Abstract
Triple-negative breast cancer (TNBC) is an aggressive subtype with a relatively poor prognosis, and its natural history without therapeutic intervention remains understudied. The purpose of this study was to investigate the natural history of untreated TNBC and construct a prognostic model utilizing data from the SEER database. Data from patients diagnosed with TNBC between 2010 and 2021 were analyzed. Median survival times for each tumor stage were calculated to estimate disease progression time (defined as the difference in median survival between consecutive stages). Cox regression was employed to identify independent prognostic factors in a training set, and a prognostic nomogram was developed based on these factors. Results showed that median survival times for untreated TNBC were 65 months (stage I), 28 months (stage II), 11 months (stage III), and 3 months (stage IV), with estimated progression times from stage I to II, II to III, and III to IV being 37 months, 17 months, and 8 months, respectively. Age, multifocal breast cancer, T stage, M stage, liver metastasis, and brain metastasis were identified as independent risk factors. The nomogram demonstrated excellent predictive performance, with a C-index of 0.795 in the training set and 0.759 in the validation set, supported by favorable calibration plots and decision curve analysis (DCA). In conclusion, untreated TNBC exhibited accelerated progression and deteriorating prognosis with increasing tumor stage, with age and tumor burden serving as key prognostic indicators, and the constructed nomogram provided a reliable tool for predicting survival in untreated TNBC patients.
Similar content being viewed by others
Introduction
Breast cancer represents the most prevalent aggressive cancer among women across the globe, constituting 25–30% of newly emerging cancer cases in the female population worldwide. Moreover, it stands as the principal culprit for cancer-related mortalities among women globally, accounting for 15% of such deaths1,2,3. Triple negative breast cancer (TNBC) is a distinct subtype of breast cancer distinguished by the absence of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) expression, which accounts for about 15–20% of all breast cancers4. Compared with other types of breast cancer, TNBC is more commonly diagnosed in younger patients and presents as high-grade invasive ductal carcinoma with a relatively poor prognosis, more aggressive disease, greater likelihood of recurrence and metastasis, and higher mortality5,6. The main treatment options for TNBC include chemotherapy, surgery, radiotherapy, and some patients may also benefit from immunotherapy, PARP inhibitors, or antibody–drug conjugate (ADC) drugs. However, due to the lack of specific therapeutic targets, the available systematic treatment options for TNBC are relatively limited, and chemotherapy remains the primary systemic treatment approach for TNBC7,8. Early diagnosis and timely, standardized treatment are of utomst importance for TNBC patients to achieve a favorable prognosis9,10. Nevertheless, across the globe, a small proportion of patients, hampered by factors such as scarce access to medical facilities or the sway of cultural prejudices, refrain from undergoing any treatment subsequent to a breast cancer diagnosis11. For breast cancer patients who have not received treatment, their prognosis is evidently much poorer. In most reported research, without any treatment intervention, the median survival time of patients was merely 3–4 years, and only 5–10% of patients survived for more than 10 years11.
The natural progression of a disease in the absence of any therapeutic intervention is known as the natural history of the disease12. When it comes to TNBC, delving into its natural history can deepen our comprehension of the disease and clarify its impact on the lifespan of the affected population. In addition, based on the natural history, one can clearly discern the actual benefits of a treatment regimen. This lays the foundation for further research and evaluation of treatment efficacy13. Besides, it can be utilized to compare how different treatment regimens improve patient prognosis, thereby evaluating the advantages and disadvantages of different treatment approaches. This is of paramount significance for formulating individualized disease diagnosis and treatment strategies11,12. At present, research on the natural history of TNBC is relatively scarce. The majority of existing conclusions originate from small-sample retrospective studies or case reports. These studies have limited instructive significance in clinical practice11. Moreover, there is a shortage of tools capable of effectively predicting the survival time of patients without treatment. Additionally, within the current diagnosis and treatment system, conducting large-scale clinical trials in clinical practice to observe the natural history of breast cancer is inconsistent with the existing diagnostic and treatment norms and ethical guidelines14.
The SEER (https://seer.cancer.gov/) database is a large-scale public database. It covers approximately 28% of the U.S. population and can provide multi-faceted clinicopathological information such as patient demographics, tumor characteristics, diagnosis, treatment, and prognosis15. The SEER database contains a wealth of information on untreated TNBC patients, along with relatively complete follow-up data and a sufficiently long follow-up time span. Therefore, in this study, we utilized the SEER database to conduct a statistical analysis of the natural history of untreated TNBC. We explored the relevant factors influencing the prognosis of TNBC and constructed a prognostic model based on this, with the hope of providing valuable guidance for clinical diagnosis and treatment.
