Abstract
Stable isotope analysis (SIA) is a powerful tool for assessing resource use and trophic structure, enhancing understanding of coexistence mechanisms among sympatric species. In this study, we analyzed carbon (δ¹³C) and nitrogen (δ¹⁵N) stable isotopes in bone collagen of three coexisting anuran species—Bombina orientalis, Rana uenoi, and R. huanrenensis—from South Korean forest to evaluate their isotopic niches, resource partitioning, and within-population variation. Rana uenoi and R. huanrenensis utilized a broad range of resources with high overlap, suggesting they likely share dietary habits and occupy similar trophic roles. In contrast, relative δ¹⁵N enrichment of B. orientalis indicates a distinct trophic niche, although its resource utilization was not entirely segregated from that of the two Rana species. Furthermore, large groups of both Rana species tended to exploit a wider variety of resources than smaller ones, whereas B. orientalis showed no isotopic sex differences. All three species displayed ontogenetic niche shifts, with B. orientalis increasingly relying on aquatic-origin resources, while the two Rana species shifting toward terrestrial-origin resources. These findings provide insight into the trophic dynamics of forest-dwelling anurans and may serve as a baseline for ecological and conservation research in the future.
Similar content being viewed by others
Introduction
Ecological niche refers to the unique role and position of a species within an ecosystem, conceptualized as an n-dimensional hypervolume that encompasses the species’ habitat, food, and interactions with the environment1,2,3,4,5,6. According to the competitive exclusion principle, it is impossible for two distinct species to have complete overlap in their ecological niche, and they coexist by partitioning resources or habitats7,8. In other words, niche differentiation is a key mechanism that enables species to coexist3,9,10,11,12, and interspecific interactions contribute to the stability and biodiversity of ecosystems13,14,15.
Biological activities are a crucial part of ecosystem cycling16,17, and stable isotope analysis (SIA) has been extensively applied in related studies18. Stable isotopes allow for the quantitative estimation of trophic positions by analyzing the relationships between organisms and their food, which can be described as an ‘isotopic niche’19. In addition, because turnover rates vary across tissues due to metabolic processes20,21,22, SIA provides the flexibility to select biological materials that reflect specific time periods, thereby facilitating the interpretation of ecological information at the desired temporal scale. For example, carbon stable isotope ratios (δ13C) vary depending on the photosynthetic metabolism in plants23, with C3 plants ranging from -35 to -22‰ and C4 plants from -17 to -11‰. These variations create distinct isotopic signatures across habitat types, serving as ‘ecological tracers’ to distinguish environmental origins. Furthermore, terrestrial environments typically exhibit higher δ13C values than freshwater ecosystems, which helps identify the habitat types utilized by organisms24,25,26,27. The smaller isotopic fractionation between consumers and their dietary sources also enables tracing the origins of consumed resources and assessing feeding frequency18,27,28,29,30,31. Meanwhile, nitrogen stable isotope ratios (δ15N) tend to increase by approximately 3–4‰ with each trophic level, providing a means to estimate the ‘bionomic axis’32,33,34,35. Overall, SIA offers valuable insight into the trophic roles of organisms36,37 and has been widely used in studies of resource partitioning and ecological interactions, including niche differentiation, overlap, and potential competition38,39,40.
Anurans thrive in forests with high biomass and contribute to ecosystem stability by occupying both terrestrial and aquatic habitats throughout their unique life cycles41,42,43. During the larval stage, they consume algae, detritus, and suspended particles44,45,46, while as adults, they prey on various insects and terrestrial invertebrates, thus linking multiple trophic levels within the food web18,47,48. By occupying various trophic positions across life stages, anurans play a key role in the cycling of matter and energy within ecosystems49,50,51. Furthermore, given the co-occurrence of many anuran species, identifying the trophic levels and ecological niches through resource partitioning aids in understanding community structure and ecosystem functions, ultimately supporting the establishment of effective conservation and management strategies52,53,54,55,56. However, studies using SIA to investigate the trophic positions and resource use of anurans have been limited in South Korea.
This study focused on three sympatric anuran species in South Korean forests: the Oriental fire-bellied toad (Bombina orientalis), the Ueno’s brown frog (formerly known as the Korean brown frog; Rana uenoi), and the Huanren brown frog (R. huanrenensis). These species inhabit a wide range of microhabitats, from lowlands to high-altitude mountain streams, and are representative of the domestic forest ecosystem57,58,59,60. Notably, R. uenoi and R. huanrenensis, which are genetically related and morphologically similar, differ markedly in their reproductive ecology, including breeding site selection and reproductive behaviors57,61,62. In contrast, B. orientalis, which breeds during the summer, exhibits distinct ecological characteristics and is expected to occupy a different trophic niche from the two Rana species59. Although populations are generally considered as an ecologically homogeneous unit, they consist of individuals with diverse ecological strategies, and variation in resource use among individuals is a key factor in determining the niche breadth and structure at the population level6,38,63,64,65. For example, body size directly influences the range and selection of resources38,49,66,67,68,69,70,71, and sex is closely associated with reproductive behavior and energy requirements; in species exhibiting sexual size dimorphism (SSD), these differences may be further pronounced, potentially leading to sex-based differences in foraging strategies72,73,74,75,76,77,78. In this study, we analyzed the δ13C and δ15N values of toe bone collagen, which reflects biological activity over the past several months to years20,79,80, to test the following four hypotheses. First, the two Rana species, which share similar ecological traits, are expected to exhibit a high degree of isotopic niche overlap, whereas B. orientalis is expected to occupy a distinct niche. Second, the breadth of resource use and trophic levels in the two Rana species are expected to vary with individual body size. Third, B. orientalis, which lacks SSD, will show no sex-based differences in isotopic niche. Fourth, all three species are expected to exhibit ontogenetic niche shifts as they grow.
