Abstract
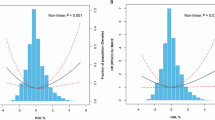
Coronary artery calcification (CAC) is a well-established cardiovascular disease (CVD) pathogenesis marker closely associated with dysregulated glucose and lipid metabolism. The present study investigated the relationship between the glycated hemoglobin A1c (HbA1c)/high-density lipoprotein cholesterol (HDL-C) ratio and CAC. A total of 1608 eligible participants were enrolled in the study. The HbA1c/HDL-C ratio was calculated by dividing HbA1c by HDL-C. LASSO regression, logistic regression, and receiver operating characteristic curve (ROC) analysis were performed to examine the relationship between the HbA1c/HDL-C ratio and CAC. The nomogram incorporating the HbA1c/HDL-C ratio was further established by multivariate logistic regression and evaluated. The HbA1c/HDL-C ratios in the CAC group were significantly higher than the control group [4.73 (4.01, 5.56) vs. 4.34 (3.67, 5.05), p < 0.001]. Subjects with an elevated HbA1c/HDL-C ratio (≥ 4.99) exhibited a higher prevalence of CAC [146/486 (30.04) vs. 200/1122 (17.83), p < 0.001]. Furthermore, an elevated HbA1c/HDL-C ratio was an independent effect factor for CAC [odds ratio, 1.135; 95% confidence interval (CI), 1.008–1.279; p = 0.037]. The area under the ROC curve of the HbA1c/HDL-C ratio was 0.630 (95% CI 0.596–0.663), and the model incorporating age, gender, body mass index, hypertension, diabetes mellitus, and the HbA1c/HDL-C ratio was 0.718 (95% CI 0.686–0.751). The constructed nomogram based on this model demonstrated favorable discrimination and clinical utility. In conclusion, the HbA1c/HDL-C ratio is closely associated with CAC and is an independent factor for CAC in asymptomatic adults without CVD.
Similar content being viewed by others
Introduction
Vascular calcification is characterized by the pathological deposition of calcium phosphate crystals within the vascular wall, leading to structural and functional arterial impairment1. This process exerts a critical determinant role in the pathogenesis of diverse cardiovascular disorders and markedly amplifies the risk of adverse cardiovascular outcomes. It is frequently observed in chronic diseases such as atherosclerosis, diabetes mellitus (DM), chronic kidney disease (CKD), and hypertension, all of which are key contributors to the progression and clinical burden of cardiovascular disease (CVD)2. Coronary artery calcification (CAC), a specific form of vascular calcification, is an essential biomarker for assessing atherosclerotic CVD. The CAC score (CACS) quantitatively assesses coronary calcium burden via imaging modalities such as computed tomography (CT)3. Studies have shown that higher CACS correlates with an elevated likelihood of future cardiovascular events, which are independently associated with traditional factors such as hypertension and hypercholesterolemia4,5,6. Notably, in asymptomatic individuals, the CACS has been demonstrated to significantly predict future all-cause mortality and cardiovascular events, including myocardial infarction, heart failure, and cardiovascular mortality7,8. Therefore, the timely identification of CAC and its associated effect factors is essential for mitigating cardiovascular morbidity and mortality.
Glycated hemoglobin A1c (HbA1c) is a widely accepted and standardized biomarker for long-term glycemic control, reflecting average plasma glucose concentrations over the preceding 2 to 3 months9. Elevated HbA1c levels may contribute to vascular calcification in multiple systems through alterations in calcium-phosphate homeostasis, promotion of oxidative stress, and endothelial dysfunction, thereby increasing the probability of cardiovascular events10,11. Several studies have demonstrated a positive association between HbA1c levels and CACSs12. Additionally, high-density lipoprotein cholesterol (HDL-C) plays a protective role in cardiovascular health through its involvement in reverse cholesterol transport, facilitating the transfer of cholesterol from peripheral tissues to the liver for metabolism and excretion, thereby inhibiting lipid accumulation and attenuating atherogenesis and vascular calcification13,14. However, emerging evidence indicates that elevated HDL-C levels do not always correspond to less vascular calcification occurrence in specific subpopulations or disease contexts14. Thus, the relationship between HDL-C and vascular calcification remains complex and is currently debated. Moreover, interactions between glucose and lipid metabolism appear to be critically involved in vascular calcification pathogenesis. Hyperglycemia-induced dyslipidemia and dyslipidemia-induced hyperglycemia, along with associated pro-inflammatory and oxidative stress pathways, exacerbate endothelial injury and promote osteogenic transdifferentiation of vascular smooth muscle cells15,16,17,18,19. Given the pivotal roles of both glycemic and lipid metabolic pathways in CAC pathophysiology, a combined assessment of HbA1c and HDL-C may provide valuable insights into the identification of CAC.
