Abstract
5,10-methylenetetrahydrofolate reductase (MTHFR) deficiency is the most common folate metabolism disorder. MTHFR is a key enzyme in the folate cycle that catalyzes the conversion of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate (5-methyl THF). A definitive diagnosis of MTHFR deficiency relies on enzymatic studies of fibroblasts and/or molecular genetic analyses. The accurate measurement of the MTHFR enzyme activity is crucial for diagnosing and predicting disease severity, which is traditionally performed using high-performance liquid chromatography with fluorescence detection. We developed a novel method for assessing the MTHFR enzymatic activity using liquid chromatography-tandem mass spectrometry (LC-MS/MS). The MTHFR enzymatic reaction was conducted in fibroblasts, and the product, 5-methyl THF, was quantified by LC-MS/MS. The MTHFR activity was evaluated in 13 control individuals and five individuals with MTHFR deficiency. The 5-methyl THF concentration was successfully measured in all cases, and the validation trial demonstrated adequate accuracy and precision. Control fibroblasts exhibited an MTHFR activity ranging from 240.1 to 624.0 pmol/min/mg protein (mean = 338.5 pmol/min/mg), while MTHFR-deficient fibroblasts showed a markedly lower activity (2.99–51.3 pmol/min/mg protein). Although our study is associated with some limitations, we present a sensitive and reliable LC-MS/MS based assay for diagnosing MTHFR deficiency.
Similar content being viewed by others
Introduction
5,10-methylenetetrahydrofolate reductase (MTHFR) deficiency, caused by mutations in the MTHFR gene, is the most common disorder of the folate metabolism. Folates are water-soluble compounds essential for normal tissue growth and development. They act as a cofactor in various one-carbon-unit transfer reactions involved in the biosynthesis of purines, pyrimidines, serine, and methionine as well as in the degradation of histidine in vivo1. Once taken up by cells, folates are retained intracellularly through poly-γ-glutamylation and reduced to tetrahydrofolate (THF) using NADPH. THF transforms into various intermediates during the folate cycle, facilitating specific metabolic pathways2. The folate cycle also plays a role in oxidative stress defense and NADPH production, which are critical metabolic pathways for cell proliferation3.
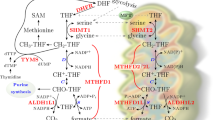
MTHFR is a cytosolic enzyme in the folate cycle that catalyzes NADPH-mediated conversion of 5,10-methylenetetrahydrofolate (5,10-methylene THF) to 5-methyltetrahydrofolate (5-methyl THF) (Fig. 1). This 5-methyl THF serves as a methyl donor in the methionine synthase-catalyzed reaction that converts homocysteine to methionine1. In the methionine cycle, methionine is further processed to form S-adenosylmethionine (SAM), a crucial methyl group donor for intracellular methylation reactions including DNA and RNA methylation, creatine synthesis, and myelin formation4,5. Thus, the remethylation of homocysteine is vital for maintaining the organism’s methylation capacity and reducing homocysteine levels.
Folate metabolic pathway; connection between the folate cycle and methionine cycle. MTHFR catalyzes the reduction of 5,10-methylene THF to the active form of folate, 5-methyl THF, using NADPH as a cofactor. In MTHFR deficiency, 5-methyl THF levels are reduced, leading to decreased methionine and SAM levels along with homocysteine accumulation. DHF, Dihydrofolate; THF, Tetrahydrofolate; 5,10-methylene THF, 5,10-methylenetetrahydrofolate; MTHFR, 5,10-methylenetetrahydrofolate reductase; 5-methyl THF, 5-methyltetrahydrofolate; FAD, flavin adenine dinucleotide; SAM, S-adenosyl-methionine; SAH, S-adenosyl-homocysteine; BHMT, Betaine-Homocysteine Methyltransferase; DMG, Dimethylglycine; CBS, Cystathionine β-synthase; CTH, Cystathionine γ-lyase.