Materials and methods
Data source and study population
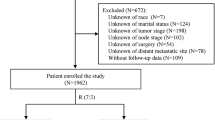
We obtained data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program. This study protocol was approved by the Ethics Committee of Suining Central Hospital. All information from the SEER program is available and free for public, and all patient data are deidentified, so informed consent is not required. Patient data from the SEER database (Version: 8.4.3, https://seer.cancer.gov/data-software/), SEER Research Data, 17 registries, Nov 2023 Sub (2000–2021) were screened. Patients with newly diagnosed TNBC from 2010 to 2021 were initially enrolled in this study. The inclusion and exclusion criteria of patients with breast cancer were as follows. The patient screening flow chart is shown in Fig. 1.
Criteria for inclusion comprised
-
1.
Pathologically diagnosed as triple negative breast cancer
-
2.
The diagnosis was made during the period from 2010 to 2021.
-
3.
The primary origin of the tumor was determined to be within the breast.
-
4.
Patients had surpassed the age of 18 at the time of diagnosis.
Exclusion criteria
-
1.
Concurrent presence of other malignant tumors.
-
2.
Incomplete follow up data.
-
3.
Special pathological types of breast cancer.
-
4.
Male breast cancer.
-
5.
Bilateral breast cancer.
-
6.
Demographic or clinicopathological information was incomplete.
Assessing variables
The most major and widely applied treatment regimens for TNBC include chemotherapy, surgery, and radiotherapy. Besides, the treatment information about TNBC obtainable in the SEER database only covers chemotherapy, surgery, and radiotherapy. Therefore, in this study, patients who have not received chemotherapy, surgery, or radiotherapy are defined as untreated patients. Demographic parameters included age at diagnosis (categorized as ≤ 50 years old, 51–70 years old, > 70 years old), race (including White, Black, Others), and marital status (including married, unmarried). Clinicopathological parameters included tumor size (categorized as 0–20 mm, 21–50 mm, > 50 mm), the number of primary tumors (categorized as unifocal breast cancer, multifocal breast cancer), laterality (including left-side, right-side), histological type (including invasive ductal carcinoma, invasive lobular carcinoma, invasive ductal and lobular carcinoma), histological grade (including I, II, III), AJCC stage (adjusted to the 7th edition of the AJCC staging system, including I, II, III, IV), T-stage (including T1, T2, T3, T4), N-stage (including N0, N1, N2, N3), and M-stage (including M0, M1). According to the inclusion and exclusion criteria, a total of 46,722 triple-negative breast cancer patients were included in the analysis. Among them, 45,695 patients received treatment, while 1,027 patients did not receive any treatment.
Survival analysis
The primary endpoint of this study was the overall survival of breast cancer patients, which was defined as the time from the date of breast cancer diagnosis to the patient’s death. The median survival time (MST) was defined as the time at which 50% of the patients reached the survival endpoint. To ascertain “life loss,” the progression duration was discerned by subtracting the MST of a more severe stage from its preceding stage12. In this study, we used the estimated time to disease progression to evaluate the natural history of TNBC. Additionally, the Kaplan–Meier survival curve and survival rate were used to compare the survival differences of breast cancers with different characteristics. To evaluate the impact of the treatment on prognosis, we divided patients into the treated group and the untreated group for survival analysis to compare survival differences between the two groups. Additionally, due to differences in clinicopathological characteristics between the two groups, we performed propensity score matching (PSM) on variables with significant discrepancies. We used a caliper value of 0.01 to balance covariates between the treated and the untreated groups.
Construction and validation of the nomogram
To construct and validate the nomogram, the untreated patients were randomly divided into training and validation sets in a ratio of 7:3. Univariate cox regression was used to identify risk factors linked to the prognosis of the untreated patients in the training set. Subsequently, statistically significant variables were incorporated into multivariate cox regression analysis. Ultimately, variables with p < 0.05 in multivariate cox regression were determined as independent risk factors and employed to construct a nomogram for predicting the prognosis of the untreated patients with TNBC. The concordance index (C-index) and the receiver operating characteristic curves (ROC) were applied to evaluate the discriminative ability of the nomogram. The calibration curves were used to compare the association between the actual outcomes and predicted probabilities. The clinical utility and benefits of the predictive model were estimated via decision curve analysis (DCA).