In recent years, there has been growing interest in using composite biomarkers to enhance the assessment of CAC. Several indices integrating lipid components, such as the atherogenic index of plasma (AIP), the low-density lipoprotein cholesterol to HDL-C (LDL-C/HDL-C) ratio, and the triglyceride to HDL-C (TG/HDL-C) ratio, have demonstrated clinical utility in predicting atherosclerosis burden and are associated with CAC progression20,21. However, these lipid-centric indices do not consider glycemic status, potentially underestimating the contribution of hyperglycemia to CAC pathogenesis. The triglyceride-glucose (TyG) index, derived from fasting plasma glucose and triglyceride levels, has emerged as a surrogate marker for insulin resistance and has been shown to correlate positively with CAC severity22. Despite the growing use of the TyG index, the research on the association between composite glycemic-lipid indices and CAC remains limited. Moreover, the TyG index depends on fasting plasma glucose and is subject to intra-individual variability and various pre-analytical influences. In contrast, HbA1c provides a stable and integrative measure of chronic glycemic exposure, offering enhanced reproducibility and reliability23. Recent studies have found that the HbA1c/HDL-C ratio is associated with several cardiometabolic and vascular conditions, including carotid atherosclerosis, stroke, diabetic retinopathy, and metabolic-associated fatty liver disease (MAFLD)23,24,25,26,27. However, no studies have examined the association between the HbA1c/HDL-C ratio and CAC. Therefore, the present study aims to investigate the potential relationship between the HbA1c/HDL-C ratio and CAC and whether this novel biomarker has identification utility for CAC in asymptomatic adults without CVD.
Methods
Study design and populations
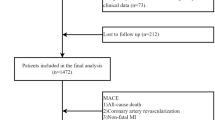
This study represents a secondary analysis of data derived from a cross-sectional cohort of Korean individuals who underwent CACS via multi-detector CT at the Seoul National University Hospital Healthcare System Gangnam Center from January 2014 to March 2016. The original study aimed to identify single-nucleotide polymorphisms (SNPs) associated with severe CAC through a genome-wide association study (GWAS) in asymptomatic individuals without a prior history of CVD28. In the initial study, 2188 participants without CVD were enrolled, and 500 individuals with CACS between the observed 50th and 90th percentiles for age and sex were excluded. In the present research, the participants with more than 20% missing data, particularly those lacking HbA1c or HDL-C values, were also excluded. Ultimately, a total of 1608 participants were included in this study (Fig. 1). The original study was approved by the ethics committee of the Seoul National University Hospital, and informed consent was waived owing to the study’s retrospective nature. All study procedures adhered to the principles outlined in the Declaration of Helsinki. Detailed information regarding the initial study design and methodology has been described previously28.
Data collection and definition
The data utilized in this study were extracted from a publicly accessible repository (https://figshare.com). According to the data sharing policy, all researchers are free to download the original data and use the data for secondary analysis without harming the rights and interests of the authors. Following the policy, we needed to cite data sources when using these data29. The original data variables we required in this study were age, gender, body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), hypertension, DM, smoking, creatinine, estimated glomerular filtration rate (eGFR), total cholesterol (TC), TG, LDL-C, HDL-C, glucose, and HbA1c. The detailed data collection and measurement methods were mentioned in the original research by Choi et al.28.
HbA1c/HDL-C ratio was calculated as HbA1c (%) divided by HDL-C (mmol/L). Based on the optimal cut-off value derived from receiver operating characteristic (ROC) curve analysis, participants were categorized into two groups: HbA1c/HDL-C ratio < 4.99 group (n = 1122) and HbA1c/HDL-C ratio ≥ 4.99 group (n = 486). The eGFR was calculated as 175 × creatinine-1.154 × age-0.203 × [0.742 (if female)], where the unit of creatinine is mg/dL.
According to self-reported habits, smoking status was categorized as never, former, or current. Hypertension was defined as SBP ≥ 130 mmHg, DBP ≥ 80 mmHg, a documented history of hypertension, or current use of antihypertensive medications. DM was defined as fasting blood glucose ≥ 7.0 mmol/L (≥ 126 mg/dL), HbA1c ≥ 6.5%, a prior diagnosis of diabetes, or current use of diabetes drugs.
