Abstract
The development of decellularized vascular tissues for tissue engineering and vascular implants presents a promising approach to creating functional blood vessels. However, effective endothelialization with human endothelial cells remains challenging. This study examined the endothelialization of decellularized porcine aortas using human induced pluripotent stem (hiPS) cell-derived endothelial cells. Various decellularization methods were used to create vessels with different luminal surface properties. hiPS-derived endothelial cells seeded on these vessels showed adhesion and alignment, particularly on those decellularized with the high hydrostatic pressure (HHP) method. These cells expressed higher levels of EphrinB2 compared to those cultured on flat surfaces, suggesting they respond to the vessel’s topographical features. The results indicate that hiPS-derived endothelial cells can adhere to and orient on decellularized porcine aortas, mimicking arterial endothelial behavior. For translational applications, achieving complete endothelial coverage by hiPSC-derived endothelial cells requires the establishment of optimal culture and seeding conditions. Nevertheless, these results highlight the importance of luminal surface topography and suggest that decellularized vascular tissues and implants can be designed with patterned surfaces to support endothelial behavior.
Similar content being viewed by others
Introduction
Cardiovascular disease remains one of the primary causes of mortality worldwide1. Surgical procedures, such as cardiovascular tissue replacement using artificial valves and vessels, are necessary when heart valves and blood vessels malfunction. Mechanical valves are more durable and have a longer service life but require long-term anticoagulant medication due to the risk of blood clot formation. In contrast, bioprosthetic valves, typically made from porcine or bovine pericardium and cross-linked with glutaraldehyde to reduce immunogenicity, are prone to calcification2. Since artificial valves do not grow, children may need multiple open-heart surgeries before reaching adulthood. These surgeries pose a risk to patient safety, with mortality rates ranging from 2 to 30%3,4.
Polyethylene terephthalate fiber (PET or Dacron) and expanded polytetrafluoroethylene (ePTFE) are the main materials used for artificial vessels. Although high patency rates have been achieved for medium and large diameters of 5 mm or more, both materials are non-degradable, remaining in vivo after implantation. They also lack autologous cell infiltration, limiting their growth potential and increasing the risk of infections due to encapsulation reactions5. In contrast, allogeneic human tissues are reported to have high anti-thrombogenic properties, lower susceptibility to infection, and growth potential because they integrate with the host tissue through recipient cell infiltration after transplantation6. Despite their excellent performance, as with organ transplantation, the number of available donors is limited, making these tissues less commonly used in treatment. Furthermore, while reports indicate that artificial valves and vessels have been successfully implanted for over 10 years, those suitable for lifelong use are largely limited to autologous tissues. Given these challenges, there is a need for the development of prosthetic valves and vessels that do not require reoperation and offer growth potential.
Decellularized tissues, which are extracellular matrix (ECM) obtained by removing cellular components and antigenic sites from human or heterologous animal tissues, are gaining attention as scaffold materials for fabricating artificial valves and vessels suitable for lifelong use7,8,9. These tissues are widely used in tissue regeneration due to their high biocompatibility, functionality, and growth potential. To address the limited availability of human tissues, heterologous animal tissues are often utilized. In order to realize the clinical application, re-cellularization of the basement membrane surface of decellularized tissues with human cells is necessary to make xenogeneic cardiovascular tissues, and this has not yet been fully achieved. Additionally, endothelialization of the luminal surface of decellularized vascular tissues before in vivo transplantation is crucial to provide endothelial function to the graft and prevent acute thrombus formation post-transplantation. Studies have shown that small-caliber artificial vessels with REDV peptides immobilized in the lumen of decellularized vessels exhibit high anti-thrombotic properties due to effective endothelialization10,11. Another study demonstrated that decellularized small intestinal submucosal tissue, formed into tubes to create artificial vessels and coated with vascular endothelial growth factor (VEGF) on the inner surface, facilitated the transformation of trapped monocytes into endothelial cells, thereby acquiring anti-thrombogenic properties12,13.