Results
Comparison of stable isotope values among three Anuran species
For δ¹³C, the values (mean ± SD) were -23.86 ± 0.41‰ for B. orientalis, -24.18 ± 0.55‰ for R. uenoi, and -24.00 ± 0.54‰ for R. huanrenensis (Kruskal–Wallis test: χ² = 10.332, p = 0.006; Table 1), with a significant difference observed between B. orientalis and R. uenoi (Dunn’s test: p = 0.002). For δ¹⁵N, the values were 3.16 ± 0.44‰ for B. orientalis, 2.13 ± 0.95‰ for R. uenoi, and 2.41 ± 0.82‰ for R. huanrenensis (Kruskal–Wallis test: χ² = 20.984, p < 0.001), with no significant difference between the two Rana species (Dunn’s test: p = 0.444).
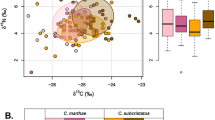
R. huanrenensis exhibited a broader isotopic niche (total hull area: TA = 3.24, standard ellipse area: SEA = 1.03, corrected standard ellipse area: SEAc = 1.08) compared to R. uenoi (TA = 2.96, SEA = 0.99, SEAc = 1.03) and B. orientalis (TA = 2.33, SEA = 0.58, SEAc = 0.60) (Table 1; Fig. 1).
The isotopic niche overlap between R. uenoi and R. huanrenensis was 88.19–89.48%, while that between B. orientalis and the two Rana species was 43.7 – 54.60%. B. orientalis overlapped 78.96 – 85.35% with the two Rana species (Fig. 2).
(a) Isotopic niches of B. orientalis (deep blue circle), R. uenoi (deep red circle), and R. huanrenensis (deep green circle), shown with 95% standard ellipse areas (SEA; solid lines) and total hull areas (TA; dashed gray lines) and (b) Boxplots of the Bayesian SEA (black point, ‰2) for three anuran species with 50%, 75%, and 95% credible intervals from inner to outer, and red cross representing sample size-corrected SEA (SEAc).
NicheROVER plots of three anuran species. (a) Ten random elliptical plots representing the trophic niche region (NR), one-dimensional density plots, and two-dimensional scatter plots of raw data for each pair of isotopes, (b) the posterior distribution of the probabilistic niche overlap metric (%) for a specified NR of 95%.
Comparison of stable isotope values between size-based groups in two Rana species
For R. uenoi, the large SVL group had δ¹³C and δ¹⁵N of -23.68 ± 0.57‰ and 3.04 ± 0.88‰, respectively, whereas the small SVL group showed -24.49 ± 0.19‰ and 1.55 ± 0.38‰ (Mann–Whitney U test, δ¹³C: U = 13.000, p < 0.001; δ¹⁵N: U = 12.500, p < 0.001; Table 1). For R. huanrenensis, the large SVL group had δ¹³C and δ¹⁵N of -23.65 ± 0.60‰ and 3.16 ± 0.59‰, respectively, whereas the small SVL group showed -24.27 ± 0.28‰ and 1.82 ± 0.34‰ (Mann–Whitney U test, δ¹³C: U = 28.500, p = 0.009; δ¹⁵N: U = 4.000, p < 0.001).
The large SVL group of R. uenoi (TA = 2.24, SEA = 1.30, SEAc = 1.46) exhibited a broader isotopic niche than the small SVL group (TA = 0.50, SEA = 0.23, SEAc = 0.24) (Table 1; Fig. 3). Similarly, the large SVL group of R. huanrenensis (TA = 1.71, SEA = 0.78, SEAc = 0.87) exhibited a broader niche than the small SVL group (TA = 0.69, SEA = 0.31, SEAc = 0.34).
In R. uenoi, the small SVL group overlapped 76.60% within the isotopic niche of the large SVL group, whereas the reverse overlap was 11.08% (Fig. 4). In R. huanrenensis, the small SVL group overlapped 39.59% within the isotopic niche of the large SVL group, while the reverse overlap was 12.24%. When comparing the large SVL groups of the two Rana species, the niche overlap was 69.57% for R. uenoi within the isotopic niche of R. huanrenensis, while the reverse overlap was 90.63%. Among the small SVL groups, R. uenoi overlapped 75.91% within the isotopic niche of R. huanrenensis, with a reverse overlap of 65.20%.
(a) Isotopic niches of large and small snout-vent length (SVL) groups of Rana uenoi (medium and light red circles) and R. huanrenensis (medium and light green circles), shown with 95% standard ellipse areas (SEA; solid lines) and total hull areas (TA; dashed gray lines) and (b) boxplots of the Bayesian SEA (black point, ‰2) of two Rana species, with 50%, 75%, and 95% credible intervals from inner to outer, and the red cross representing sample size-corrected SEA (SEAc).
NicheROVER plots of large and small snout-vent length (SVL) groups of two Rana species. (a) Ten random elliptical plots representing the trophic niche region (NR), one-dimensional density plots, and two-dimensional scatter plots of raw data for each pair of isotopes, (b) the posterior distribution of the probabilistic niche overlap metric (%) for a specified NR of 95%.
Comparison of stable isotope values between sex-based groups in B. orientalis
For B. orientalis, female had δ¹³C and δ¹⁵N of -23.88 ± 0.32‰ and 3.06 ± 0.36‰, respectively, whereas male showed -23.83 ± 0.47‰ and 3.24 ± 0.49‰ (Mann–Whitney U test, δ¹³C: U = 154.000, p = 0.824; δ¹⁵N: U = 117.500, p = 0.168; Table 1).
Male (TA = 2.28, SEA = 0.76, SEAc = 0.81) exhibited a broader isotopic niche than female (TA = 1.00, SEA = 0.38, SEAc = 0.40) (Table 1; Fig. 5). Furthermore, female overlapped 96.82% within the isotopic niche of male, with a reverse overlap of 67.89% (Fig. 6).