All participants underwent coronary artery CT by a 256-slice multidetector CT scanner (Brilliance iCT 256; Philips Medical Systems, Cleveland, Ohio) or a 16-slice scanner (Somatom Sensation 16; Siemens Medical Solutions, Forchheim, Germany) for CAC measurement28. CACS was calculated using dedicated analysis software (Rapidia 2.8; INFINITT, Seoul, Republic of Korea) based on the Agatston scoring method28. Image interpretation and scoring were performed by experienced radiologists specialized in cardiovascular imaging according to standard guidelines. The Agatston score quantifies CAC by identifying lesions with a CT value of more than 130 HU and applying the formula: ∑ (calcification area × density coefficient determined by CT value intervals)30,31. In line with previous studies, the presence of CAC is defined as CACS > 030. Accordingly, in this study, participants were divided into two groups: the non-CAC group (CACS = 0; n = 1262) and the CAC group (CACS > 0; n = 346).
Statistical analysis
Data processing and analysis were performed using R software (version 4.4.0; R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org) and Zstats software (version 1.0; Weijun Zheng, Hangzhou, China; http://www.zstats.net). Multiple imputation was utilized to handle missing values. Continuous variables were summarized using median (IQR) due to abnormal distribution, as checked by the Shapiro–Wilk test, and categorical variables using frequencies and percentages. Differences in continuous variables were compared by the Mann–Whitney U-test and categorical variables by the chi-square test or Fisher’s exact test. The correlation between the HbA1c/HDL-C ratio and clinical variables was evaluated by Spearman’s correlation test. The Least Absolute Shrinkage and Selection Operator (LASSO) regression, combined with the optimal λ value, was used to screen the potential influencing factors of CAC. CAC-relevant effect factors were examined using univariate and backward conditional multivariate logistic regressions. The ROC curve was used to evaluate the predictive value of the HbA1c, HDL-C, and HbA1c/HDL-C ratio for CAC, and the optimal cut-off value was determined by Youden’s index. The clinical predictive model was constructed based on multivariate logistic regression analysis results, and a nomogram was established to represent the model visually using the rms R package. The area under the curve (AUC) determined by ROC is used to test the discriminative ability of the models and variables, and the DeLong method was utilized to compare the AUCs by the pROC R package. The bootstrap resampling method was used for internal validation. The consistency index (C-index), calibration curve, and decision curve analysis (DCA) were used to estimate the model’s discriminatory ability, prediction accuracy, and clinical utility. A two-sided p < 0.05 was considered statistically significant.
Results
Baseline characteristics of the study populations
Table 1 summarizes the baseline clinical characteristics of the study participants. The median age of participants was 52 years, and 76.06% were males. Compared to participants in the control group, those in the CAC group had significantly higher HbA1c/HDL-C ratio [4.73 (4.01, 5.56) vs. 4.34 (3.67, 5.05), p < 0.001]. Additionally, the patients with CAC were older and had higher BMI, SBP, DBP, TG, glucose, and HbA1c levels, and lower levels of TC and HDL-C (all p < 0.05). The patients with DM, hypertension, smoking, and male patients were more prone to CAC.
Participants were further categorized into two groups based on the optimal cut-off value of the HbA1c/HDL-C ratio in the ROC curve analysis (Table 2). Notably, patients with an elevated HbA1c/HDL-C ratio (≥ 4.99) exhibited a higher prevalence of CAC compared to those with lower ratios [146/486 (30.04) vs. 200/1122 (17.83), p < 0.001]. Significant differences in gender, BMI, SBP, DBP, hypertension, DM, smoking, creatinine, TC, TG, HDL-C, glucose, and HbA1c were also observed between the two groups (all p < 0.05).
Correlation between the HbA1c/HDL-C ratio and clinical variables
The correlations between the HbA1c/HDL-C ratio and various clinical variables are shown in Table 3. The HbA1c/HDL-C ratio was correlated positively with BMI (r = 0.378, p < 0.001), SBP (r = 0.114, p < 0.001), DBP (r = 0.119, p < 0.001), hypertension (r = 0.089, p < 0.001), DM (r = 0.304, p < 0.001), smoking (r = 0.155, p < 0.001), creatinine (r = 0.165, p < 0.001), TG (r = 0.427, p < 0.001), glucose (r = 0.266, p < 0.001), while negatively with TC (r = − 0.204, p < 0.001).