In MTHFR deficiency, SAM is depleted because of the impaired methionine synthase-mediated remethylation of homocysteine, which is caused by decreased 5-methyl THF production. This depletion affects the overall methylation capacity of the organism6. In addition, elevated homocysteine levels can damage vascular endothelial cells and they are associated with cognitive dysfunction and vascular injury7.
The clinical manifestations of MTHFR deficiency vary and include cerebellar symptoms, central nervous system (CNS) disorders, and peripheral neuropathy. Neonatal and early infantile-onset cases often present with severe and diverse CNS symptoms, such as poor oral intake, impaired consciousness, apnea, muscle hypotonia, and hydrocephalus. During later infancy, symptoms, such as epileptic seizures, intellectual disability, and microcephaly, become apparent. Older children and adults may present with peripheral neuropathy, cerebellar ataxia, spasticity, behavioral abnormalities, and psychiatric symptoms. Magnetic resonance imaging frequently shows diffuse T2 hyperintense signals in the cerebral white matter, particularly in older pediatric and adult individuals8,9,10,11,12,13.
The severity of symptoms and age of onset correlate with residual enzyme activity, and early-onset cases, such as neonatal presentations, can be fatal without treatment1,6,14. Early intervention with betaine has been shown to prevent a functional decline, including neurological and cognitive impairments, in individuals with MTHFR deficiency15. Therefore, identifying affected individuals and initiating treatment before irreversible neurological damage occurs is critical.
A definitive diagnosis of MTHFR deficiency relies on enzymatic studies of fibroblasts and/or molecular genetic analyses. Genetic analyses, including sequencing of all MTHFR exons, exon-intron boundaries, and promoter regions, have identified over 100 pathogenic mutations16. Most mutations are private, and synonymous mutations can also cause diseases. In addition, the large number of single-nucleotide polymorphisms (SNPs) in MTHFR complicates the performance of mutational analyses. The pathogenicity and functional impact of missense variants have been clarified in a recent study17. Thus, enzyme assays are essential for characterizing the functional consequences of novel variants identified through molecular genetic techniques, such as next-generation sequencing7.
The enzymatic reaction catalyzed by MTHFR is irreversible in vivo. Although they can be measured in the reverse direction under in vitro conditions18, reverse assays have limitations, including incomplete recovery due to organic solvent extraction and uncertain specificity. These challenges make it difficult to accurately measure the enzyme activity, particularly in samples with low residual activity. Subsequently, a forward assay technique using fibroblasts was developed to overcome these limitations and is now the standard method7,19,20. This assay requires NADPH as an electron donor and flavin adenine dinucleotide (FAD) as a coenzyme1,21,22. In the forward assay method, the reaction product 5-methyl THF was measured using high-performance liquid chromatography with fluorescence detection (HPLC-FLD)19; however, no studies have previously used liquid chromatography-tandem mass spectrometry (LC-MS/MS).
LC-MS/MS is well regarded for its higher accuracy and lower experimental error than HPLC-FLD, and it enables the measurement of trace amounts of analytes. In the context of newborn screening, flow injection analysis tandem mass spectrometry (FIA-MS/MS) is typically used for the first-tier analysis of primary markers, whereas LC-MS/MS is employed in second-tier testing to reduce false positives and confirm abnormalities. Moreover, LC-MS/MS has recently been employed for diagnostic screening of conditions such as Fabry disease by assessing enzyme activity in dried blood spots23,24. Although measuring folate cycle metabolites in biochemical matrices via LC-MS/MS is challenging owing to their low chemical stability and high interconvertibility, recent methods have been developed to address these issues2,25.
Building on previous forward assay methods, we herein report the establishment of a method for measuring enzyme activity in fibroblasts using LC-MS/MS, which could pave the way for future diagnostic screening using dried blood spots.