Statistical analysis
The chi-square test or Fisher exact test was performed for comparison of counting data, while t test was utilized for comparison of measurement data. Cumulative survival time was calculated using the Kaplan–Meier method, and the differences in survival curves were analyzed via the log-rank test. Univariate and multivariate Cox proportional hazards regression analyses were conducted to identify variables that significantly influenced the prognosis of the untreated patients with TNBC in the training set. All statistical analyses were executed using R Software (version 4.4.1). P values < 0.05 were considered to denote statistically significant differences.
Results
Clinical and pathological characteristics
Based on the inclusion and exclusion criteria, 46,722 patients with TNBC were enrolled from the SEER database. To assess the impact of treatment on prognosis, patients were categorized into treated (n = 45,695, 97.8%) and untreated (n = 1,027, 2.2%) groups according to their treatment history. The median age of all patients was 58 years. In terms of racial composition and marital status, White patients accounted for the highest proportion (70.1%), and 54.0% of patients were married. Regarding the clinicopathological features, the majority of patients had tumors less than 50 mm in size. Among them, 42.2% of patients had tumors smaller than 20 mm, and 46.5% had tumors sized between 21 and 50 mm, and 91.4% of patients had unifocal breast cancer. Invasive ductal carcinoma was the most prevalent histological type, comprising as much as 97.5% of all patients. With respect to histological grade, 82.5% of patients were at grade III. The number of patients with tumors in the left-breast and right-breast was almost equal. In terms of staging, the majority of patients were in stage I (34.5%) or stage II (41.5%). Notably, the tumor stage of patients in the untreated group was markedly later than that of the treated group. During the follow-up period of the study, a total of 10,642 (22.8%) patients died, among whom 9,961 (21.8%) were in the treated group and 681 (66.3%) were in the untreated group. There was a significant difference in survival rates between the two groups. The distribution of demographic and clinicopathological characteristics of patients in the treated group and the untreated group varied significantly. Therefore, we conducted PSM with a caliper of 0.01 on the variables with significant differences to compare the impact of treatment factors on the prognosis of TNBC patients. The demographic and clinicopathological characteristics before and after PSM were presented in Tables 1 and 2.
Impact of treatment on prognosis of TNBC patients
Within this study cohort, 1,027 patients (2.2%) did not receive any form of treatment. Among those who did, as many as 42,709 patients (91.4%) underwent surgical intervention, 26,864 (57.5%) received radiotherapy, and 37,176 (79.6%) received chemotherapy. The research outcomes demonstrate that treatment is consistently a critical factor influencing the prognosis of patients with triple-negative breast cancer, both before and after PSM. It is clearly evident from Fig. 2 that the treated group exhibited higher overall survival rates and cancer-specific survival rates compared to the untreated group, the survival rate of the treated group was higher than that of the untreated group. This result intuitively highlights the important influence of treatment on the prognosis of patients with TNBC.
The natural history of untreated TNBC patients
Kaplan–Meier survival analysis was performed on TNBC of different stages in the untreated group, as shown in Fig. 3. The prognosis of patients in the untreated group deteriorated as the disease stage advanced. As indicated in Table 3, the median survival time for all untreated patients was 14 (95% CI 12–16) months. Among them, the median survival time for stage I patients was 65 (95% CI 48–106) months, for stage II patients it was 28 (95% CI 23–33) months, for stage III patients it was 11 (95% CI 10–15) months, and for stage IV patients it was 3 (95% CI 3–3) months. The occurrence of breast cancer typically commences with gene mutations in cells, and then gradually develops into early-stage breast cancer. In the absence of treatment intervention, it further advances into intermediate-stage and late-stage breast cancer, presenting as a step-by-step process16. In this study, we calculated the disease progression time of TNBC by subtracting the median survival time of patients with higher-stage TNBC from that of patients with lower-stage TNBC. As shown in Table 3 and Fig. 4, the estimated progression times from stage I to stage II, from stage II to stage III, and from stage III to stage IV were 37 months, 17 months, and 8 months, respectively.