(a) Isotopic niches of male and female Bombina orientalis (medium blue and light blue circles), shown with 95% standard ellipse areas (SEA; solid lines) and total hull areas (TA; dashed gray lines) and (b) boxplots of the Bayesian SEA (black point, ‰2) of B. orientalis with 50%, 75%, and 95% credible intervals from inner to outer and the red cross representing sample size-corrected SEA (SEAc).
NicheROVER plots of male and female Bombina orientalis. (a) Ten random elliptical plots representing the trophic niche region (NR), one-dimensional density plots and two-dimensional scatter plots of raw data for each pair of isotopes, (b) the posterior distribution of the probabilistic niche overlap metric (%) for a specified NR of 95%.
Ontogenetic niche shifts of three Anuran species
All three anuran species exhibited variation in stable isotope values (δ¹³C and δ¹⁵N) associated with increasing SVL (Fig. 7). For δ¹⁵N, B. orientalis (p = 0.004) and the two Rana species (p < 0.001) showed a significant increase with SVL. For δ¹³C, B. orientalis exhibited a significant decrease (p = 0.015), whereas the two Rana species showed a significant increase (p < 0.001).
Discussion
SIA of bone collagen reflects long-term patterns of resource use within a community and can be effectively employed to evaluate the trophic ecology79,80. In this study, we conducted SIA of δ13C and δ15N to compare resource utilization among three anuran species. The result showed that R. uenoi and R. huanrenensis exhibited broader isotopic niches compared to B. orientalis, indicating the use of a variety of resources. However, no significant differences in stable isotope values were observed between the two Rana species, and their niche overlap was also high, ranging from 88.19 to 89.48%. This suggests that R. uenoi and R. huanrenensis likely consume comparable types of prey and occupy overlapping trophic levels, implying that their ecological roles could be similar81. In contrast, B. orientalis showed relatively higher values of δ15N, suggesting that it occupies a different trophic niche82. Nevertheless, its resource use appears to partially overlap with that of the two Rana species. However, SIA has limitations in fully determining the exact trophic positions and feeding habits of species, so future research is needed to verify the actual prey items consumed by these species.
Body size influences prey selection and foraging ability in organisms, leading to differences in resource range and selection strategies depending on the growth stage66,68,71,83. A size-based comparison of R. uenoi and R. huanrenensis showed that the large SVL groups had broader isotope niches than small SVL groups, with significant differences in stable isotope values between the two groups within each species. These results suggest that larger individuals utilize a broader range of food resources from various trophic levels and origins compared to smaller individuals84,85. In contrast, small SVL groups exhibited lower isotopic variation among individuals, indicating a more restricted range of resource use. However, the partial niche overlap between small and large SVL groups suggests that smaller individuals may share some resources with larger ones. These findings imply the presence of size-related intraspecific trophic niche differentiation49,86.
Sexual differences in anurans may influence resource use patterns due to varying reproductive strategies and associated ecological requirements76,77,78,87. However, in B. orientalis, no significant differences in stable isotope values were observed between males and females, and their isotopic niche overlap was high (67.89–96.82%). This suggests that there are no distinct differences in resource use or feeding behavior between the sexes in B. orientalis. Nevertheless, males exhibited a broader isotopic niche than females, which may indicate the exploitation of a wider range of resources.
In many organisms, growth is accompanied by shifts in resource utilization patterns, along with morphological, physiological, and behavioral changes. This phenomenon can be interpreted as an ‘ontogenetic niche shift’, which is closely related to resource competition and increased energy efficiency, and functions as a strategy for individuals to establish an ecological niche and maximize fitness38,67,70,71,88,89,90,91. In this study, ontogenetic niche shifts were observed in all three anuran species. δ15N values significantly increased with SVL across all three species, suggesting that larger individuals are more likely to consume food resources from higher trophic levels30,38,84,92. Meanwhile, δ13C values showed different trends among species. In R. uenoi and R. huanrenensis, δ13C values increased with SVL, indicating a shift in resource use from aquatic to terrestrial origins. This is likely because the two larger Rana individuals migrate to the mountains after breeding in early spring and predominantly inhabit terrestrial environments such as leaf litter. In contrast, smaller individuals, being less mobile, tend to stay near aquatic habitats and actively forage during the summer, thereby relying more on the aquatic-origin resources38,61,71,86. Conversely, in B. orientalis, δ13C values decreased with increasing SVL. This pattern is likely due to larger individuals spending more time near aquatic environments during the breeding season, thereby increasing their opportunities to encounter aquatic-origin resources such as aquatic insects. These species-specific differences reflect variations in ecological traits and microhabitat use, suggesting that the direction and ecological implications of ontogenetic niche shifts may differ among species69,70. However, as stable isotope values alone cannot fully elucidate the ontogenetic shifts in resource use throughout growth, future research should integrate additional data to confirm changes in habitat selection and dietary composition across growth stages.
Conclusion
This study is significant as it applied SIA to the under-explored field of anuran trophic ecology in South Korea, evaluating the resource use and trophic partitioning patterns of three sympatric anuran species—B. orientalis, R. uenoi, and R. huanrenensis—coexisting in forest ecosystems. The results revealed a high degree of isotopic niche overlap between the two genetically related Rana species, suggesting a tendency for ‘niche conservatism’, in which ecological traits are retained from a common ancestor. Furthermore, in both Rana species, larger body size was associated with an expansion of ecological function, whereas B. orientalis showed no significant sex-based differences in isotopic niche, implying that sexual size monomorphism may be associated with limited ecological differentiation between males and females. These findings challenge the traditional view of populations as ecologically homogeneous units and suggest that ecological roles may vary with individual traits. Additionally, ontogenetic niche shifts were observed in all three species, indicating that their ecological roles and positions within the food web change throughout their life stages. However, as this study was conducted only in a specific area over a limited time period, it was constrained in providing a detailed interpretation of the species’ ecological roles and positions. Future research should adopt a more quantitative approach across broader spatial and temporal scales to enable more precise and comprehensive analysis. Our study offers foundational insights into the trophic ecology of forest-dwelling anurans and is expected to contribute significantly to future conservation planning and ecological vulnerability assessments.