Results
Validating the measurement of 5-methyl THF with LC-MS/MS
The LC-MS/MS method for measuring 5-methyl THF was validated using quality control (QC) specimens. The mean 5-methyl THF values, coefficients of variation (CV%), and relative errors (RE%) were analyzed for each sample (Table 1). The accuracy and precision of the limit of quantification (LOQ) QC samples (0.1 ng/mL) were within ± 20% for both neat diluent and blank samples. Similarly, other QC samples (ranging from 0.25 to 25 ng/mL) demonstrated an accuracy and precision within ± 15%. Calibration curves (ranging from 0.1 to 50.0 ng/mL) were linear with a mean coefficient of regression (R2) of 0.9998 (n = 15). No unexpected fragment ion was identified in the mass spectrum, indicating no degradation or interference from the other folate metabolites.
Confirmation of the MTHFR assay method in fibroblasts
The MTHFR forward assay method used in this study was fully validated in a previous study19. In our study, the substrate amount, protein amount, and reaction time were determined using control fibroblasts.
Dependence on reaction times and protein amount
The relationship between the reaction time and 5-methyl THF production, as well as between the enzyme extract protein amount and reaction velocity, is shown in Figs. 2 and 3. The assay curve was almost linear for up to 40 min in the reaction time experiment and for up to 60–80 µg in the protein amount experiment. Based on these results, a reaction time of 20 min and a protein concentration of 10–70 µg were selected, consistent with a previous report.
Kinetics of 5-methyl THF production over reaction time. Changes in 5-methyl-THF production were observed over various reaction times (2.5 to 80 min). The substrate (5,10-methylene THF) concentration was maintained at 100 µmol/L, whereas a fixed amount of enzyme extract protein ranging from 10 to 70 µg was used. 5-methyl THF, 5-methyltetrahydrofolate; 5,10-methylene THF, 5,10-methylenetetrahydrofolate.
Enzyme assay velocity as a function of enzyme extract protein amount. The enzymatic assay velocity (pmol/min) as a function of the enzyme extract protein content varied from 2 to 200 µg. The reaction time was fixed at 20 min, with a substrate (5,10-methylene THF) at a concentration of 100 µmol/L. V, enzymatic assay velocity; 5,10-methylene THF, 5,10-methylenetetrahydrofolate.
Dependence on 5,10-methylene THF concentrations
The Michaelis-Menten kinetics curve is shown in Fig. 4. The enzymatic reaction rate (enzyme activity) was examined at substrate concentrations up to 400 µmol/L. The Michaelis-Menten constant (Km) was calculated as 41.8 µmol/L (95% confidence interval [CI]: 29.5–58.1). Based on this result, 100 µmol/L of 5,10-methylene THF was selected for the MTHFR assay, similar to a previous report.
Michaelis-Menten kinetics curve. MTHFR activity as a function of substrate (5,10-methylene THF) concentration. MTHFR activity was analyzed at substrate concentrations ranging from 5 to 400 µmol/L. The reaction time was fixed at 20 min, and the enzyme extract protein amounts were fixed at 10–70 µg. 5,10-methylene THF, 5,10-methylenetetrahydrofolate.
MTHFR activities in fibroblasts of control individuals
MTHFR assays were conducted on 13 control individuals. Each assay used 10–30 µL of enzyme extract, containing 31.6–62.2 µg of protein, with an assay time of 20 min. The assay products were diluted 100-fold or 1000-fold and analyzed using LC-MS/MS. Dilution rates were selected to maintain concentrations within the calibration curve. The concentration of the reaction product (5-methyl THF) was successfully measured in all cases (Fig. 5a). The amount of 5-methyl THF produced ranged from 80.1 to 201.1 ng (mean = 154.8, standard deviation [SD] = 38.8). MTHFR activity ranged from 240.1 to 624.0 pmol/min/mg protein (mean = 338.5, SD = 96.5; Table 2). All samples were analyzed in duplicate using LC-MS/MS, and the mean values were subsequently used for data interpretation (Supplementary Table S1).