Prognosis factors of untreated TNBC patients
In this study, 1,027 untreated TNBC patients were randomly partitioned into a training set (715 patients) and a validation set (312 patients) at a 7:3 ratio. There were no substantial differences in the distribution of demographic and clinicopathological characteristics between the training set and the validation set, indicating the comparability of the two sets. In the training set, the log-rank test and univariate Cox regression analysis were employed to evaluate the variables associated with the prognosis of untreated TNBC patients. The results revealed that age, marital status, tumor size, multifocal breast cancer, T stage, N stage, M stage, bone metastasis, lung metastasis, liver metastasis, and brain metastasis were correlated with the overall survival of untreated TNBC. All variables with statistical significance in the univariate analysis were incorporated into the multivariate Cox regression model. The results demonstrated that age, multifocal breast cancer, T stage, M stage, liver metastasis, and brain metastasis were independent predictors affecting the overall survival of untreated TNBC patients. The results are presented in Table 4.
Construction and validation of a nomogram
Leveraging the independent risk factors affecting the prognosis of untreated TNBC patients identified by Cox regression analysis, a Nomogram model was developed to predict the 1-year, 5-year, and 10-year overall survival rates of untreated TNBC patients (Fig. 5). The Nomogram revealed that age had the most significant impact on the prognosis of untreated TNBC patients. Following age, the T stage and M stage of the tumor also exerted a considerable influence, with brain metastasis and liver metastasis having a relatively significant impact as well. By aggregating the scores of all prognostic indicators, a total score is computed. This enables the estimation of the 1-year, 5-year, and 10-year survival probabilities for each untreated TNBC patient.
To assess the predictive accuracy of the nomogram, both the C-index and ROC curves were employed. The C-index of the nomogram was calculated as 0.795(95% CI 0.773–0.817) in the training set and 0.759(95% CI 0.730–0.788) in the validation set. The ROC curves of the nomogram (Fig. 6) were consistent with the C-index, suggesting excellent predictive ability of the model. As depicted in Fig. 7, the 95% CI of calibration belt in both training and validation sets scarcely cross the diagonal bisector line. This observation implied that the calibration plot exhibited favorable concordance between the predicted probabilities and the actual observed outcomes. The DCA was implemented to compare the usability and advantages of the nomogram model with the AJCC TNM stage system (Fig. 8). The findings indicate that the nomogram displayed greater practicality in predicting the prognosis of patients with untreated TNBC when compared to the TNM stage system.
Discussion
TNBC is a distinct subtype of breast cancer, characterized by high malignancy, pronounced susceptibility to recurrence and metastasis, absence of specific therapeutic targets, and marked propensity for drug-resistance development17,18. These characteristics ultimately contribute to an unfavorable prognosis for patients with TNBC. Consequently, this disease has become a focal area of research in the scientific community in recent years19. Currently, the classification of TNBC is mainly based on pathological classification through immunohistochemistry (IHC). However, this classification method may lead to inaccurate assessment of TNBC subtypes, resulting in bias in prognosis evaluation20. Genomic and transcriptomic analyses have uncovered that TNBC comprises multiple subtypes, each with unique genomic drivers that play a crucial role in tumorigenesis and development and thereby enable researchers to design targeted therapies21,22. These novel treatment regimens are significantly reshaping the therapeutic landscape of TNBC21,23. Nevertheless, due to the limitations of the SEER database, the treatment information available for TNBC is restricted to surgery, chemotherapy, and radiotherapy. Therefore, in our study, TNBC patients who had not undergone surgery, chemotherapy, or radiotherapy were defined as untreated patients. This was to investigate the impact of treatment on the prognosis of TNBC patients and to explore the natural history of TNBC.