Materials and methods
Field survey and sample collection
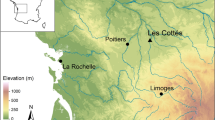
Surveys and capture of three anuran species—the Oriental fire-bellied toad (Bombina orientalis Boulenger, 1980), the Ueno’s brown frog (Rana uenoi Matsui, 2014), and the Huanren brown frog (R. huanrenensis Fei, Ye and Huang, 1991)—were conducted within the Danmoknyeong forest genetic resource conservation area in Mt. Jeombong, Gangwon, South Korea (Figs. 8 and 9), between April and October 2022. Mt. Jeombong, located at the boundary of the southern and northern limits of native plants on the Korean Peninsula, was designated as South Korea’s first UNESCO Biosphere Reserve in 1982, due to its exceptional natural landscape and significant conservation value93. Specimens were collected by walking along streams, wetlands, leaf litter, and grasslands considering their habitat preferences. Using a digital vernier caliper (CD-15APX, Mitutoyo, Japan), we measured the snout-vent length (SVL) and collected specimens only from individuals with an SVL of 30 mm or more. For SIA, the tip of the third longest front toe was clipped using sterilized dissecting scissors94,95,96. The clipped area was disinfected with animal-specific povidone iodide, and the individuals were allowed to rest for a few minutes before being released back into their original habitats. The toe samples were stored in 1.5 ml tubes in an icebox during transport to the laboratory and subsequently kept at -80 °C in ultra-low temperature (ULT) freezers until analysis.
During the study period, 36 individuals of B. orientalis, 26 individuals of R. uenoi, and 25 individuals of R. huanrenensis were collected (Table 1). All individuals of B. orientalis could be distinguished by sex, while both adult and juvenile specimens were collected for two Rana species. For group analysis, B. orientalis was divided into two groups based on sex. Rana uenoi and R. huanrenensis were divided into two groups based on size, using an SVL of approximately 45 mm as a criterion, which is estimated to approximate the mean size of the collected population and as a criterion to distinguish between adults and juveniles97.
Location of the study area at Danmoknyeong in Mt. Jeombong, Gangwon, South Korea. The figure was generated though the QGIS 3.32.3 software (https://qgis.org/).
Species identification
Morphological identification of the R. uenoi and R. huanrenensis species was initially conducted during the period. The species were identified correctly based on criteria such as the size and body shape, eardrum diameter relative to eye size, snout shape, hind leg web development degree, dorsal surface color and tubercles presence, black ventral patches and their color, and vocal sacs presence57,98,99,100,101,102,103. Next, a portion of the morphologically classified individuals within the remaining samples were randomly selected for genetic species identification through genomic DNA analysis. For specimens lacking bone samples after stable isotope analysis, oral epithelium cell DNA was collected for species identification. Oral epithelium cell was obtained by gently swabbing the inside of the individual’s mouth with a sterile swab for approximately 30 s to 1 min after fixing the individual’s mouth with a spatula, and then immediately storing it in a -80 °C ULT freezer for subsequent analysis.
For sample extraction and genomic DNA analysis, the DNeasy Blood and Tissue Kit (Qiagen, CA, USA) was used following the provided protocol. Primers used for species identification targeted regions of Cytb (735 bp) and CO1 (600 bp) (Supplementary Table S1), with two pairs each employed104,105,106,107,108. For polymerase chain reaction (PCR), a total volume of 25 µl per sample was prepared with sterile ddH2O 16.9 µl, 10 X Buffer 2.5 µl, dNTP Mix 2.5 mM 2.5 µl, 10 µM each of forward and reverse primers, 1 µl of template DNA, and Takara Ex Taq™ (5 U/µL) (Takara Bio, Shiga, Japan). PCR conditions for both Cytb and CO1 were set as follows: Initial denaturation at 94 °C for 5 min, followed by denaturation at 94 °C for 30 s, annealing at 50 °C for 45 s, and extension at 72 °C for 1 min. This cycle was repeated 35 times, excluding the initial denaturation, and finalized with a 10 min extension at 72 °C. PCR amplification products were visualized by staining with ethidium bromide (Thermo Fisher Scientific, Seoul, Korea) and analyzed for fragment size on a 2% agarose gel (TAE buffer). Subsequently, the obtained DNA was sequenced by Cosmogenetech (Seoul, Korea). The sequences were aligned using Geneious Prime (2023.2), and both Cytb and CO1 sequences were verified for accurate morphological identification using NCBI’s BLASTn program.
SIA of δ13C and δ15N
The frozen-preserved toe bone samples of anurans underwent a series of procedures: they were rinsed in ultrapure water, and then the skin and other tissues surrounding the bones were removed using a dissecting knife and forceps. The toe bones were then immersed in 0.5 M HCl (Samchun chemicals, Gyeonggi, Korea) for 24 h at 4 °C for demineralization109. Subsequently, they were dried and homogenized for over 48 h at 50 °C using a dry oven (C-DOA3; Changshin Science, Korea). Samples with a C: N ratio less than 4.0 underwent no lipid extraction process, while those with a C:N ratio of 4.0 or higher were excluded from the analysis110. The pre-treated samples were analyzed using a stable isotopes ratio mass spectrometer system with elemental analyzer (Isoprime, Vision-EA, UK) at the Stable Isotope Laboratory of the National Instrumentation Center for Environmental Management (NICEM) at Seoul National University, South Korea.
The stable isotope ratios of the samples were calculated using the following formula30.
Here, δX represents δ13C or δ15N, and Rsample/Rstandard denotes the corresponding ratio of stable carbon or nitrogen isotopes (δ13C/δ12C or δ15N/δ14N) between the sample and the chemical standard material.