LC-MS/MS chromatographic separation of 5-methyl THF. (a) A mass chromatogram in a control fibroblast containing 20.8 ng/mL 5-methyl THF. (b) A mass chromatogram in a MTHFR-deficient fibroblast (Case 3) containing 0.38 ng/mL 5-methyl THF. The x-axis represents time (min), and the y-axis represents the ion counts in the MRM channel normalized to the maximum signal (100%).
MTHFR activities in fibroblasts of MTHFR-deficient individuals
Five individuals with MTHFR deficiency underwent MTHFR assays. Each assay used 10–30 µL of enzyme extract, containing 41.6–62.4 µg of protein, with an assay time of 20 min. The assay products were diluted 100-fold and analyzed using LC-MS/MS. Dilution rates were selected to maintain the concentrations within the calibration curve. The concentration of the reaction product (5-methyl THF) was successfully measured in all the cases (Fig. 5b). The amount of 5-methyl THF produced ranged from 1.14 to 29.4 ng. MTHFR activity ranged from 2.99 to 46.6 pmol/min/mg protein (Table 2). All samples were analyzed in duplicate using LC–MS/MS, and the mean values were used for data interpretation (Supplementary Table S1). Our measurements revealed a significant reduction in enzyme activity, which is consistent with previous results obtained using HPLC-FLD.
MTHFR assays without FAD were also performed to confirm the dependency of the residual enzyme activity on FAD. In control fibroblasts, the activity ranged from 213.9 to 575.4 pmol/min/mg protein (mean = 333.2, SD = 85.4) (Table 2). The ratio of MTHFR activity with FAD to without FAD ranged from 0.89 to 1.15 (mean = 1.00) in control fibroblasts and from 0.94 to 1.31 (mean = 1.10) in MTHFR-deficient fibroblasts.
Discussion
Diagnosing MTHFR deficiency can be challenging because the clinical manifestations and imaging findings are often nonspecific. Suspicion typically arises when blood amino acid analysis reveals elevated homocysteine and reduced methionine levels. Enzyme activity measurements are crucial for the definitive diagnosis, disease severity assessment, and pathogenicity testing of genetic polymorphisms.
Various cell types (e.g., skin fibroblasts, lymphocytes, lymphoblasts, and hepatocytes) have been used to measure MTHFR activity. However, discrepancies in enzyme activity values across sample types raise concerns regarding interpretation20,26,27. In 2002, Suormala et al. introduced a stable method for measuring enzyme activity using fibroblasts in a forward assay with HPLC-FLD, which is now widely used to compare residual enzyme activity19. Our study employed and validated this forward assay method to determine the amount of substrate, protein, and reaction time. These results are consistent with prior findings, and we adopted the same forward assay method.
The accuracy and reliability of the LC-MS/MS validation methods are listed in this study (Table 1). The mass transitions (m/z) of the product ions were consistent with those in previous reports2. Suormala et al. reported MTHFR activity in control fibroblasts using HPLC-FLD, ranging from 242 to 910 pmol/min/mg protein (mean = 431, SD = 150), whereas Burda et al. reported values of 230–884 pmol/min/mg protein (mean = 421, SD = 140)19,28. Although our control sample size was small, our results showed a similar mean enzyme activity. The residual activity in MTHFR-deficient individuals was distinctly reduced, consistent with the results of previous studies (Table 2). The LC-MS/MS method demonstrated less variation in the control fibroblast activity than the HPLC method; however, further validation with a larger sample size is necessary.
MTHFR catalyzes the irreversible reduction of 5,10-methylene THF to 5-methyl THF, which requires FAD as a cofactor and NADPH as an electron donor22. Previous studies have noted a two- to three-fold difference in enzyme activity in reverse assays, depending on the presence of FAD. No such differences were observed in our forward assay, which is consistent with previous findings19.