The association of the HbA1c/HDL-C ratio with CAC
LASSO regression was used to screen for potential influencing factors of CAC. The optimal λ value of 0.036 was determined through tenfold cross-validation, resulting in the selection of seven variables associated with CAC: age, gender, BMI, hypertension, DM, glucose, and HbA1c/HDL-C ratio (Fig. 2). Univariate and multivariate logistic regression analyses were performed based on the above variables (Table 4). In univariate logistic regression analysis, HbA1c/HDL-C ratio, age, male, BMI, hypertension, DM, and glucose were significantly associated with CAC (all p < 0.05). The multivariate logistic regression analysis further found that the HbA1c/HDL-C ratio [odds ratio (OR), 1.135; 95% confidence interval (CI), 1.008–1.279; p = 0.037], age (OR, 1.056; 95% CI 1.038–1.075; p < 0.001), male (OR, 1.992; 95% CI 1.338–2.762; p < 0.001), BMI (OR, 1.067; 95% CI 1.013–1.124; p = 0.014), hypertension (OR, 1.849; 95% CI 1.419–2.411; p < 0.001), and DM (OR, 2.298; 95% CI 1.601–3.300; p < 0.001) were independent effect factors for CAC.
The LASSO regression illustrates the effect of regularization on predictor coefficients via a coefficient path plot (a), identifies the optimal penalty strength through cross-validation (b), and ranks clinical variables by importance based on absolute regression coefficients (c). LASSO, Least Absolute Shrinkage and Selection Operator; DM, diabetes mellitus; HbA1c, glycated hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; BMI, body mass index.
Subgroup and interaction analyses were performed to delve deeper into the association between the HbA1c/HDL-C ratio and CAC (Table 5). The results of subgroup analyses based on age, BMI, hypertension, or DM were in line with the findings of the overall group. Notably, the association between HbA1c/HDL-C ratio and CAC was stronger in females than males (p for interaction = 0.011).
The identification ability of the HbA1c/HDL-C ratio for CAC
ROC curve analysis demonstrated that the HbA1c/HDL-C ratio was a good predictor for CAC. The AUC was 0.630 (95% CI 0.596–0.663). The accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the HbA1c/HDL-C ratio were 68.0%, 74.3%, 45.5%, 83.1%, and 32.9%, respectively, when the optimum cut-off value was 4.99 (Fig. 3a and Table 6). Additionally, the AUC of CAC predicted by the HbA1c/HDL-C ratio was higher than HbA1c (AUC, 0.630 vs. 0.587, p = 0.036) and HDL-C (AUC, 0.630 vs. 0.591, p < 0.001) alone (Fig. 3b,c and Table 6).
ROC curves of the HbA1c/HDL-C ratio (a), HbA1c (b), HDL-C (c), and baseline model + HbA1c/HDL-C (d) on identifying CAC. AUC, the area under the curve; CI, confidence interval; ROC, receiver operating characteristic; HbA1c, glycated hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; CAC, coronary artery calcification.
Nomogram for CAC identification based on the HbA1c/HDL-C ratio
According to the results of the multivariate logistic regression analysis, a nomogram was developed for CAC identification. The baseline model, excluding the HbA1c/HDL-C ratio, yielded an AUC of 0.700 (95% CI 0.668–0.732). Incorporation of the HbA1c/HDL-C ratio into the baseline model had an incremental effect on the predictive value for CAC (AUC, 0.718 vs. 0.700, p = 0.009) (Fig. 3d and Table 6), indicating an enhanced discriminative capacity of the model. The resulting nomogram is depicted in Fig. 4a. Among the included variables, age, BMI, and the HbA1c/HDL-C ratio were the primary contributors in the model, while gender, hypertension, and DM had comparatively smaller contributions. The higher the total score, the greater the probability of CAC.
Internal validation was conducted using the bootstrap resampling method to evaluate the performance and clinical utility of the nomogram. The C-index was calculated to be 0.717, indicating the model’s good discrimination ability. The calibration curve is closely distributed along the ideal diagonal line (Fig. 4b), suggesting excellent agreement between predicted and actual outcomes. The Hosmer–Lemeshow test yielded a non-significant result (χ2 = 4.567, p = 0.803), further supporting the model’s calibration accuracy. DCA reflects that the model provided a net clinical benefit across a wide range of threshold probabilities (Fig. 4c), indicating substantial clinical utility for predicting CAC among asymptomatic adults without CVD.
Discussion
This study mainly demonstrated the potential association between the HbA1c/HDL-C ratio and CAC and its utility in CAC identification. To the best of our knowledge, this is the first study to evaluate the association of the HbA1c/HDL-C ratio with CAC in asymptomatic adults without CVD.