The natural history of a disease refers to the natural progression and manifestations of the disease in the complete absence of any treatment intervention12. For any disease, understanding the natural history of untreated cases can provide a real and highly valuable background basis for accurately evaluating the true advantages of a certain treatment method11. Breast cancer, as the most invasive cancer currently impacting the health of women worldwide, lacks large-scale prospective studies to explore its natural history. Moreover, there are no studies specifically reporting the natural history and progression of TNBC without treatment. Existing research on the natural history of breast cancer is mostly based on small-sample observational studies or case reports. A retrospective study summarized multiple reports on the natural history of breast cancer. The research found that, compared with most other cancers, the process of breast cancer leading to patient death is relatively slow, and patients often ultimately die due to various complications triggered by breast cancer11. Based on the research data of patient groups from different geographical regions, the estimated survival times exhibit a high degree of similarity. In most related reports, the vast majority of breast cancer patients can survive around 3–4 years when they receive no treatment11,24. Nevertheless, in the extreme situation of complete lack of treatment, a tiny fraction of breast cancer patients show strikingly good prognoses. Approximately 5–10% of untreated breast cancer patients manage to survive for over 10 years24,25. In our study, we found that the median survival time of untreated TNBC patients was merely 14 months (95% CI 12–16 months). This data is significantly lower than the survival time of the majority of breast cancer patients reported in other studies. This substantial difference is closely related to the unique disease characteristics of TNBC itself. Compared with other types of breast cancer, TNBC typically exhibits greater invasiveness, is more prone to recurrence and metastasis, and has a relatively poorer prognosis26. In terms of long-term survival rate, 18.7% of TNBC patients survived for more than 10 years. This is essentially similar to the long-term survival rate of breast cancer without treatment reported in other studies11,24. This unexpectedly high survival rate may be due to TNBC’s heterogeneity, which aligns with the finding from genomic and transcriptomic analyses that TNBC has multiple subtypes21,27,28.
The natural history of breast cancer can be explored through various methods. Perhaps the most straightforward one is to describe the survival time and survival rate of breast cancer patients without treatment, as we described previously. However, such a description is overly general and fails to reflect individual differences. Another intuitive approach is to describe it through clinical manifestations. As reported in existing studies, the primary clinical manifestation of most breast cancer patients is the discovery of a breast mass. The mass usually appears 3–12 months prior to diagnosis. In the absence of treatment, the tumor generally invades the skin approximately 14–16 months after diagnosis, then skin ulcers occur about 6 months later, and it becomes fixed to the chest wall about 2 months after that. It often invades the other breast as well11. In women with axillary lymph node metastasis, the average time for axillary lymph node enlargement is around 15 months11. Among all these untreated patients, approximately 25% develop obvious distant metastases within one year, and death usually ensues after the appearance of obvious metastases11. However, such a description is evidently subjective. To objectively depict the natural history of TNBC patients, we reflected its progression by estimating the progression time of TNBC at different tumor stages. As is widely acknowledged, tumor staging is one of the most crucial factors influencing the prognosis of breast cancer and an important quantitative indicator representing the severity of breast cancer29. Investigating the time it takes for untreated breast cancer patients to progress from one stage to the next can relatively objectively reveal the natural history of untreated breast cancer. Thus, we deduced the progression time of untreated TNBC patients at different stages by comparing the median survival times of patients in different stages, thereby demonstrating the natural history of TNBC. This study discovered that, without treatment, the median survival times of TNBC were 65 (95% CI 48–106) months for stage I, 28 (95% CI 23–33) months for stage II, 11 (95% CI 10–15) months for stage III, and 3 (95% CI 3–3) months for stage IV. Therefore, we deduced that the progression times from stage I to stage II, from stage II to stage III, and from stage III to stage IV of TNBC were 37 months, 17 months, and 8 months respectively. The disease progresses at an accelerating pace as the stage increases.
To gain a more in-depth understanding of the natural history of TNBC and the prognostic factors in untreated cases, we conducted COX regression analyses on potentially relevant factors. The outcomes indicated that age, tumor T stage, tumor M stage, multifocal breast cancer, liver metastasis, and brain metastasis were independent risk factors for the prognosis of untreated TNBC patients. Based on these factors, a Nomogram model was developed to forecast the survival probability of untreated TNBC. Validation through internal subgrouping revealed that the model demonstrated robust predictive ability and high accuracy. Compared with the commonly-used TNM staging system, this Nomogram model exhibited superior accuracy and practicality. The factors employed in constructing this model are all easily-obtainable clinicopathological factors, which possess good accessibility and practical value and can be extensively applied in clinical practice. In the nomogram of this study, the main prognostic factors for untreated TNBC patients are age and tumor burden, and these factors have also been corroborated by many other studies as important for treated patients5,30,31. In contrast, factors like histological grade, race, and marital status, which other studies found affected the prognosis of treated TNBC patients5,30,31, had no significant impact on untreated patients in this study. This might be attributed to the much worse prognosis and rapid disease progression in untreated patients, overshadowing the influence of these factors.