We have previously reported that the high hydrostatic pressure (HHP) decellularization technique maintains a higher degree of ECM integrity compared to chemical decellularization methods14,15,16. HHP decellularization is a physical tissue decellularization method that utilizes extremely high pressure (typically around 100–1000 MPa) to rupture cellular membranes. In the HHP process, tissues are placed in a pressure chamber filled with isotonic solution and subjected to high hydrostatic pressure for a specific duration. This pressure disrupts cellular membranes and causes intracellular components to leak out, which can then be removed through subsequent washing steps. Importantly, because no harsh chemicals are used, HHP-treated tissues exhibit better biocompatibility and structural preservation. Allogeneic and orthotopic transplantation of decellularized porcine aortas using the HHP method demonstrated effective vascular endothelial cell coating and achieved a 1-year patency rate without the need for anti-clotting agents17. Additionally, the luminal surface of decellularized vessels is assumed to be subject to external damage, including intraoperative handling and drying, leading to thrombus formation and intimal thickening18,19. In decellularized blood vessels produced using the HHP method, the shape and function of the basement membrane within the vascular lumen are well-preserved14,15,18. Consequently, it is believed that the recruitment of recipient cells, such as endothelial cells, contributes to the high anti-thrombotic properties. Futhermore, we have been developing methods to induce cardiovascular cell lineages from human induced pluripotent stem (iPS) cells with high efficiency as a human cardiovascular cell source20,21. To date, we have found that administering a high concentration of VEGF during differentiation culture results in the production of highly purified endothelial cells, which can be used to investigate the cellular behavior and morphology of human endothelial cells.
Based on these studies, this research investigates the endothelialization of the luminal basement membrane surface of decellularized aortas prepared using various decellularization methods, with human iPS cell-derived vascular endothelial cells (hiPSC-ECs). The aim of this study is to explore the necessary elements for preparing decellularized cardiovascular tissues from xenogeneic animal sources.
Materials and methods
Preparation of decellularized porcine aortas
Decellularized porcine aortas were prepared as previously reported. Fresh porcine aortas were sourced from Tokyo Shibaura Zouki (Tokyo, Japan) and stored at 4℃ until use. They were circularly cut to an inner diameter of 15 mm using a hollow punch. Decellularization was carried out using two methods: the detergent method (SDS method) and the high hydrostatic pressure (HHP) method. For the SDS method, native aortic slices were first immersed in 10% isodine solution (Shionogi healthcare, Osaka, Japan) for 10 min, followed by a 1% gentamicin solution (Tokyo Chemical Industry Com, Ltd, Tokyo, Japan) in saline overnight at 4 °C for sterilization. The slices were then incubated in Tris buffer containing 0.1% ethylenediaminetetraacetic acid (EDTA) (Dojindo, Kumamoto, Japan) for 24 h at 4 °C, and subsequently washed with 0.1% EDTA in phosphate-buffered saline (PBS) (FUJIFILM Wako Pure Chemical Corp., Osaka, Japan). The aortas were further incubated with 0.5% agarose and 1.25% trypsin for 4 h at 37 °C, followed by another wash with 0.1% EDTA in PBS for 2 h. The aortas were then soaked in 10 mM Tris (Sigma-Aldrich Co. Ltd, USA) buffer containing 0.1% EDTA and 0.1% SDS (FUJIFILM Wako Pure Chemical Corp., Osaka, Japan) for 24 h at room temperature and washed with PBS. They were rinsed in 50 mM Tris buffer containing 50 U/mL DNase (Roche Diagnostics, Tokyo, Japan), 1 U/mL RNase (Roche Diagnostics, Tokyo, Japan), 50 µg/mL bovine serum albumin (BSA) (Sigma-Aldrich Co. Ltd, USA.), and 10 mM MgCl2 (FUJIFILM Wako Pure Chemical Corp., Osaka, Japan) for 4 h at 37 °C, and subsequently washed in 0.1% EDTA in PBS at 37 °C for 72 h. For the HHP method, decellularization was achieved by applying hydrostatic pressure of 1000 MPa at 30 °C for 10 min using a hydrostatic pressure system (Dr. Chef, Kobelco, Kobe, Japan). This was followed by a 7-day wash with DNase (0.2 mg/mL) and MgCl2 (50 mM) in saline at 4 °C, and a 3-day wash in 80% ethanol in saline at 4 °C to remove cellular debris. Dr. Chef is a high hydrostatic pressure (HHP) device that uses water as a pressure-transmitting medium to apply uniform, isotropic pressure—up to approximately 980 MPa—to samples in a sealed chamber. Originally developed for food sterilization, the device has been adapted for biomedical applications such as decellularization of biological tissues17,22.