Statistical analysis
To compare stable isotope values among three species of anurans, the Stable Isotope Bayesian Ellipses in R (SIBER) was employed111. δ13C and δ15N were designated as x and y axes, respectively, to generate isotopic biplots using 95% confidence interval ellipses, enabling visual comparison of the isotopic niche areas and resource utilization diversity. This package calculated total hull area (TA), standard ellipse area (SEA), and corrected standard ellipse area (SEAc) to determine the breadth of resource use112. Additionally, to ascertain significant differences in δ13C and δ15N values among the three anuran species, a Kruskal–Wallis test was conducted with species as the independent variable and each stable isotope values as the dependent variable. Post-hoc analysis using Dunn’s test was performed for pairwise comparisons. Furthermore, comparisons between groups within the populations of the two Rana species and B. orientalis were verified using Mann–Whitney U test. These statistical methods were chosen after testing for normality (Shapiro–Wilk test) and homogeneity of variances (Levene’s test) within each group.
To assess niche overlap (%) within stable isotope niches among groups, the nicheROVER package in R was utilized113. Bayesian analysis generated 10 random ellipses for stable isotope pairs, and Monte–Carlo simulations were conducted to determine posterior distributions and overlap coefficients within the 95% confidence interval. The simulations were run 10,000 times for high accuracy, and overlap coefficients indicate the probability of one species’ individuals being found within another species’ niche space.
Finally, using the Generalized Linear Model (GLM), trends in stable isotope values corresponding to size-related growth processes were examined to confirm intrapopulation variations in trophic niches.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Hutchinson, G. E. Concluding remarks. Cold spring harbor sympos. Quantit Biol. 22, 415–427 (1957).
Pianka, E. R. The structure of Lizard communities. Ann. Rev. Ecol. Syst. 4, 53–74 (1973).
Polechová, J. & Storch, D. Ecological niche. in Ecological Niche. Vol. 2 (eds Jørgensen, S. E. & Fath, B. D.) 1088–1097 (Elsevier, 2008).
Ricklefs, R. E. Evolutionary diversification, coevolution between populations and their antagonists, and the filling of niche space. Proc. Natl. Acad. Sci. USA 107, 1265–1272 (2010).
Peterson, A. T. Ecological niche conservatism: a time-structured review of evidence. J. Biogeogr. 38, 817–827 (2011).
Ingram, T., Costa-Pereira, R. & Araújo, M. S. The dimensionality of individual niche variation. Ecology 99, 536–549 (2018).
Hardin, G. The competitive exclusion principle: an Idea that took a century to be born has implications in ecology, economics, and genetics. Science 131, 1292–1297 (1960).
Darlington, J. P. Competition, competitive repulsion, and coexistence. Proc. Natl. Acad. Sci. USA. 69, 3151–3155 (1972).
MacArthur, R. H. Population ecology of some warblers of Northeastern coniferous forests. Ecology 39, 599–619 (1958).
Schoener, T. W. Resource partitioning in ecological communities: research on how similar species divide resources helps reveal the natural regulation of species diversity. Science 185, 27–39 (1974).
Wilson, D. S. The adequacy of body size as a niche difference. Am. Nat. 109, 769–784 (1975).
Schoener, T. W. The controversy over interspecific competition: despite spirited criticism, competition continues to occupy a major domain in ecological thought. Am. Sci. 70, 586–595 (1982).
Thompson, J. N. The evolution of species interactions. Science 284, 2116–2118 (1999).
Andresen, E., Arroyo-Rodríguez, V. & Escobar, F. Tropical biodiversity: The importance of biotic interactions for its origin, maintenance, function, and conservation. in Ecological networks in the tropics (eds Dáttilo, W. & Rico-Gray, V.) 1–13 (Springer, 2018).
Luna, P., Corro, E. J., Antoniazzi, R. & Dáttilo, W. Measuring and linking the missing part of biodiversity and ecosystem function: the diversity of biotic interactions. Diversity 12, 86. https://doi.org/10.3390/d12030086 (2020).
Seale, D. B. Influence of amphibian larvae on primary production, nutrient flux, and competition in a pond ecosystem. Ecology 61, 1531–1550 (1980).
Whiles, M. R. et al. The effects of amphibian population declines on the structure and function of Neotropical stream ecosystems. Front. Ecol. Environ. 4, 27–34 (2006).
Peterson, B. J. & Fry, B. Stable isotopes in ecosystem studies. Ann. Rev. Ecol. Syst. 18, 293–320 (1987).
Bearhop, S., Adams, C. E., Waldron, S., Fuller, R. A. & MacLeod, H. Determining trophic niche width: a novel approach using stable isotope analysis. J. Anim. Ecol. 73, 1007–1012 (2004).
Tieszen, L. L., Boutton, T. W., Tesdahl, K. G. & Slade, N. A. Fractionation and turnover of stable carbon isotopes in animal tissues: implications for δ13C analysis of diet. Oecologia 57, 32–37 (1983).
Codron, D. et al. The confounding effects of source isotopic heterogeneity on consumer-diet and tissue-tissue stable isotope relationships. Oecologia 169, 939–953 (2012).
Vander Zanden, M. J., Clayton, M. K., Moody, E. K., Solomon, C. T. & Weidel, B. C. Stable isotope turnover and Half-Life in animal tissues: A literature synthesis. PloS One. 10, e0116182 (2015).
Smith, B. N. & Epstein, S. Two categories of 13C/12C ratios for higher plants. Plant. Physiol. 47, 380–384 (1971).
Clementz, M. T. & Koch, P. L. Differentiating aquatic mammal habitat and foraging ecology with stable isotopes in tooth enamel. Oecologia 129, 461–472 (2001).
Clementz, M. T., Holroyd, P. A. & Koch, P. L. Identifying aquatic habits of herbivorous mammals through stable isotope analysis. Palaios 23, 574–585 (2008).
Bennett, P. M. & Hobson, K. A. Trophic structure of a boreal forest arthropod community revealed by stable isotope (δ13C, δ15N) analyses. Entomol. Sci. 12, 17–24 (2009).
