in the testis tissue of rats continuously exposed to drinking water containing 0.3% PbAc over a 35-day period, in comparison to the control group. As illustrated in Fig. 7, although lead concentration was reduced in the group that received GBA in conjunction with PbAc compared to the group that was solely exposed to PbAc for 35 days, but this reduction was not statistically significant (P > 0.05).
Lead concentration in testis tissue. The lead concentration significantly increased (P < 0.05) in the testis tissue of rats in comparison to the control group. lead concentration was reduced in the group that received GBA in conjunction with PbAc compared to the group that was solely exposed to PbAc for 35 days, but this relationship was not statistically significant (P > 0.05). (Control: Deionized drinking water, PbAc: Lead acetate, PbAc + GBA: Lead acetate + Galbanic acid, GBA: Galbanic acid, *: Significant difference with Control group; * P < 0.05, ** P < 0.01).
Relative expression of apoptotic genes in experimental groups
We analyzed the expression levels of Bcl-2 and Bax using RT-PCR. The result demonstrated that lead exposure down-regulated the expression of Bcl-2 and concomitantly up-regulate the Bax expression compared to the control group. Treatment with GBA, in GBA + PbAc group, significantly up-regulated BAX expression and non-significantly down-regulated BCL-2 in comparison PbAc group. However, the expression ratio of BAX to Bcl-2 was significantly decreased in the GBA + PbAc group compared to the PbAc group (Fig. 8).
The expressions of Bax (A) and Bcl-2 (B) gene and Bax/Bcl-2 ratio (C) in the testis tissues were measured using RT-PCR. The expressions of Bax significantly upregulated (P < 0.05) and the expression of Bcl-2 significantly downregulated (P < 0.001) in PbAc group as compared to the control group. In the PbAc + GBA group, the expression of Bax decreased significantly compared with PbAc group (P < 0.001). Bax/Bcl-2 ratio expression in PbAc + GBA group decreased significantly compared with PbAc group (P < 0.001). (Control: Deionized drinking water, PbAc: Lead acetate, PbAc + GBA: Lead acetate + Galbanic acid, GBA: Galbanic acid, *: Significant difference with Control group; #: Significant difference with PbAc group, * P < 0.05, ** P < 0.01, ***,### P < 0.001).
Discussion
The effect of environmental contaminants on fertility is of great concern. There are accumulating evidence showing that lead exposure impacts negatively on male reproduction with significant alterations in both structure and functions of reproductive organs26. In this study, we found that lead acetate administration increased the ROS production and induced following oxidative stress in testis tissue. The depletion of glutathione and protein bound sulfhydryl groups and the changes in the activity of various antioxidant enzymes have been implicated in lead induced oxidative tissue damage27,28,29. However, the raise in ROS production was reduced following co-administration of GBA along with lead acetate. These results are in line with previous researches which GBA exhibited free radical-scavenging activity in DPPH and ABTS tests and also enhanced the cellular redox state in the human dermal fibroblasts by up-regulating SOD, CAT, and GPx genes30.
At physiological levels, ROS are actively engaged in the control of spermatogenesis and fertilization31. However, the overproduction of ROS triggers Oxidative Stress in spermatozoa by reacting with polyunsaturated fatty acids (PUFA), which are abundant in spermatozoal lipid membranes. Testis are the main target organs of oxidative stress due to the presence of abundant polyunsaturated fatty acid in the biomembrane, which makes them more prone to lipid peroxidation32. This leads to the initiation of lipid peroxidation chain reactions resulting in the production of deleterious products which bind to the nucleophilic centers of DNA and proteins, resulting in significant cellular damage and impaired semen parameters33,34. In this study, lead acetate induced lipid peroxidation and increased testicular MDA concentration. The increase in MDA level following lead administration was also observed in previous studies35,36,37. When co-administered with Pb, GBA significantly decreased testicular MDA level in comparison with PbAc group, which could be due to its antioxidant properties. Previously, ethanol extract from seeds and 50% water-ethanol extracts from roots of Ferula assa-foetida L., (which contain GBA) were prepared into so-called “Masculine” tablets, have also substantially decreased the level of lipid peroxidation in human sperm cells at a concentration of 50 µg/ml38.