Histological analysis
The decellularized aortas obtained via the SDS and HHP methods were first immersed in a neutral buffered solution (pH 7.4) containing 10% formalin in PBS for 24 h, then soaked in dehydrated ethanol. The samples were subsequently transferred to xylene and then embedded in paraffin. Four-µm thick sections were cut from each paraffin block using a microtome for hematoxylin and eosin (H&E) staining and Type IV collagen staining. As a quantitative analysis, binarization was performed on Collagen IV staining images using ImageJ software, and the percentage of the stained positive area was measured.
Scanning electron microscopy (SEM) observation
The surface structure of the decellularized aortas was evaluated using a scanning electron microscope (S-4500/EMAX-700, Hitachi, Ltd, Tokyo, Japan). Samples were fixed with 2.5% glutaraldehyde (TAAB Laboratories Equipment, Berkshire, UK) in PBS and then dehydrated through a series of ethanol solutions. The dehydrated samples were immersed in tert-butyl alcohol (FUJIFILM Wako Pure Chemical Corp., Osaka, Japan) overnight and subsequently vacuum-dried. Before observation, the surfaces of the vacuum-dried decellularized samples were coated with gold.
Human iPS cell culture and differentiation
The present study used human iPSCs line 201B6 established at the Center for iPS Cell Research and Application (CiRA, Kyoto, Japan). The human iPSC line 201B6 was derived from human skin fibroblasts using 4 factors (OCT3/4, SOX2, KLF4, and c-MYC) via retroviral transduction23. The cell line was distributed from Kyoto University to RIKEN under a material transfer agreement. The maintenance of human iPSCs and differentiation of cardiovascular cells was conducted following our previous studies24 with modifications. In brief, iPSCs were expanded and maintained with StemFit AK02N medium (Ajinomoto, Tokyo, Japan). At confluence, the cells were dissociated with TrypLE Select (Thermo Fisher Scientific, Waltham, MA, USA), dissolved in 0.5 mM ethylenediaminetetraacetic acid in PBS (1:1) and passaged as single cells (5,000–8,000 cells/cm2) every 7 days in AK02N containing iMatrix-511 silk (FUJIFILM Wako Pure Chemical Corp., Osaka, Japan) (0.125 µg/cm2) (uncoated laminin fragment) and ROCK inhibitor (Y-27632, 10 µM, FUJIFILM Wako, Osaka, Japan). For cardiovascular cell differentiation, single iPSCs were seeded onto Matrigel-coated plates (1:60 dilution) at a density of 300,000–400,000 cells/cm2 in AK02N with Y-27,632 (10 µM). At confluence, the cells were covered with Matrigel (Corning, NY, USA) (1:60 dilution in AK02N) one day before induction. We replaced the AK02N medium with RPMI + B27 medium (RPMI 1640, Thermo Fisher; 2 mM L-glutamine, Thermo Fisher; 1× B27 supplement without insulin, Thermo Fisher) supplemented with 10 ng/mL Activin A (R&D or Mitsubishi Chemical Corporation, Tokyo, Japan) (differentiation day 0; d0) and 5 µM CHIR99021 (Tocris Bioscience, Bristol, UK) was added for 24 h, which was followed with supplementation with 10 ng/mL bone morphogenetic protein 4 (BMP4; R&D) and 10 ng/mL basic fibroblast growth factor (bFGF; FUJIFILM Wako) (d1) for 4 days without culture medium change. At d5, the culture medium was replaced with RPMI1640 medium supplemented with 200 ng/ml of VEGF165, 1 mM 8-Bromoadenosine-3',5'-cyclic monophosphate sodium hydrate (cAMP) (Nacalai Tesque, Inc., Kyoto, Japan) and was refreshed every other day with RPMI1640 supplemented with 200 ng/ml VEGF. At d13, the cultured cells were dissociated using Accutase (Nacalai Tesque), and a portion of them was used for evaluation by flow cytometry (see the section"Flow cytometry"). The remaining cells were cryopreserved using STEM-CELLBANKER (Takara Bio Inc., Kusatsu, Japan) per instruction from the manufacturer. The preparation of hiPSC-ECs up to this point was carried out at RIKEN (Kobe, Japan), and the cryopreserved vials were then sent to Tohoku University (Sendai, Japan).