Coronary atherosclerosis has been recognized as a leading cause of CVD, and CAC serves as a highly reliable and robust biomarker for coronary atherosclerosis, closely associated with traditional CVD effect factors32,33. Hyperglycemia is considered a well-established contributor to CAC34. Elevated blood glucose level increases oxidative stress through enhanced glucose oxidation, while the resulting free radicals contribute to CAC development by triggering vascular inflammation and endothelial dysfunction35. In vitro studies have confirmed that hyperglycemic environments induce calcification in vascular smooth muscle cells, while anti-hyperglycemic agents can significantly attenuate this process36. Clinically, suboptimal glycemic control is strongly associated with accelerated CAC progression in patients with DM, even in the absence of overt coronary artery disease or symptoms. Conversely, tighter glycemic control has been shown to reduce the progression of CAC in asymptomatic patients with DM34,37. HbA1c represents a glycated form of hemoglobin, and its synthesis rate is proportional to glucose concentrations in the environment surrounding red blood cells, thereby directly reflecting the level of blood glucose metabolism in the body25. A recent study has identified a correlation between serum calcification propensity and HbA1c in patients with type 2 DM38. Furthermore, elevated HbA1c in individuals with pre-DM was independently correlated with increased CACS12. HDL-C, known as “good cholesterol”, plays a protective role in atherosclerosis by removing cholesterol from macrophages within the coronary arterial walls and transporting it to hepatocytes, slowing the progression and calcification of atherosclerotic lesions13. A recent study has found that lower HDL-C levels are associated with the presence and progression of CAC, whereas higher HDL-C levels confer protection against CAC. However, a few studies have failed to confirm this relationship14.
The interplay between glucose and lipid metabolism in the pathogenesis of vascular calcification is mediated through several key mechanisms. Under hyperglycemic conditions, glucose and lipoproteins undergo non-enzymatic glycation to form advanced glycation end products (AGEs). These AGEs bind to endothelial cell receptors, activating intracellular signaling cascades that induce oxidative stress and chronic inflammation. This process exerts dual detrimental effects: on the one hand, it causes endothelial dysfunction by increasing vascular permeability and calcium deposition; on the other hand, it induces osteogenic differentiation of vascular smooth muscle cells, directly driving calcification progression39,40,41,42. Hyperglycemia also perturbs lipid metabolism, often resulting in elevated LDL-C and reduced HDL-C levels. LDL-C accumulation within the vascular wall contributes to calcification, while diminished HDL-C compromises its atheroprotective and anti-inflammatory properties. HDL-C reduction further elevates plasma glucose levels by suppressing insulin secretion and inhibiting AMPK signaling in skeletal muscle19,24. Furthermore, hyperglycemia is frequently accompanied by insulin resistance, which not only sustains elevated glucose levels but also exacerbates dyslipidemia. This combined metabolic disturbance amplifies pro-inflammatory signaling, thereby intensifying vascular inflammation and calcification43,44. Despite the crucial role that hyperglycemia and dyslipidemia play in the development of CAC, clinical observations revealed that CAC is still prevalent in individuals with normal HbA1c or HDL-C levels14, which suggests that relying solely on a single blood glucose or lipid indicator may be insufficient for accurately reflecting the condition. To enhance the precision of CAC identification, we have been exploring additional biomarkers for diagnostic support. The HbA1c/HDL-C ratio, a novel composite marker integrating glucose and lipid metabolism, has been found to be positively associated with the prevalence of MAFLD. Compared to the single indicators of HbA1c and HDL-C, the HbA1c/HDL-C ratio has shown significantly greater accuracy in screening for MAFLD26. Additionally, the HbA1c/HDL-C ratio has been linked to carotid atherosclerosis and plaque formation in patients with and without DM, and it may serve as a valuable tool for monitoring high-risk patients in the primary prevention of atherosclerosis24. Our study demonstrated that the HbA1c/HDL-C ratio was significantly elevated in CAC subjects and was independently associated with CAC, highlighting its essential role and value in vascular disease. Subgroup analyses demonstrated consistent associations between the HbA1c/HDL-C ratio levels and CAC across most stratified factors, reinforcing the robustness and generalizability of the present findings and underscoring their clinical relevance to broader populations. Notably, gender-based disparities were observed in this association. These differences may be partially attributed to variations in hormonal profiles, metabolic pathways, and metabolic rates between sexes45, which may influence the determination of HbA1c/HDL-C levels. ROC curve analysis found that the HbA1c/HDL-C ratio outperformed HbA1c or HDL-C alone in identifying CAC. Although the AUC was moderate, this finding is consistent with the multifactorial nature of CAC pathogenesis. Beyond glycemic and lipid dysregulation, CAC is also driven by inflammation, oxidative stress, and genetic predisposition16, which cannot be fully captured by this ratio alone. Nonetheless, from a clinical perspective, the HbA1c/HDL-C ratio offers a pragmatic and integrative assessment of glucose-lipid metabolic status. A high ratio suggests concomitant hyperglycemia and dyslipidemia, reflecting impaired metabolic homeostasis24 and an elevated probability of vascular calcification. Conversely, a low ratio signifies more stable metabolic control, which can effectively maintain vascular health. As such, it is a convenient and practical clinical reference for physicians to initially screen patients with CAC and guide further diagnostic evaluations. Compared to traditional lipid-related indices such as the AIP index, LDL-C/HDL-C ratio, and TG/HDL-C ratio, the HbA1c/HDL-C ratio uniquely incorporates glycemic assessment in evaluating CAC. In contrast to the TyG index, HbA1c provides a more stable measure of long-term glycemic exposure23. Most importantly, we also found that the HbA1c/HDL-C ratio exhibits a higher AUC in identifying CAC than lipid-derived and glycemic-lipid composite indices such as the TyG index (Supplementary Fig. 1). Furthermore, incorporating the HbA1c/HDL-C ratio into the baseline model for predicting CAC had an incremental effect, indicating that the HbA1c/HDL-C ratio may offer a more reliable basis for the early detection and assessment of CAC.