A significant decrease in the number of different cells at seminiferous tubules (spermatogonia, primary spermatocytes, spermatozoa and leydig cells) in PbAc treated rats was observed in present study. Such effects can be due to lead-induced inhibition of spermatogenesis by decreasing stages length related to spermiation and beginning of mitosis39. Actually, lead exposure induce ROS generation and defect in mechanism of sperms production with apoptosis of germ cells, which finally lead to decrease in number of primary and secondary spermatocytes40. It has been reported that Ferula assa‑foetida oleo gum resin, which GBA extracted from it, significantly increased the number and viability of sperms as well as spermatogenesis process and numbers of Leydig cells41. In accordance with previous research, administration of GBA along with lead acetate prevent the reduction in the number different cells at seminiferous tubules, which can be related to antioxidant properties of GBA. Cholinergic molecules are expressed in Leydig and Sertoli cells of the male reproductive system. It is reported that AChE decreases the level of testosterone in Leydig cells42. Lead exposure which significantly increased penile AChE activity43 can also decrease testosterone level in these cells. GBA reverse such adverse effect on cells of male reproductive system by inhibition of AChE which have previously been reported in several studies44,45.
Moreover, in the present study, a significant decrease was observed in the different parameters related to seminiferous tubule including seminiferous outer diameters, seminiferous epithelial height and number of seminiferous tubule in PbAc group compared with control group. Such effects have also reported in Asadpour et al., study which was prevented by antioxidant like N-acetyl cystein46. However, GBA inhibit the decrease in above mentioned parameters in group which received GBA along with lead acetate. Increase in seminiferous epithelial height have previously demonstrated with Ferula assa‑foetida oleo gum resin in male wistar rats41.
There is evidence that lead can pass through the blood-testis barrier, and by accumulating in the testis and epididymis, affects the sperm quantity and quality, respectively47. Beside, lead toxicity increases the spontaneous acrosome loss and decreases the ability of spermatozoa to undergo acrosome reaction48. Consistent with previous studies, we also observed significant decrease in the mean percentage of sperm acrosome integrity in PbAc group as compared to control group. The mean percentage of sperm acrosome integrity significantly increased in PbAc + GBA group compared with PbAc group.
Pb can easily cross the biomembrane and accumulate in the ovary, testis, and placenta49. The results of the lead measurement in our research show that the administration of Pb significantly increased the testicular Pb level when compared with the control group. This observation is in line with previous studies, which showed that exposure to Pb causes an increase in serum Pb level and accumulation in the testis50. The observed increase in testicular Pb level was non-significantly decreased when GBA were co-administered along with lead acetate, suggesting a weak chelating effect of GA on lead acetate. It imply that protective effects of GA on neurotoxicity of lead acetate was mainly through another mechanisms.