Bishop, M. R., Drewes, R. C. & Vredenburg, V. T. Food web link ages demonstrate importance of terrestrial prey for the threatened California Red-Legged frog. J. Herpetol. 48, 137–143 (2014).
DeNiro, M. J. & Epstein, S. Influence of diet on the distribution of carbon isotopes in animals. Geochim. Cosmochim. Acta. 42, 495–506 (1978).
Szepanski, M. M., Ben-David, M. & Van Ballenberghe, V. Assessment of anadromous salmon resources in the diet of the Alexander Archipelago Wolf using stable isotope analysis. Oecologia 120, 327–335 (1999).
Post, D. M. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83, 703–718 (2002).
Tiunov, A. V. Stable isotopes of carbon and nitrogen in soil ecological studies. Biol. Bull. 34, 395–407 (2007).
DeNiro, M. J. & Epstein, S. Influence of diet on the distribution of nitrogen isotopes in animals. Geochim. Cosmochim. Acta. 45, 341–351 (1981).
Minagawa, M. & Wada, E. Stepwise enrichment of 15N along food chains: further evidence and the relation between δ15N and animal age. Geochim. Cosmochim. Acta. 48, 1135–1140 (1984).
Cabana, G. & Rasmussen, J. B. Modelling food chain structure and contaminant bioaccumulation using stable nitrogen isotopes. Nature 372, 255–257 (1994).
Vander Zanden, M. J., Casselman, J. M. & Rasmussen, J. B. Stable isotope evidence for the food web consequences of species invasions in lakes. Nature 401, 464–467 (1999).
Jung, J. H. Habitat characteristics of three salamanders (Caudata: Amphibia) in forests and genetic diversity of Karsenia koreana. PhD thesis (Seoul Natl Univ., 2020).
García-Vernet, R., Borrell, A., Víkingsson, G., Halldórsson, S. D. & Aguilar, A. Ecological niche partitioning between Baleen whales inhabiting Icelandic waters. Prog Oceanogr. 199, 102690 (2021).
Cloyed, C. S. & Eason, P. K. Niche partitioning and the role of intraspecific niche variation in structuring a guild of generalist anurans. R Soc. Open. Sci. 4, 170060 (2017).
Marshall, H. H. et al. Stable isotopes are quantitative indicators of trophic niche. Ecol. Lett. 22, 1990–1992 (2019).
Oh, H. J. et al. Trophic ecology of endangered gold-spotted pond frog in ecological wetland park and rice paddy habitats. Animals 11, 967 (2021).
Beard, K. H., Vogt, K. A. & Kulmatiski, A. Top-down effects of a terrestrial frog on forest nutrient dynamics. Oecologia 133, 583–593 (2002).
Hocking, D. J. & Babbitt, K. J. Amphibian contributions to ecosystem services. Herpetol Conserv. Biol. 9, 1–17 (2014).
Huckembeck, S. et al. Feeding ecology and basal food sources that sustain the paradoxal frog Pseudis minuta: a multiple approach combining stomach content, prey availability, and stable isotopes. Hydrobiologia 740, 253–264 (2014).
Altig, R., Whiles, M. R. & Taylor, C. L. What do tadpoles really eat? Assessing the trophic status of an understudied and imperiled group of consumers in freshwater habitats. Freshw. Biol. 52, 386–395 (2007).
Montaña, C. G. et al. Revisiting what do tadpoles really eat? A 10-year perspective. Freshw. Biol. 64, 2269–2282 (2019).
Antoniazzi, C. E. et al. Trophic ecology of tadpoles in floodplain wetlands: combining gut contents, selectivity, and stable isotopes to study feeding segregation of syntopic species. Hydrobiologia 847, 3013–3024 (2020).
Gardner, T. Declining amphibian populations: a global phenomenon in conservation biology. Anim. Biodiv Conserv. 24, 25–44 (2001).
Hopkins, W. A. Amphibians as models for studying environmental change. ILAR J. 48, 270–277 (2007).
Werner, E. E. & Gilliam., J. F. The ontogenetic niche and species interactions in size-structured populations. Ann. Rev. Ecol. Syst. 15, 393–425 (1984).
Lima, A. P. & Moreira, G. Effects of prey size and foraging mode on the ontogenetic change in feeding niche of Colostethus stepheni (Anura: Dendrobatidae). Oecologia 95, 93–102 (1993).
Hirai, T. Ontogenetic change in the diet of the pond frog, Rana nigromaculata. Ecol. Res. 17, 639–644 (2002).
Toft, C. A. Resource partitioning in amphibians and reptiles. Copeia 1–21 (1985).
Duré, M. I., Kehr, A. I. & Schaefer, E. F. Niche overlap and resource partitioning among five sympatric bufonids (Anura, Bufonidae) from Northeastern Argentina. J. Herpetol. 8, 27–39 (2009).
Cajade, R., Schaefer, E. F., Duré, M. I. & Kehr, A. I. Trophic and microhabitat niche overlap in two sympatric dendrobatids from La selva, Costa Rica. Cuad. Herpetol. 24, 81–92 (2010).
Vignoli, L. & Luiselli, L. Dietary relationships among coexisting Anuran amphibians: a worldwide quantitative review. Oecologia 169, 499–509 (2012).
Molina-Burgos, B. E., Valenzuela-Sánchez, A., Alvarado-Rybak, M., Klarian, S. & Soto-Azat, C. Trophic ecology of the endangered darwin’s frog inferred by stable isotopes. Endanger. Species Res. 36, 269–278 (2018).
Song, J. Y. A field guide book of amphibian & reptile in the Korea National Park. National Park Research Institute, Annual report No. 1–77 (2007).
Song, J. Y. & Lee, I. Elevation distribution of Korean amphibians. Korean J. Herpetol. 1, 15–19 (2009). (in Korean with English abstract).
Ko, S. B., Chang, M. H., Song, J. Y., Yang, K. S. & Oh, H. S. Comparison of the stomach contents of Pre- and Post-hibernation Rana dybowskiiin Jeju Island. Korean J. Herpetol. 5, 27–32 (2013). (in Korean with English abstract).