Flow cytometry
Flow cytometry was conducted by our previous study with modifications24 Differentiated cardiovascular cells and cardiac tissue sheets were dissociated by incubation with Accutase as indicated at the Section"Human iPS cell culture and differentiation". and stained with one or a combination of the following surface markers: anti-PDGFRβ conjugated with phycoerythrin (PE), clone 28d4, 1:100 (BD) for parietal cells, and anti-VE-cadherin conjugated with phycoerythrin (PE), clone 55-7h1, 1:100 (BD) for hiPS-ECs. To eliminate dead cells, cells were stained with the LIVE/DEAD fixable Aqua dead cell staining kit (Thermo Fisher). For cell surface markers, staining was carried out in PBS with 5% FBS. For intracellular proteins, staining was carried out in cells fixed with 4% paraformaldehyde (PFA) in PBS. Cells were stained with the anti-cardiac isoform of troponin T (cTnT) (clone 13 − 11) (Thermo Fisher) labelled with APC using Zenon technology (Thermo Fisher) (1:50) for cardiomyocytes. The staining was performed in PBS with 5% FBS and 0.75% saponin (Nacalai Tesque). The stained cells were analyzed by CytoFLEX S (Beckman Coulter, Brea, CA, USA). Data was collected from at least 10,000 events. Data was analyzed with CytExpert software (Beckman Coulter).
Immunofluorescent imaging
Cells were fixed with 4% paraformaldehyde (PFA) and permeabilized with 1% Triton X-100 in PBS. Subsequently, the cells were incubated with 1% BSA in PBS. After washing, the cells were stained with CD31 antibody (#9G11, R&D systems, Minneapolis, USA) and Alexa488 antibody (ab150113, Abcam, Cambridge, UK). Nuclei were stained using DAPI (340–07971, Dojindo,). Stained cells were photographed with an all-in-one fluorescent microscopic system, (BZ-X710, Keyence Corp., Osaka, Japan).
Human induced pluripotent stem cell-derived endothelial cells (hiPSC-ECs) culture
Six cm dish was coated with 0.1% gelatin at 37 °C for 1 h. Six mL of endothelial cell medium (EBM-2) (Lonza, Walkersville, USA) warmed to 37 °C was prepared in a 15 mL conical tube. A frozen vial taken from liquid nitrogen was thawed until just a bit of ice remained, and then it was quickly suspended in the medium. The cell was centrifuged at 1200 rpm, 4 °C for 5 min, and the supernatant was aspirated. An appropriate amount of medium was added, and the cells were seeded at 1.9–3.8 × 104 cells/cm2.
Cell seeding and proliferation measurement
The aortas (15 mm internal diameter) were placed in a 24-well tissue culture plate. hiPSC-ECs were seeded at a density of 2 × 104 cells/cm² on the surface of the decellularized aortas and cultured at 37 °C for 7 days. Cell viability was assessed on days 1 and 7 by staining the hiPS-ECs with calcein-AM (Dojindo, Kumamoto, Japan). The treated cells were observed using a fluorescence microscope (BZ-X710, Keyence Corp., Osaka, Japan). Cell density was determined by counting the number of cells.
Cell alignment
Approximately 20 cells were randomly selected. The angle of each cell relative to the longitudinal axis of the aorta was measured using ImageJ software. These angles were then plotted to illustrate the distribution of cell orientations throughout the decellularized aorta. A smaller angle indicates that the cells are aligned along the longitudinal axis of the aorta. The punched-out circular segments of the porcine aorta exhibited inward curling on the luminal side, which enabled identification of the longitudinal axis of the vessel.
Gene expression
Total RNA was extracted from hiPSC-ECs cultured on TCPS and on HHP decellularized aortas using ISOGEN and a spin column (Nippon Gene, Tokyo, Japan), following the manufacturer’s instructions. GAPDH was used as a reference gene for normalization of gene expression. Quantitative PCR was performed using Power SYBR Green PCR Master Mix on a StepOnePlus system (Thermo Fisher Scientific), employing the ΔCT method. The forward and reverse primer sequences are provided in Table 1.