Apoptosis is a programmed cell death able to cause different biochemical and morphological events, such as DNA fragmentation, chromatin condensation, cell shrinkage, membrane blebbing and the appearance of apoptotic bodies51. In the seminiferous tubules, during the normal process of spermatogenesis, apoptotic cellular death is crucial in its regulation to maintain the fertility potential of males52. Oxidative stress arises as a result of excessive generation of ROS and disruption of an antioxidant defense system is one of the main reasons for reproductive tissue apoptosis53. Apoptosis can also be induced via the mitochondrial pathway in germ cells with up-regulation of bcl-2-associated-X-protein (Bax), and down-regulation of B-cell lymphoma 2 (Bcl-2) genes. Although Bcl-2 inhibits apoptosis linking to the pro-apoptotic effector proteins, Bax modulates mitochondrial outer membrane permeabilization, thus causing apoptosis. In our study, the expression of Bax was significantly increased in PbAc group in comparison with control group. Conversely, the expression of Bcl2 was significantly decreased in in PbAc group in comparison with control group. The raise in Bax expression and collapse in BCL-2 expression following Pb administration has been reported in previous research54. Such alteration make cell prone to apoptosis, as previous studies revealed a testicular damage and apoptosis in the spermatogenic cells following Pb exposure55,56. Anyway, in rat which received GBA along with lead acetate, GBA significantly inhibited the Pb-induced up-regulation in Bax expression and down-regulation in Bcl2 expression and as a result DNA fragmentation significantly decreased in PbAc + GBA in comparison with PbAc group. Ammar et al.,57 reported elevated AChE activity in teratozoospermia cases of infertility and found an association with apoptosis. Lead exposure also significantly increased penile AchE activity43. GBA which is an inhibitor of AChE activity44,45 can prevent apoptosis in male rat reproductive system through inhibitory effects on AChE.
Conclusion
The finding of the present study demonstrated that GBA mitigate lead-induced seminiferous tubules damage and inhibition of spermatogenesis. GBA alleviate the adverse effects of Pb actate on the testis through modulation of ROS production, MDA level, Bax and Bcl-2 expression. Considering the results of previous studies inhibitory effects of GBA on AChE can also contribute to the protective effect of this natural sesquiterpenes on male reproductive system. Further in-depth studies are required to elucidate the precise mechanisms by which GBA reduces lead-induced male reproductive toxicity, and to evaluate the safety and efficacy of different doses.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Mattison, D. R. et al. Reproductive toxicity: male and female reproductive systems as targets for chemical injury. Med. Clin. North Am. 74 (2), 391–411 (1990).
Wirth, J. J. & Mijal, R. S. Adverse effects of low level heavy metal exposure on male reproductive function. Syst. Biology Reproductive Med. 56 (2), 147–167 (2010).
Bhardwaj, J. K., Paliwal, A. & Saraf, P. Effects of heavy metals on reproduction owing to infertility. J. Biochem. Mol. Toxicol. 35 (8), e22823 (2021).
Elgawish, R. A. R. & Abdelrazek, H. M. Effects of lead acetate on testicular function and caspase-3 expression with respect to the protective effect of cinnamon in albino rats. Toxicol. Rep. 1, 795–801 (2014).
Hsu, P-C-H-C-C., Chen, M-Y-L-L-Y. & Guo, Y. L. Lead-induced changes in spermatozoa function and metabolism. J. Toxicol. Environ. Health Part. A. 55 (1), 45–64 (1998).
He, Y. et al. Heavy metal exposure, oxidative stress and semen quality: exploring associations and mediation effects in reproductive-aged men. Chemosphere 244, 125498 (2020).
Panner Selvam, M. K., Sengupta, P. & Agarwal, A. Sperm DNA fragmentation and male infertility. Genetics of Male Infertility: A Case-Based Guide for Clinicians. :155 – 72. (2020).
Aitken, R. J. & De Iuliis, G. N. Origins and consequences of DNA damage in male germ cells. Reprod. Biomed. Online. 14 (6), 727–733 (2007).
Hales, D. B. et al. Mitochondrial function in Leydig cell steroidogenesis. Ann. N. Y. Acad. Sci. 1061 (1), 120–134 (2005).
Naughton, C. K., Nangia, A. K. & Agarwal, A. Varicocele and male infertility: part II: pathophysiology of varicoceles in male infertility. Hum. Reprod. Update. 7 (5), 473–481 (2001).
Takeshima, T. et al. Oxidative stress and male infertility. Reproductive Med. Biology. 20 (1), 41–52 (2021).