This study utilized LASSO regression to identify seven candidate effectors associated with CAC. These variables were subsequently incorporated into a multivariate logistic regression model, and a nomogram was developed integrating age, sex, BMI, hypertension, DM, and the HbA1c/HDL-C ratio. All included variables were validated as independent correlates of CAC in alignment with findings from prior studies22,46,47. The model demonstrated good discriminative ability according to the C-index. Calibration analysis further validated the model’s reliability, showing a strong concordance between predicted probabilities and actual outcomes. DCA confirmed the clinically meaningful utility of the nomogram in identifying CAC among asymptomatic adults without CVD, providing actionable insights to optimize clinical decision-making and resource allocation in practice. Notably, all variables included in the nomogram are readily accessible through routine clinical assessments, rendering the model a practical and efficient tool for rapid CAC evaluation in clinical practice.
This study has several limitations. First, the relatively small sample size and single-center design may affect the generalizability and robustness of the results. Second, this cross-sectional study cannot establish a causal relationship between the HbA1c/HDL-C ratio and CAC. Third, the study does not include data on medications that treat glucose and lipid abnormalities, which may omit potential confounding factors. Lastly, the absence of external validation for the nomogram restricts the ability to assess its generalizability and clinical utility in broader populations.
Conclusions
In conclusion, the HbA1c/HDL-C ratio is closely associated with CAC and is an independent factor for CAC in asymptomatic adults without CVD. These relationships deserve further research.
Data availability
Data can be downloaded from a public database (https://figshare.com)29.
Abbreviations
- DM:
-
Diabetes mellitus
- CKD:
-
Chronic kidney disease
- CVD:
-
Cardiovascular disease
- CAC:
-
Coronary artery calcification
- CACS:
-
CAC score
- CT:
-
Computed tomography
- HbA1c:
-
Glycated hemoglobin A1c
- HDL-C:
-
High-density lipoprotein cholesterol
- AIP:
-
Atherogenic index of plasma
- LDL-C:
-
Low-density lipoprotein cholesterol
- TG:
-
Triglyceride
- TyG:
-
Triglyceride-glucose
- MAFLD:
-
Metabolic-associated fatty liver disease
- GWAS:
-
Genome-wide association study
- SNPs:
-
Single-nucleotide polymorphisms
- BMI:
-
Body mass index
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- eGFR:
-
Estimated glomerular filtration rate
- TC:
-
Total cholesterol
- ROC:
-
Receiver operating characteristic
- LASSO:
-
Least absolute shrinkage and selection operator
- AUC:
-
Area under the curve
- C-index:
-
Consistency index
- DCA:
-
Decision curve analysis
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- PPV:
-
Positive predictive value
- NPV:
-
Negative predictive value
- AGEs:
-
Advanced glycation end products
References
Giachelli, C. M. The emerging role of phosphate in vascular calcification. Kidney Int. 75(9), 890–897 (2009).
Gui, Z., Shao, C., Zhan, Y., Wang, Z. & Li, L. Vascular calcification: High incidence sites, distribution, and detection. Cardiovasc. Pathol. 72, 107667 (2024).
Savo, M. T. et al. Comparative prognostic value of coronary calcium score and perivascular fat attenuation index in coronary artery disease. J. Clin. Med. 13(17), 5205 (2024).
Taylor, A. J. et al. Coronary calcium independently predicts incidents of premature coronary heart disease over measured cardiovascular risk factors: Mean three-year outcomes in the Prospective Army Coronary Calcium (PACC) project. J. Am. Coll. Cardiol. 46(5), 807–814 (2005).