Statistical analysis
The quantification of collagen IV-positive area in decellularized aortas (Fig. 1) and the quantitative analysis of qRT-PCR (Fig. 5) were expressed using the mean with standard deviation (SD). The Student’s t-test was used to determine significant differences. The graphs were generated using GraphPad Prism software.
Results and discussion
Porcine aortas were decellularized using two methods: the SDS method, which uses a detergent, and the HHP method, which uses high hydrostatic pressure. The surface properties of the aortas were then evaluated for each method. Figure 1 shows microscopic images of decellularized porcine aorta stained with hematoxylin and eosin (H&E) and Type IV collagen. H&E staining indicates that the purple-stained nuclei are removed after decellularization treatment. The SDS-decellularized aorta exhibits a wide gap between elastin fibers (Fig. 1B), whereas the HHP-decellularized aorta retains its elastin fibers even after decellularization (Fig. 1C). Staining for type IV collagen, a key component of the basement membrane, reveals that the basement membrane is diminished in the SDS-decellularized aorta (Fig. 1E) but preserved after HHP decellularization (Fig. 1 F). As a quantitative analysis, the percentage of Collagen IV-positive areas in immunostained images was measured using ImageJ software. The results showed that the HHP-treated group retained a significantly higher amount of Collagen IV compared to the SDS-treated group (Fig. 1G).
Figure 2 shows SEM images of untreated and decellularized aortas. The luminal surface of the untreated aorta is covered by endothelial cells with a basement membrane, with visible longitudinal surface grooves. After decellularization, both treated aortas reveal more longitudinally elongated fibers. This suggests that decellularization removed the endothelial cells and thinned the basement membrane, exposing the underlying fibers. Notably, fine fibers are more prominently exposed in the SDS-decellularized aorta (Fig. 2B). The longitudinal surface grooves are visible on the HHP-decellularized aorta, while they are less distinct and appear flat on the SDS-decellularized aorta. Regarding HHP decellularization, although the surface morphology appears to be relatively preserved (Fig. 2C), it has been reported that treatment at 1000 MPa, similar to the conditions in this study, preserves type IV collagen in the basement membrane, while type VII collagen is damaged at 500 MPa25. Therefore, it is predicted that the components are affected by the high pressure.
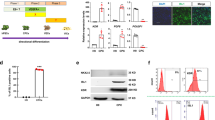
Figure 3 shows the results of the characterization of the generated hiPSCs. The generated hiPSCs were evaluated by flow cytometry using three different antibodies for cardiomyocytes, vascular endothelial cells and vascular mural cells, and it was found that endothelial cells were obtained with a high purity and yield of 94.7%. In addition, in Fig. 3B, fluorescence staining with CD31, a marker of vascular endothelial cells, showed a single-layer high-density culture of vascular endothelial cells. Thus, most of the hiPSC-EC phenotypes showed endothelial cell phenotypes before seeding onto decellularized tissue.