As far as we are aware, there are extremely few studies that establish a predictive model capable of accurately assessing the prognosis of untreated TNBC patients. However, constructing such a model to evaluate the prognosis of these patients remains a highly meaningful undertaking. The construction of this model is not merely limited to estimating the survival probability of TNBC patients without treatment. More importantly, it can be utilized to evaluate the survival benefits that TNBC patients can derive from various treatment regimens. Furthermore, it plays a pivotal role in aiding doctors to precisely select treatment plans for specific patients. For instance, when considering the elderly or patients with comorbid special diseases, understanding the survival time and probability of TNBC without treatment can assist in formulating suitable treatment plans for these groups.
Limitations
This study does have certain limitations that ought to be taken into consideration. Firstly, as a retrospective study, there might be selection bias in the included cases. Specifically, some cases could have been excluded on account of incomplete data. Secondly, certain factors potentially related to the prognosis of triple-negative breast cancer, like patients’ comorbidity information, Ki-67, etc., couldn’t be analyzed owing to the absence of these factors in the SEER database. Besides, The definition in this study for patients who did not receive radiotherapy or chemotherapy might not be sufficiently precise, owing to the sensitivity issues of the SEER database pertaining to radiotherapy and chemotherapy. Finally, given that both the training set and the validation set of the model are sourced from the SEER database, there is a risk of overfitting. Consequently, external validation on other datasets is necessary to ensure the reproducibility of the model.
Conclusions
Treatment represents a pivotal factor that exerts a profound influence on the prognosis of TNBC patients. The prognosis of patients in the treated group is markedly superior to that of patients in the untreated group. In the absence of treatment, the disease progression rate of TNBC escalates significantly as the tumor stage advances, and the prognosis gradually deteriorates. Age, T stage, M stage, multifocal breast cancer, liver metastasis, and brain metastasis are identified as independent risk factors that impact the prognosis of untreated TNBC patients. The predictive model developed based on these factors demonstrates a commendable predictive ability regarding the prognosis of untreated TNBC patients.
Data availability
The original data for this study can be obtained from the SEER (https://seer.cancer.gov/) database, further inquiries can be directed to the corresponding author.
References
Siegel, R. L., Giaquinto, A. N. & Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 74(1), 12–49 (2024).
Sung, H. et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71(3), 209–249 (2021).
Houghton, S. C. & Hankinson, S. E. Cancer progress and priorities: Breast cancer. Cancer Epidemiol. Biomark. Prev. 30(5), 822–844 (2021).
Pareja, F. et al. Triple-negative breast cancer: The importance of molecular and histologic subtyping, and recognition of low-grade variants. NPJ Breast Cancer 2, 16036 (2016).
Xie, N. et al. Clinicopathological characteristics and treatment strategies of triple-negative breast cancer patients with a survival longer than 5 years. Front. Oncol. 10, 617593 (2020).
Meng, X., Cai, Y., Chang, X. & Guo, Y. A novel conditional survival nomogram for monitoring real-time prognosis of non-metastatic triple-negative breast cancer. Front. Endocrinol. (Lausanne) 14, 1119105 (2023).
Leon-Ferre, R. A. & Goetz, M. P. Advances in systemic therapies for triple negative breast cancer. BMJ 381, e071674 (2023).
Gadi, V. K. & Davidson, N. E. Practical approach to triple-negative breast cancer. J. Oncol. Pract. 13(5), 293–300 (2017).
Sun, X. et al. Nanomaterials for the diagnosis and treatment of triple-negative breast cancer. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 16(6), e2019 (2024).
Mendes, T. F., Kluskens, L. D. & Rodrigues, L. R. Triple Negative breast cancer: Nanosolutions for a big challenge. Adv. Sci. (Weinh) 2(11), 1500053 (2015).
Galmarini, C. M., Tredan, O. & Galmarini, F. C. Survivorship in untreated breast cancer patients. Med. Oncol. 32(2), 466 (2015).
Wu, J. et al. The natural history of breast cancer: A chronological analysis of breast cancer progression using data from the SEER database. Ann. Transl. Med. 10(6), 365 (2022).
Tan, K. H. et al. Quantifying the natural history of breast cancer. Br. J. Cancer 109(8), 2035–2043 (2013).
Loibl, S. et al. Early breast cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann. Oncol. 35(2), 159–182 (2024).
Liu, R. et al. Cancer-specific survival outcome in early-stage young breast cancer: Evidence from the SEER database analysis. Front. Endocrinol. (Lausanne) 12, 811878 (2021).