Barati, E., Nikzad, H. & Karimian, M. Oxidative stress and male infertility: current knowledge of pathophysiology and role of antioxidant therapy in disease management. Cell. Mol. Life Sci. 77, 93–113 (2020).
Amadi, C. N., Offor, S. J., Frazzoli, C. & Orisakwe, O. E. Natural antidotes and management of metal toxicity. Environ. Sci. Pollut. Res. 26, 18032–18052 (2019).
Rajak, C., Singh, N. & Parashar, P. Metal toxicity and natural antidotes: prevention is better than cure. Environ. Sci. Pollut. Res. 27, 43582–43598 (2020).
Kasaian, J., Iranshahy, M. & Iranshahi, M. Synthesis, biosynthesis and biological activities of Galbanic acid–A review. Pharm. Biol. 52 (4), 524–531 (2014).
Sajjadi, M., Karimi, E., Oskoueian, E., Iranshahi, M. & Neamati, A. Galbanic acid: induced antiproliferation in Estrogen receptor-negative breast cancer cells and enhanced cellular redox state in the human dermal fibroblasts. J. Biochem. Mol. Toxicol. 33 (11), e22402 (2019).
Girgis, S. M., ElRaouf, A. A. & Abdou, H. S. Protective effect of Ferula hermonis root extract against cycraminduced DNA, biochemical and testicular damage in rats. Jordan J. Biol. Sci. ;14(1). (2021).
Kumar, V. L. Ameliorative effects of ferulic acid against lead acetate-induced oxidative stress, mitochondrial dysfunctions and toxicity in prepubertal rat brain. Neurochem. Res. 39 (12), 2501 (2014).
Kim, K-H. et al. Galbanic acid isolated from Ferula assafoetida exerts in vivo anti-tumor activity in association with anti-angiogenesis and anti-proliferation. Pharm. Res. 28, 597–609 (2011).
Mohammed, E-E-M. et al. Acridine orange and flow cytometry: which is better to measure the effect of varicocele on sperm DNA integrity? Advances in urology. 2015;2015.
Feng, H. L., Han, Y. B., Hershlag, A. & Zheng, L. J. Impact of Ca2 + flux inhibitors on acrosome reaction of hamster spermatozoa. J. Androl. 28 (4), 561–564 (2007).
Khadivi, F., Razavi, S. & Hashemi, F. Protective effects of zinc on rat sperm chromatin integrity involvement: DNA methylation, DNA fragmentation, ubiquitination and protamination after bleomycin Etoposide and cis-platin treatment. Theriogenology 142, 177–183 (2020).
Nam, H. H. et al. Protective effects of an aqueous extract of Protaetia brevitarsis seulensis larvae against radiation-induced testicular injury in mice. Food Sci. Nutr. 10 (11), 3969–3978 (2022).
Ohkawa, H., Ohishi, N. & Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 95 (2), 351–358 (1979).
Lozi, A. A. et al. Comparative toxicology of heavy metals arsenate, arsenite, cadmium, Chrome vi, lead, and nickel in the testis of adult Swiss mice after acute exposure. (2022).
Undaryati, Y. M., Sudjarwo, S. A. & I’thisom, R. Literature review: effect of lead toxicity on reproductive system. J. Global Res. Public. Health. 5 (1), 1–8 (2020).
Ercal, N. et al. A role for oxidative stress in suppressing serum Immunoglobulin levels in lead-exposed fisher 344 rats. Arch. Environ. Con Tox. 39, 251–256 (2000).
Gürer, H., Özgünes, H., Neal, R., Spitz, D. R. & Erçal, N. Antioxidant effects of N-acetylcysteine and succimer in red blood cells from lead-exposed rats. Toxicology 128 (3), 181–189 (1998).
Patra, R. C., Swarup, D. & Dwivedi, S. K. Antioxidant effects of α tocopherol, ascorbic acid and L-methionine on lead induced oxidative stress to the liver, kidney and brain in rats. Toxicology 162 (2), 81–88 (2001).