Figure 4 presents hiPSC-ECs seeded on porcine aorta cultured for 7 days, and stained with Calcein-AM. On SDS decellularized aortas, only a small number of hiPSC-ECs adhered, likely due to cell damage from residual detergent. Residual SDS amounts in the tissues were evaluated using methylene blue, revealing that a significantly higher amount of SDS remained in SDS-treated vessels compared to untreated and HHP-treated vessels (Supplement Fig. 1). Other researchers have also said the residual SDS in the decellularized ECM is difficult to remove, compromising recellularization due to its high cytotoxicity26. Repeated washing with PBS to remove residual SDS has been tried, however, it is reported that even 0.01% SDS is difficult to remove completely27. While reports suggest that cardiovascular tissues decellularized with SDS are effective in vivo28,29 it can be assumed that SDS is not suitable for decellularizing tissues intended for transplantation after recellularization. To develop tissues for transplantation after in vitro recellularization, other reports recommend considering the use of an ionic detergent that is less aggressive than SDS, such as sodium deoxycholate, in the future8,30. The dead cells were considered to have detached from the surface of the aorta and were floating in the culture medium. Therefore, the cells present in the medium were collected and stained with Ethidium homodimer-1 (EtHD-1), resulting in a significantly increased staining intensity in the SDS-treated group (data not shown). In contrast, while cells adhered to the HHP-decellularized aortas, the number of adherent cells was lower than those on tissue culture polystyrene (TCPS). The morphology of the adherent cells differed. They were more pavement-like on TCPS and spindle-shaped on the decellularized aortas. As shown in Methods 3–9, the orientation angle of the adherent cells was measured from fluorescence microscopy images. The closer the angle is to 0, the more the cells are aligned along the longitudinal axis of the aorta. While cells on TCPS did not show alignment, cells on HHP-decellularized aortas were aligned along the long axis of the vessel (Fig. 4B). This suggests that the luminal surface of HHP-decellularized aortas is oriented in the long-axis direction, and hiPSC-ECs specifically respond to this vascular alignment by adjusting their morphology accordingly. For the application of decellularized aortas as implantable materials, complete endothelial coverage is critical to prevent platelet activation and thrombogenesis, thereby ensuring proper vascular function and immune homeostasis31. At day 7, which represents an early time point, intercellular junctions may not have fully matured. Furthermore, suboptimal seeding density or culture conditions cannot be excluded as contributing factors to inadequate cell attachment and spreading. These observations underscore the need for further optimization, including ECM surface pre-treatment (e.g., laminin or VEGF coating), dynamic culture conditions implementation, and seeding density reevaluation.
The expression of vascular endothelial cell and arterial vascular endothelial markers was evaluated in hiPSC-ECs cultured on TCPS and on HHP-decellularized aortas (Fig. 5). These three markers were used in this study because they are also used for expression evaluation during hiPSC-EC induction20. Especially, the expression levels of EphrinB2 markers were found to be higher in hiPSC-ECs on HHP-decellularized aortas compared to those on TCPS. This suggests that the orientation of hiPSC-ECs along the luminal surface structure of the decellularized aorta enhances the expression of genes specific to the vascular endothelium. This phenomenon is consistent with the concept of contact guidance, where cells align and move along a directional substrate. It is known as essential step in cell adhesion, alignment and migration and has been observed in organ and tissue regeneration process32. In recent years, research has been advancing to understand how the phenotype of endothelial cells is influenced by topography33,34,35. Endothelial cells respond to substrate shape by promoting the extension of focal adhesions in the direction of the substrate while limiting the growth of focal adhesions in the orthogonal direction. This leads to the formation of aligned actin stress fibers and the generation of anisotropic stress that aligns and elongates cells in the direction of the topography, proposing a model where cells align and elongate accordingly36. EphrinB2 has been reported as a powerful regulator of endothelial cell morphology and motility. EphrinB2 induces endothelial cell behavior by repeating cycles of actomyosin-dependent cell contraction and extension even in the absence of Eph receptors37,38. Therefore, it is thought that hiPSC-ECs on HHP-decellularized vessels recognize the shape of the lumen surface and align and elongate along the major axis, leading to an increase in EphrinB2 expression compared to that on flat TCPS.
Conclusion
In this study, using high-purity human iPS cell-derived endothelial cells, we investigated the endothelialization of the luminal membrane surface of decellularized porcine aortas and explored the necessary elements for creating decellularized vascular tissues from xenogeneic animals. The decellularization methods allowed the preparation of decellularized vessels with different luminal surface properties. Seeding high-purity human iPS cell-derived ECs onto these decellularized vessels revealed that, on HHP-decellularized vessels, initial adhesion and alignment of endothelial cells in the long axis direction were observed. Additionally, the expression of EphrinB2 in endothelial cells on the decellularized vessels was higher compared to that on flat TCPS. This suggests that hiPS-derived ECs recognize the grooves in the long axis direction of the lumen surface shape of the decellularized vessels, leading to increased EphrinB2 expression associated with cell motility and orientation. These results indicate that human iPS cell-derived ECs can attached and orient on porcine derived decellularized vessels, demonstrating arterial endothelial behavior. This highlights the importance of the luminal surface topography in the functional expression of hiPSC-ECs when preparing decellularized vascular tissues from xenogeneic animals. The findings of this study are expected to be useful not only for decellularized tissue but also in designing vascular implants with topographically patterned surfaces. Furthermore, there is potential to control the differentiation and polarity of undifferentiated hiPS cells based on luminal surface shape.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Wilcox, N. et al. Cardiovascular disease and cancer: shared risk factors and mechanisms. Nat. Rev. Cardiol. 21, 617–631 (2024).