Do, M. S. et al. The study on habitat analysis and ecological niche of Korean brown frogs (Rana dybowskii, R. Coreana and R. huanrensis) using the species distribution model. Korean J. Herpetol. 9, 1–11 (2018). (in Korean with English abstract).
Yoon, I. B., Kim, J. I. & Yang, S. Y. Life history of Rana temporaria dybowskii in Korea. Korean J. Environ. Ecol. 17, 401–405 (1999). (in Korean with English abstract).
Joo, Y. D. Study on Feeding Preference of Order Salientia (Amphibia) in Korea. PhD thesis (Incheon Natl Univ., 2015).
Bolnick, D. I., Yang, L. H., Fordyce, J. A., Davis, J. M. & Svanbäck, R. Measuring individual-level resource specialization. Ecology 83, 2936–2941 (2002).
Bolnick, D. I. et al. The ecology of individuals: incidence and implications of individual specialization. Am. Nat. 161, 1–28 (2003).
Takola, E. & Schielzeth, H. Hutchinson’s ecological niche for individuals. Biol. Philos. 37, 25 (2022).
Loman, J. Food, feeding rates and prey-size selection in juvenile and adult frogs, Rana arvalis nilss. and R. temporaria L. Ekol Pol. 27, 581–601 (1979).
Olson, M. H. Ontogenetic niche shifts in largemouth bass: variability and consequences for first-year growth. Ecology 77, 179–190 (1996).
Kramer, D. L. & Chapman, M. R. Implications of fish home range size and relocation for marine reserve function. Environ. Biol. Fishes. 55, 65–79 (1999).
Scharf, F. S., Juanes, F. & Rountree, R. A. Predator size-prey size relationships of marine fish predators: interspecific variation and effects of ontogeny and body size on trophic-niche breadth. Mar. Ecol. Prog Ser. 208, 229–248 (2000).
Wells, K. D. The Ecology and Behavior of Amphibians (University of Chicago Press, 2007).
Schalk, C. M. & Fitzgerald, L. A. Ontogenetic shifts in ambush-site selection of a sit-and-wait predator, the Chacoan horned frog (Ceratophrys cranwelli). Can. J. Zool. 93, 461–467. https://doi.org/10.1139/cjz-2014-0320 (2015).
Howard, R. D. Sexual dimorphism in bullfrogs. Ecology 62, 303–310 (1981).
Frayer, D. W. & Wolpoff, M. H. Sexual dimorphism. Ann. Rev. Anthropol. 14, 429–473 (1985).
Shine, R. Ecological causes for the evolution of sexual dimorphism: a review of the evidence. Q. Rev. Biol. 64, 419–461 (1989).
Houston, D. & Shine, R. Sexual dimorphism and niche divergence: feeding habits of the Arafura Filesnake. J. Anim. Ecol. 62, 737–748 (1993).
Butler, M. A., Schoener, T. W. & Losos, J. B. The relationship between sexual size dimorphism and habitat use in greater Antillean Anolis lizards. Evolution 54, 259–272 (2000).
Perry, G. & Garland, T. Lizard home ranges revisited: effects of sex, body size, diet, habitat, and phylogeny. Ecology 83, 1870–1885 (2002).
Radu, N. R., Bogdan, H. V., Bata, Z., Popa, C. & Osvat-Szabo, E. G. The trophic spectrum of a Bombina bombina (Linnaeus, 1761) population from the cermei region (Arad county, Romania). Herpetol Roman. 1, 17–21 (2007).
Ambrose, S. H. Preparation and characterization of bone and tooth collagen for isotopic analysis. J. Archaeol. Sci. 17, 431–451 (1990).
Medeiros, L., da Silveira Monteiro, D., Petitet, R. & Bugoni, L. Effects of lipid extraction on the isotopic values of sea turtle bone collagen. Aquat. Biol. 23, 191–199 (2015).
Yoon, I. B., Lee, S. J. & Yang, S. Y. The study on food habits and life history of Hyla japonica and Bombina orientalis (Amphibia; Salientia). Korean J. Environ. Ecol. 14, 81–94 (1996). (in Korean with English abstract).
Plaza, J. L., Metillo, E. B. & Sanguila, M. B. Trophic ecology of syntopic anurans of tropical stream communities. J. Trop. Ecol. 37, 109–117 (2021).
Werner, T. K. & Sherry, T. W. Behavioral feeding specialization in Pinaroloxias inornata, the darwin’s Finch of Cocos island, Costa Rica. Proc. Natl. Acad. Sci. USA. 84, 5506–5510 (1987).
Kupfer, A., Langel, R., Scheu, S., Himstedt, W. & Maraun, M. Trophic ecology of a tropical aquatic and terrestrial food web: insights from stable isotopes (15N). J. Trop. Ecol. 22, 469–476 (2006).
del Martínez, C., Wolf, N., Carleton, S. A. & Gannes, L. Z. Isotopic ecology ten years after a call for more laboratory experiments. Biol. Rev. 84, 91–111 (2009).
Perkins, M. W., Cloyed, C. S. & Eason, P. K. Intraspecific dietary variation in niche partitioning within a community of ecologically similar snakes. Evol. Ecol. 34, 1017–1035 (2020).
Magalhães, R. F., Garda, A. A., Marques, N. C. S. & Brandao, R. A. Sexual dimorphism and resource utilisation by the Veadeiros waterfall frog Bokermannohyla pseudopseudis (Anura: Hylidae). Salamandra 52, 171–177 (2016).
Hammerschlag-Peyer, C. M., Yeager, L. A., Araujo, M. S. & Layman, C. A. A hypothesis-testing framework for studies investigating ontogenetic niche shifts using stable isotope ratios. PloS One 6, e27104 (2011).
Nakazawa, T. Ontogenetic niche shifts matter in community ecology: a review and future perspectives. Popul. Ecol. 57, 347–354 (2015).