Sajjadi, M., Karimi, E., Oskoueian, E., Iranshahi, M. & Neamati, A. Galbanic acid: induced antiproliferation in Estrogen receptor-negative breast cancer cells and enhanced cellular redox state in the human dermal fibroblasts. J. Biochem. Mol. Toxic. 33 (11), e22402 (2019).
Baskaran, S., Finelli, R., Agarwal, A. & Henkel, R. Reactive oxygen species in male reproduction: A Boon or a bane? Andrologia 53 (1), e13577 (2021).
Telišman, S., Čolak, B., Pizent, A., Jurasović, J. & Cvitković, P. Reproductive toxicity of low-level lead exposure in men. Environ. Res. 105 (2), 256–266 (2007).
Aitken, R. J., Bromfield, E. G. & Gibb, Z. Oxidative stress and reproductive function: the impact of oxidative stress on reproduction: A focus on gametogenesis and fertilization. Reproduction 164 (6), F79–F94 (2022).
Chakraborty, S. & Roychoudhury, S. Pathological Roles of Reactive Oxygen Species in Male Reproduction. Oxidative Stress and Toxicity in Reproductive Biology and Medicine: A Comprehensive Update on Male Infertility-Volume Onep. 41–62 (Springer, 2022).
Oyeyemi, W. A., Daramola, O-O., Akinola, A. O., Idris, A. O. & Aikpitanyi, I. Hepatic and reproductive toxicity of sub-chronic exposure to Dichlorvos and lead acetate on male Wistar rats. Asian Pac. J. Reprod. 9 (6), 283–290 (2020).
Oyeyemi, W. A. et al. Vitamin E and Quercetin attenuated the reproductive toxicity mediated by lead acetate in male Wistar. Bull. Natl. Res. Centre. 46 (1), 22 (2022).
El-Magd, M. et al. A potential mechanism associated with lead‐induced testicular toxicity in rats. Andrologia 49 (9), e12750 (2017).
Kassis, E. et al. Efficacy and safety assessments of Ferula assa-foetida L., traditionally used in Greco-Arab herbal medicine for enhancing male fertility, libido and erectile function. Open. Complement. Med. J. ;1(1). (2009).
Leiva, K. P., Rubio, J., Peralta, F. & Gonzales, G. F. Effect of Punica granatum (pomegranate) on sperm production in male rats treated with lead acetate. Toxicol. Mech. Methods. 21 (6), 495–502 (2011).
Makhlouf, M., Eldien, H., Zagloul, D., Dief, E. A. & ElHaliem, N. The effect of lead acetate on testicular structure and protective effect of vitamin E in adult albino rat. Egypt. J. Histol. 31 (2), 406–418 (2008).
Bagheri, S. M. et al. Effect of Ferula assa-foetida Oleo gum resin on spermatic parameters and testicular histopathology in male Wistar rats. J. Ayurveda Integr. Med. 6 (3), 175 (2015).
Mor, I. & Soreq, H. Cholinergic Toxicity and the Male Reproductive System. InReproductive and Developmental Toxicology 2011 Jan 1 (pp. 863–870 ). Academic.
Besong, E. E., Ashonibare, P. J., Akhigbe, T. M., Obimma, J. N. & Akhigbe, R. E. Sodium acetate abates lead-induced sexual dysfunction by upregulating testosterone-dependent eNOS/NO/cGMP signaling and activating Nrf2/HO-1 in male Wistar rat. Naunyn. Schmiedebergs Arch. Pharmacol. 397 (2), 1233–1243 (2024).
Razzaghi-Asl, N., Sepehri, S., Ebadi, A., Miri, R. & Shahabipour, S. Molecular Docking and quantum mechanical studies on biflavonoid structures as BACE-1 inhibitors. Struct. Chem. 26, 607–621 (2015).