Fioretta, E. S., Dijkman, P. E., Emmert, M. Y. & Hoerstrup, S. P. The future of heart valve replacement: recent developments and translational challenges for heart valve tissue engineering. J. Tissue Eng. Regen. Med. 12(1), E323–E35 (2018).
Bahaaldin Alsoufi, C. M. et al. Outcomes and associated risk factors for mitral valve replacement in children. European J. Cardio-Thorac. Surg. 543–551 (2011).
Hofferberth, S. C. et al. A geometrically adaptable heart valve replacement. Sci. Transl. Med. 12, 531 (2020).
Drews, J. D., Miyachi, H. & Shinoka, T. Tissue-engineered vascular grafts for congenital cardiac disease: clinical experience and current status. Trends Cardiovasc. Med. 27 (8), 521–531 (2017).
Furlough, C. L. et al. Peripheral artery reconstructions using cryopreserved arterial allografts in infected fields. J. Vasc. Surg. 70 (2), 562–568 (2019).
Barbulescu, G. et al. Decellularized extracellular matrix scaffolds for cardiovascular tissue engineering: current techniques and challenges. Int. J. Mol. Sci. 23, (2022).
Ramm, R. et al. Decellularization combined with enzymatic removal of N-linked glycans and residual DNA reduces inflammatory response and improves performance of Porcine xenogeneic pulmonary heart valves in an ovine in vivo model. Xenotransplantation. 27(2), (2020).
Sarikouch, S. et al. Decellularized fresh homografts for pulmonary valve replacement: a decade of clinical experience. Eur. J. Cardiothorac. Surg. 50 (2), 281–290 (2016).
Mahara, A. et al. Tissue-engineered acellular small diameter long-bypass grafts with neointima-inducing activity. Biomaterials 58, 54–62 (2015).
Mazloomnejad, R. et al. Angiogenesis and Re-endothelialization in decellularized scaffolds: Recent advances and current challenges in tissue engineering, Front. Bioeng. Biotechnol. 11, (2023)
Smith, R. J. Jr. et al. Endothelialization of arterial vascular grafts by circulating monocytes. Nat. Commun. 11(1), 1622 (2020).
Nasiri, B., Row, S., Smith, R. J., Swartz, D. D. & Andreadis, S. T. Cell-Free vascular grafts that grow with the host. Adv. Funct. Mater. 30(48), (2020).
Kobayashi, M. et al. In vitro evaluation of surface biological properties of decellularized aorta for cardiovascular use. J. Mater. Chem. B. 8, 48 (2020).
Wu, P. L. et al. Decellularized Porcine aortic intima-media as a potential cardiovascular biomaterial. Interact. Cardiovasc. Thorac. Surg. 21 (2), 189–194 (2015).
Ho, W. et al. A novel approach for the endothelialization of xenogeneic decellularized vascular tissues by human cells utilizing surface modification and dynamic culture. Sci. Rep. 12(1), (2022).
Funamoto, S. et al. The use of high-hydrostatic pressure treatment to decellularize blood vessels. Biomaterials 31 (13), 3590–3595 (2010).
Sébastien Déglise, C. B. & Allagnat, F. Vascular smooth muscle cells in intimal hyperplasia, an update. Front. physiol. 1081881 (2022).
Kurokawa, S. et al. In vivo recellularization of xenogeneic vascular grafts decellularized with high hydrostatic pressure method in a Porcine carotid arterial interpose model. Plos One . 16(7), (2021).
Ikuno, T. et al. Efficient and robust differentiation of endothelial cells from human induced pluripotent stem cells via lineage control with VEGF and cyclic AMP. Plos One 12, e0173271 (2017).
Kozue Murata, A. M., Tomonaga, K. & Masumoto, H. Predicted Risk of Heart Failure Pandemic Due To Persistent SARS-CoV-2 Infection Using a three-dimensional Cardiac Model. iScience. (2024).