Strandberg, R., Czene, K., Eriksson, M., Hall, P. & Humphreys, K. Estimating distributions of breast cancer onset and growth in a swedish mammography screening cohort. Cancer Epidemiol. Biomark. Prev. 31(3), 569–577 (2022).
Hudis, C. A. & Gianni, L. Triple-negative breast cancer: An unmet medical need. Oncologist 16(Suppl 1), 1–11 (2011).
Prado-Vazquez, G. et al. A novel approach to triple-negative breast cancer molecular classification reveals a luminal immune-positive subgroup with good prognoses. Sci. Rep. 9(1), 1538 (2019).
Yang, C. et al. Research hotspots and frontiers of neoadjuvant therapy in triple-negative breast cancer: A bibliometric analysis of publications between 2002 and 2023. Int. J. Surg. 110(8), 4976–4992 (2024).
Kim, H. K. et al. Discordance of the PAM50 intrinsic subtypes compared with immunohistochemistry-based surrogate in breast cancer patients: Potential implication of genomic alterations of discordance. Cancer Res. Treat. 51(2), 737–747 (2019).
Jiang, Y. Z. et al. Genomic and transcriptomic landscape of triple-negative breast cancers: Subtypes and treatment strategies. Cancer Cell 35(3), 428-440.e5 (2019).
Bareche, Y. et al. Unravelling triple-negative breast cancer molecular heterogeneity using an integrative multiomic analysis. Ann. Oncol. 29(4), 895–902 (2018).
Guo, L. et al. Breast cancer heterogeneity and its implication in personalized precision therapy. Exp. Hematol. Oncol. 12(1), 3 (2023).
Joseph, K. et al. Outcome analysis of breast cancer patients who declined evidence-based treatment. World J. Surg. Oncol. 10, 118 (2012).
Han, E., Johnson, N., DelaMelena, T., Glissmeyer, M. & Steinbock, K. Alternative therapy used as primary treatment for breast cancer negatively impacts outcomes. Ann. Surg. Oncol. 18(4), 912–916 (2011).
Yao, M., Wang, S., Chen, L., Wei, B. & Fu, P. Research on correlations of miR-585 expression with progression and prognosis of triple-negative breast cancer. Clin. Exp. Med. 22(2), 201–207 (2022).
Lehmann, B. D. et al. Refinement of triple-negative breast cancer molecular subtypes: Implications for neoadjuvant chemotherapy selection. PLoS ONE 11(6), e0157368 (2016).
Liu, Y. et al. Subtyping-based platform guides precision medicine for heavily pretreated metastatic triple-negative breast cancer: The FUTURE phase II umbrella clinical trial. Cell Res. 33(5), 389–402 (2023).
Shao, N. et al. Comparison of the 7th and 8th edition of American Joint Committee on Cancer (AJCC) staging systems for breast cancer patients: A surveillance, epidemiology and end results (SEER) analysis. Cancer Manag. Res. 11, 1433–1442 (2019).
Shi, H., Wang, X. H., Gu, J. W. & Guo, G. L. Development and validation of nomograms for predicting the prognosis of triple-negative breast cancer patients based on 379 Chinese patients. Cancer Manag. Res. 11, 10827–10839 (2019).
Li, Z. et al. Establishment and verification of a nomogram to predict tumor-specific mortality risk in triple-negative breast cancer: A competing risk model based on the SEER cohort study. Gland Surg. 11(12), 1961–1975 (2022).
Acknowledgements
The authors would like to thank SEER for the open access to the database.
Funding
This work was supported by Scientific research project of Suining Central Hospital (No. 2022ypj20).
Author information
Authors and Affiliations
Contributions
Dasong Wang contributed to the study conception and design. Dasong Wang, Yan Yang and Yu He participate in writing proposal, analyzed the data, wrote the result and discussion. Dasong Wang, Wei Rong, Li Fan, Hongwei Yang, Maoshan Chen and Lei Yang participate in analyzing the data, writing result and prepared manuscript. All authors commented on previous versions of the manuscript, and all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study protocol was approved by the Ethics Committee of Suining Central Hospital. All information from the SEER program is available and free for public, and all patient data are deidentified, so informed consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, D., Yang, Y., Rong, W. et al. Natural history and prognostic nomogram of untreated triple negative breast cancer based on SEER database. Sci Rep 15, 23347 (2025). https://doi.org/10.1038/s41598-025-07114-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-07114-2