Nakanishi, R. et al. All-cause mortality by age and gender based on coronary artery calcium scores. Eur. Heart J. Cardiovasc. Imaging. 17(11), 1305–1314 (2016).
Wetscherek, M. T. A. et al. Incidental coronary artery calcification on non-gated CT thorax correlates with risk of cardiovascular events and death. Eur. Radiol. 33(7), 4723–4733 (2023).
LaMonte, M. J. et al. Coronary artery calcium score and coronary heart disease events in a large cohort of asymptomatic men and women. Am. J. Epidemiol. 162(5), 421–429 (2005).
Greenland, P., LaBree, L., Azen, S. P., Doherty, T. M. & Detrano, R. C. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA 291(2), 210–215 (2004).
Su, Y. et al. Emerging biosensor probes for glycated hemoglobin (HbA1c) detection. Mikrochim. Acta. 191(6), 300 (2024).
Flammer, A. J. et al. Patients with an HbA1c in the prediabetic and diabetic range have higher numbers of circulating cells with osteogenic and endothelial progenitor cell markers. J. Clin. Endocrinol. Metab. 97(12), 4761–4768 (2012).
Eastwood, S. V. et al. Ethnic differences in cross-sectional associations between impaired glucose regulation, identified by oral glucose tolerance test or HbA1c values, and cardiovascular disease in a cohort of European and South Asian origin. Diabet. Med. 33(3), 340–347 (2016).
Scicali, R. et al. HbA1c increase is associated with higher coronary and peripheral atherosclerotic burden in non diabetic patients. Atherosclerosis 255, 102–108 (2016).
Rye, K. A., Bursill, C. A., Lambert, G., Tabet, F. & Barter, P. J. The metabolism and anti-atherogenic properties of HDL. J. Lipid Res. 50(Suppl), S195-200 (2009).
Abedi, F. et al. HDL-cholesterol concentration and its association with coronary artery calcification: a systematic review and meta-analysis. Lipids Health Dis. 22(1), 60 (2023).
Sharma, C. et al. Association of biomarkers for dyslipidemia, inflammation, and oxidative stress with endothelial dysfunction in obese youths: a case-control study. Diabetes Metab. Syndr. Obes. 17, 2533–2545 (2024).
Onnis, C. et al. Coronary artery calcification: Current concepts and clinical implications. Circulation 149(3), 251–266 (2024).
Yang, C., Liu, Z., Zhang, L. & Gao, J. The association between blood glucose levels and lipids in the general adult population: Results from NHANES (2005–2016). J. Health Popul. Nutr. 43(1), 163 (2024).
Ahmed, B., Sultana, R. & Greene, M. W. Adipose tissue and insulin resistance in obese. Biomed. Pharmacother. 137, 111315 (2021).
Drew, B. G. et al. High-density lipoprotein modulates glucose metabolism in patients with type 2 diabetes mellitus. Circulation 119(15), 2103–2111 (2009).
Hisamatsu, T. et al. Lipoprotein particle profiles compared with standard lipids in association with coronary artery calcification in the general Japanese population. Atherosclerosis 236(2), 237–243 (2014).
Yao, H. et al. Coronary artery calcification burden, atherogenic index of plasma, and risk of adverse cardiovascular events in the general population: evidence from a mediation analysis. Lipids Health Dis. 23(1), 258 (2024).
Won, K. B. et al. Different associations of atherogenic index of plasma, triglyceride glucose index, and hemoglobin A1C levels with the risk of coronary artery calcification progression according to established diabetes. Cardiovasc. Diabetol. 23(1), 418 (2024).
Huang, C. et al. Association between the hemoglobin A1c/high-density lipoprotein cholesterol ratio and stroke incidence: a prospective nationwide cohort study in China. Lipids Health Dis. 24(1), 25 (2025).
Hu, X. et al. Association between the plasma-glycosylated hemoglobin A1c/high-density lipoprotein cholesterol ratio and carotid atherosclerosis: A retrospective study. J. Diabetes Res. 2021, 9238566 (2021).
Wang, S., Pan, X., Zhang, M. & Chen, S. Correlation between glycolipid metabolism levels and diabetic retinopathy in patients with type 2 diabetes mellitus. Diabetes Metab. Syndr. Obes. 17, 1–9 (2024).
He, S. et al. The newly proposed plasma-glycosylated hemoglobin A1c/High-Density lipoprotein cholesterol ratio serves as a simple and practical indicator for screening metabolic associated fatty liver disease: An observational study based on a physical examination population. BMC Gastroenterol. 24(1), 274 (2024).
Wu, J. et al. The HbA1c/HDL-C ratio as a screening indicator of NAFLD in U.S. adults: A cross-sectional NHANES analysis (2017–2020). BMC Gastroenterol. 25(1), 369 (2025).