Karimi, G., Iranshahi, M., Hosseinalizadeh, F., Riahi, B. & Sahebkar, A. Screening of acetylcholinesterase inhibitory activity of terpenoid and coumarin derivatives from the genus Ferula. Pharmacologyonline 1, 566–574 (2010).
Asadpour, R., Shahbazfar, A., Kianifard, D., Azari, M. & Zaboli, N. Comparison of the protective effects of Garlic (Allium sativum L) extract, vitamin E and N acetyl Cystein on testis structure and sperm quality in rats treated with lead acetate. Revue Med. Vet. 164 (1), 27–41 (2013).
Anjum, M. R., Madhu, P., Reddy, K. P. & Reddy, P. S. The protective effects of zinc in lead-induced testicular and epididymal toxicity in Wistar rats. Toxicol. Ind. Health. 33 (3), 265–276 (2017).
Ramos-Treviño, J., Bassol-Mayagoitia, S., Hernández-Ibarra, J. A., Ruiz-Flores, P. & Nava-Hernández, M. P. Toxic effect of cadmium, lead, and arsenic on the Sertoli cell: mechanisms of damage involved. DNA Cell Biol. 37 (7), 600–608 (2018).
Flora, G., Gupta, D. & Tiwari, A. Toxicity of lead: a review with recent updates. Interdisciplinary Toxicol. 5 (2), 47–58 (2012).
Batra, N., Nehru, B. & Bansal, M. Influence of lead and zinc on rat male reproduction at ‘biochemical and histopathological levels’. J. Appl. Toxicology: Int. J. 21 (6), 507–512 (2001).
Bhardwaj, J. K., Panchal, H. & Saraf, P. Cadmium as a testicular toxicant: A review. J. Appl. Toxicol. 41 (1), 105–117 (2021).
Aitken, R. J., Findlay, J. K., Hutt, K. J. & Kerr, J. B. Apoptosis in the germ line. Reproduction 141 (2), 139 (2011).
Bhardwaj, J. K. & Saraf, P. N-acetyl cysteine‐mediated effective Attenuation of methoxychlor‐induced granulosa cell apoptosis by counteracting reactive oxygen species generation in caprine ovary. Environ. Toxicol. 32 (1), 156–166 (2017).
El-Khadragy, M. et al. Impact of coenzyme Q10 administration on lead acetate-induced testicular damage in rats. Oxidative Med. Cell. Longev. 2020 (1), 4981386 (2020).
Hassan, E., El-Neweshy, M., Hassan, M. & Noreldin, A. Thymoquinone attenuates testicular and spermotoxicity following subchronic lead exposure in male rats: possible mechanisms are involved. Life Sci. 230, 132–140 (2019).
He, Y. et al. Lead inhibits human sperm functions by reducing the levels of intracellular calcium, cAMP, and tyrosine phosphorylation. Tohoku J. Exp. Med. 238 (4), 295–303 (2016).
Ammar, O., Mehdi, M., Tekeya, O., Neffati, F. & Haouas, Z. Novel association between apoptotic sperm biomarkers with seminal biochemical parameters and acetylcholinesterase activity in patients with teratozoospermia. J. Assist. Reprod. Genet. 36, 2367–2378 (2019).
Acknowledgements
This study was supported by a research grant (No. 7106) from Shahrekord University of Medical Sciences, Shahrekord, Iran.
Author information
Authors and Affiliations
Contributions
M.H.Z and F.K. administered the project, designed the study; M.H.Z and H.S.S.G: reviewed & edited the original manuscript; M.H.Z, F.K. and S.K. Methodology; N.G. Wrote original draft; N.G. and M.K. carried out the experiment;
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gharibshahi, N., Gazwi, H.S.S., Khosravi, M. et al. Galbanic acid alleviates lead acetate-induced reproductive toxicity in prepubertal male wistar rats. Sci Rep 15, 22508 (2025). https://doi.org/10.1038/s41598-025-06661-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-06661-y