Glos, J., Wolter, J., Struck, U. & Rödel, M. O. Ecological shifts during larval development in two West African savanna frogs. J. Trop. Ecol. 33, 50–59 (2017).
Reshetnikov, A. N. et al. Trophic positions and niche segregation of two Anuran species in the ecosystem of a forest lake. Hydrobiologia 848, 4801–4814 (2021).
Yang, S. N., Zhang, C. L., Liao, W. B., Li, N. & Hu, J. H. Trophic niche shifts in mountain Feirana frogs under human-mediated habitat transformations. Asian Herpetol Res. 12, 234–241 (2021).
Lee, K. S. & Cho, D. S. Relationships between the Spatial distribution of vegetation and microenvironment in a temperate hardwood forest in mt. Jumbong biosphere reserve area, Korea. Korean J. Environ. Ecol. 23, 241–253 (2000). (in Korean with English abstract).
Leyse, K. E., Lind, A. J., Savage, W. K., Shaffer, H. B. & Stephens, M. R. Tissue collection protocol for genetic research. Univ. Calif. Davis 1–9 (2003).
Phillott, A. D. et al. Toe-clipping as an acceptable method of identifying individual anurans in mark recapture studies. Herpetol Rev. 38, 305–308 (2007).
Perry, G., Wallace, M. C., Perry, D., Curzer, H. & Muhlberger, P. Toe clipping of amphibians and reptiles: science, ethics, and the law1. J. Herpetol. 45, 547–555 (2011).
Ko, Y. M. Study of Life-cycle of Rana dyvowskii in Jeju Island. PhD thesis (Jeju Natl Univ., 2012).
Yang, S. Y. et al. First record of a brown frog Rana huanrenensis (Family Ranidae) from Korea. Korean J. Biol. Sci. 4, 45–50 (2000).
Kim, J. B., Min, M. S., Yang, S. Y. & Matsui, M. Genetic relationships among Korean brown frog species (Anura, Ranidae), with special reference to evolutionary divergences between two allied species Rana dybowskii and R. huanrenensis. Zool. Sci. 19, 369–382 (2002).
Song, J. Y., Chang, M. H. & Chung, K. H. Morphological comparison of four brown frogs (Amphibia; Ranidae). Korean J. Environ. Ecol. 23, 157–162 (2005). (in Korean with English abstract).
Seo, M. J. Physical Characteristics of Breeding Huanren Brown Frogs (Rana huanrenensis), and the Age Structure of their Population in the Research Forest of Kangwon National University. Master’s thesis (Kangwon Natl Univ., 2011).
NIBR (National Institute of Biological Resources). Red Data Book of Republic of Korea. Vol. 2, Amphibians and Reptiles (pp. 110–117) (NIBR, 2019).
Othman, S. N. et al. Evaluating the efficiency of popular species identification analytical methods, and integrative workflow using morphometry and barcoding bioinformatics for taxonomy and origin of traded cryptic brown frogs. Glob Ecol. Conserv. 38, e02253 (2022).
Tanaka-Ueno, T., Matsui, M., Wu, G. F., Fei, L. & Takenaka, O. Identity of Rana chensinensis from other brown frogs as assessed by mitochondrial cytochrome b sequences. Copeia 1999, 187–190 (1999).
Stuart, B. L. & Parham, J. F. Molecular phylogeny of the critically endangered Indochinese box turtle (Cuora galbinifrons). Mol. Phylogenet Evol. 31, 164–177 (2004).
Che, J. et al. Universal COI primers for DNA barcoding amphibians. Mol. Ecol. Resour. 12, 247–258 (2012).
Zhou, W. W. et al. Speciation in the Rana chensinensis species complex and its relationship to the uplift of the Qinghai-Tibetan plateau. Mol. Ecol. 21, 960–973 (2012).
Yang, B. T. et al. Diversity and phylogeography of Northeast Asian brown frogs allied to Rana dybowskii (Anura, Ranidae). Mol. Phylogenet Evol. 112, 148–157 (2017).
Conrad, C. et al. Ancient DNA analysis and stable isotope ecology of sea turtles (Cheloniidae) from the gold rush-era (1850s) Eastern Pacific ocean. Open. Quat. 4, 1–13 (2018).
Post, D. M. et al. Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 152, 179–189 (2007).
Jackson, A. L., Inger, R., Parnell, A. C. & Bearhop, S. Comparing isotopic niche widths among and within communities: SIBER–Stable isotope bayesian ellipses in R. J. Anim. Ecol. 80, 595–602 (2011).
Monadjem, A. et al. Morphology and stable isotope analysis demonstrate different structuring of bat communities in rainforest and savannah habitats. R Soc. Open. Sci. 5, 180849 (2018).
Swanson, H. K. et al. A new probabilistic method for quantifying n-dimensional ecological niches and niche overlap. Ecology 96, 318–324 (2015).
Acknowledgements
This research was funded by the National Institute of Ecology, Ministry of Environment, Republic of Korea (grant number NIE–B–2024–02).
Author information
Authors and Affiliations
Contributions
S. Y. L. and C. Y. C. conceived the ideas and designed the methodology for study. S. Y. L. conducted the field surveys and collected experimental data. S. Y. L., J. Y. S, K. S. K, J. B. S. and C. Y. C. analyzed the data together. S. Y. L. wrote the first draft of the manuscript. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical statement
The study conducted under the permission for capturing and collecting wildlife from Inje–gun office (2022–05) and was approved by Institutional Animal Care and Use Committee (IACUC) of Seoul National University (SNU–220520–2–1). The study design followed the ethical and methodological principles of the ARRIVE 2.0 guidelines.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lee, S., Song, J., Koo, K.S. et al. Trophic niche partitioning among three sympatric anuran species in a South Korean forest ecosystem based on stable isotope analysis. Sci Rep 15, 25358 (2025). https://doi.org/10.1038/s41598-025-07856-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-07856-z