Choi, S. Y. et al. Genome-wide association study of coronary artery calcification in asymptomatic Korean populations. PLoS ONE 14(3), e0214370 (2019).
CHOI SU-YEON. Clinical characteristics_CACS_GWAS_n = 1688. figshare. Dataset. 2019. https://doi.org/10.6084/m9.figshare.7853588.v1.
Agatston, A. S. et al. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 15(4), 827–832 (1990).
Leipsic, J. et al. SCCT guidelines for the interpretation and reporting of coronary CT angiography: A report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J. Cardiovasc. Comput. Tomogr. 8(5), 342–358 (2014).
Greenland, P., Blaha, M. J., Budoff, M. J., Erbel, R. & Watson, K. E. Coronary calcium score and cardiovascular risk. J. Am. Coll. Cardiol. 72(4), 434–447 (2018).
Jin, H. Y. et al. The relationship between coronary calcification and the natural history of coronary artery disease. JACC Cardiovasc. Imaging. 14(1), 233–242 (2021).
Won, K. B. et al. Impact of optimal glycemic control on the progression of coronary artery calcification in asymptomatic patients with diabetes. Int. J. Cardiol. 266, 250–253 (2018).
Nishikawa, T. et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404(6779), 787–790 (2000).
Shi, L. et al. The key role of liraglutide in preventing autophagy of vascular smooth muscle cells in high glucose conditions. Balkan Med. J. 41(1), 54–63 (2024).
Anand, D. V. et al. Determinants of progression of coronary artery calcification in type 2 diabetes role of glycemic control and inflammatory/vascular calcification markers. J. Am. Coll. Cardiol. 50(23), 2218–2225 (2007).
Mencke, R. et al. Serum calcification propensity is associated with HbA1c in type 2 diabetes mellitus. BMJ Open Diabetes Res. Care. 9(1), e002016 (2021).
Flores-Roco, A., Lago, B. M. & Villa-Bellosta, R. Elevated glucose levels increase vascular calcification risk by disrupting extracellular pyrophosphate metabolism. Cardiovasc. Diabetol. 23(1), 405 (2024).
An, Y. et al. The role of oxidative stress in diabetes mellitus-induced vascular endothelial dysfunction. Cardiovasc. Diabetol. 22(1), 237 (2023).
Yao, H. et al. Epidemiological research advances in vascular calcification in diabetes. J. Diabetes Res. 2021, 4461311 (2021).
Yahagi, K. et al. Pathology of human coronary and carotid artery atherosclerosis and vascular calcification in diabetes mellitus. Arterioscler. Thromb. Vasc. Biol. 37(2), 191–204 (2017).
Nedosugova, L. V. et al. Inflammatory mechanisms of diabetes and its vascular complications. Biomedicines. 10(5), 1168 (2022).
Freeman, A. M., Acevedo, L. A. & Pennings, N. Insulin resistance. In StatPearls. (StatPearls Publishing) Copyright © 2025. (StatPearls Publishing LLC, 2025).
Mancio, J. et al. Gender differences in the association of epicardial adipose tissue and coronary artery calcification: EPICHEART study: EAT and coronary calcification by gender. Int. J. Cardiol. 249, 419–425 (2017).
Makaryus, A. N., Sison, C., Kohansieh, M. & Makaryus, J. N. Implications of gender difference in coronary calcification as assessed by CT coronary angiography. Clin. Med. Insights Cardiol. 8(Suppl 4), 51–55 (2014).
Won, K. B. et al. Sex difference in the risk of coronary artery calcification progression related to hyperuricemia among asymptomatic 12,316 Korean adults. Sci. Rep. 14(1), 28710 (2024).
Huang, J. C. et al. Exploring the relationship between visceral fat and coronary artery calcification risk using metabolic score for visceral fat (METS-VF). Life 14(11), 1399 (2024).
Acknowledgements
We thank the investigators and the participants of the original study for their contributions.
Funding
This study was supported by the National Natural Science Foundation of China (82170433) and the Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX24_0479).
Author information
Authors and Affiliations
Contributions
Chen Chen and Wenkang Zhang contributed to the conception, design, data analysis, and interpretation. Mingkang Li and Gaoliang Yan contributed to the conception and design. Chengchun Tang contributed to the conception, and provided administrative support. All authors wrote and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants followed the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, C., Li, M., Yan, G. et al. Data-driven analysis of the relationship between the HbA1c/HDL-C ratio and coronary artery calcification: a cross-sectional study. Sci Rep 15, 31865 (2025). https://doi.org/10.1038/s41598-025-07469-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-07469-6