Negishi, J. et al. Porcine radial artery decellularization by high hydrostatic pressure. J. Tissue Eng. Regen. Med. 9 (11), E144–E51 (2015).
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131(5), 861–872 (2007).
Maihemuti, W., Murata, K., Abulaiti, M., Minatoya, K. & Masumoto, H. Simultaneous electro-dynamic Stimulation Accelerates Maturation of Engineered Cardiac Tissues Generated by Human iPS Cells. Biochemi. Biophys. Res. Commun. 733, (2024).
Morimoto, N. et al. The Alteration of the Epidermal Basement Membrane Complex of Human Nevus Tissue and Keratinocyte Attachment after High Hydrostatic Pressurization. Biomed. Res. Int. (2016).
Caamaño, S., Shiori, A., Strauss, S. H. & Orton, E. C. Does sodium Dodecyl sulfate wash out of detergent-treated bovine pericardium at cytotoxic concentrations? J. Heart Valve Dis. 18 (1), 101–105 (2009).
Syed, O., Walters, N., Day, R., Kim, H. & Knowles, J. Evaluation of decellularization protocols for production of tubular small intestine submucosa scaffolds for use in oesophageal tissue engineering. Acta Biomater. 10, 5043–5054 (2014).
da Costa, F. D. A. et al. The early and midterm function of decellularized aortic valve allografts. Ann. Thorac. Surg. 90 (6), 1854–1861 (2010).
Vafaee, T. et al. Decellularization of human donor aortic and pulmonary valved conduits using low concentration sodium Dodecyl sulfate. J. Tissue Eng. Regen. Med. 12 (2), E841–E53 (2018).
Li, J. et al. The application of composite scaffold materials based on decellularized vascular matrix in tissue engineering: a review. Biomed Eng Online. (2023).
Jana, S. Endothelialization of cardiovascular devices. Acta Biomater. 99, 53–71 (2019).
Sales, A., Holle, A. & Kemkemer, R. Initial contact guidance during cell spreading is contractility-independent. Soft Matter 13, 5158–5167 (2017).
Ana Maria Almonacid Suarez I. v. d. H., Brinker M. G. L., van Rijn P. & Harmsen M. C. Topography-driven alterations in endothelial cell phenotype and contact guidance. Heliyon. e04329 (2020).
Claire, A., Dessalles, C. L., Alessia, C., & Barakat A.I. Integration of substrate- and flow-derived stresses in endothelial cell mechanobiology. Inherit Commun. Biol. (2021).
Hinds, M. W. H. a. M. T. The effects of topographic micropatterning on endothelial colony-forming Cells. Tissue Eng. Part A. 270–281 (2021).
Claire Leclech, A. I. B. Is there a Universal mechanism of cell alignment in response to substrate Topography?. Cytoskeleton. 284–292 (2021).
Claire Leclech, A. K., Muller L., & Barakat, A. I., Distinct contact guidance mechanisms in single endothelial cells and in monolayers. Adv. Mater. Interfaces. (2023).
Bochenek M. L., Dickinson S., Astin J. W., Adams R. H. & Nobes C. D. Ephrin-B2 regulates endothelial cell morphology and motility independently of Eph-receptor binding. Adv. Mater. Interfaces J. Cell. Sci. 1235–1246 (2010).
Acknowledgements
This work was partly supported by Grants-in-Aid for Scientific Research, KAKENHI [23K17207, 24H00787, 21H04954, 22K08956, 17H04291], and a grant for the Frontier Research Institute for Interdisciplinary Sciences (TI-FRIS/FRIS) from Tohoku University.
Author information
Authors and Affiliations
Contributions
M.K., A.K., and H.M. conceptualized and designed the study. M.K. and K.M. performed the investigation. Y.H., T.K., and H.M. were responsible for the methodology. M.Y., H.M., and A.K. supervised the study. M.K. wrote the original draft. M.Y., H.M., and A.K. reviewed and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kobayashi, M., Murata, K., Yamamoto, M. et al. Recellularization of decellularized vascular grafts via aligned seeding of endothelial cells derived from human iPS cells. Sci Rep 15, 27470 (2025). https://doi.org/10.1038/s41598-025-07458-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-07458-9