MTHFR polymorphisms are associated with various diseases (e.g., cardiovascular diseases, neurodevelopmental and psychiatric conditions, diabetes mellitus, and cancer)29,30,31. Polymorphisms such as p.Ala222Val (NM_005957.5: c.665 C > T) and p.Glu429Ala (NM_005957.5: c.1286 A > C) have been reported to reduce enzymatic activity32,33. However, the standardization of measurement methods and specimens remains a challenge. Suormala et al. did not observe any reduced MTHFR activity for these polymorphisms19. Our precise residual enzyme activity measurements may provide new insights into the effects of genetic polymorphisms on various diseases.
The limitations associated with this study include the small sample size and the lack of a direct comparison between HPLC-FLD and LC-MS/MS methods. Therefore, future studies with larger sample sizes are warranted.
We herein report a novel LC-MS/MS assay for diagnosing MTHFR deficiency. This method demonstrates high accuracy and reliability. While acknowledging the limitations of this study, this approach has the potential to improve residual enzyme activity measurements compared with previous methods. In addition, it may support the development of assays for dried blood spots, facilitating newborn screening and further investigation into genetic polymorphisms and disease associations.
Materials and methods
Fibroblast cultures
Skin fibroblasts were obtained from five individuals with MTHFR deficiency and 13 control individuals. The experimental protocol was approved by the Ethics Committee of Jichi Medical University, Tochigi, Japan. Written informed consent was obtained from all the participants or their parents. All experiments were performed in accordance with the relevant guidelines and regulations. Individuals with MTHFR deficiency were previously diagnosed and their MTHFR activities were measured using the physiological forward assay described by Suormala et al., with modifications as described by Rummel et al. and Burda et al.19,28,34. The MTHFR pathogenic polymorphisms of these individuals are detailed in Table 210,11,14. Individuals with folate metabolism disorder were excluded from the control group.
Fibroblasts were cultured in Dulbecco’s Modified Eagle’s medium (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific) and 1% penicillin/streptomycin (Thermo Fisher Scientific). The cultures were grown in 6- or 10-cm disposable cell culture dishes (IWAKI, Shizuoka, Japan) and maintained at 37 °C in an atmosphere of 5% CO2 and 95% air. Fibroblasts were harvested using 0.25% trypsin (Thermo Fisher Scientific) upon reaching confluency. The cells were then washed with isotonic phosphate-buffered saline (Thermo Fisher Scientific) and stored at − 80 °C.
Enzyme extracts
Fibroblast cell pellets were suspended in 50–100 µL of ice-cold lysis buffer consisting of 1.5 g/L Lubrol (Tokyo Chemical Industry, Tokyo, Japan) and 0.01 mol/L potassium phosphate buffer (pH 6.6). The suspensions were left to stand on ice for 30 min. The cell suspensions were then centrifuged at 16,000 × g at 4 °C for 10 min and the supernatants were collected as enzyme extracts. The protein concentrations of the enzyme extracts were measured using a BCA protein assay kit (Takara Bio, Shiga, Japan).
Enzyme assays
The enzyme assay solutions were prepared as described in the forward assay method of the previous report19. The standard assay mixture consisted of 0.05 mol/L potassium phosphate buffer (pH 6.6), 100 µmol/L 5,10-methylenetetrahydrofolate (Schircks Laboratories, Bauma, Switzerland), 200 µmol/L NADPH (Merck KGaA, Darmstadt, Germany), 5 µmol/L FAD (Merck KGaA), and enzyme extract containing 10–70 µg of protein, for a final volume of 100 µL. The samples were incubated for 20 min at 37 °C. The assay was terminated by adding 50 µL stop solution (50 mL/L HClO4 in 10 g/L ascorbic acid). Blank samples were also prepared to assess the influence of 5-methyl-THF, originating from a minor impurity in the substrate 5,10-methylenetetrahydrofolate, with the enzyme extract added after the stop solution. The samples were stored at − 20 °C if an LC-MS/MS analysis was not performed immediately.
The reaction time, amount of protein, and amount of substrate were validated to determine the optimal conditions for our method. The amount of 5-methyl THF produced was examined at various reaction times up to 80 min, and the reaction velocity was analyzed with enzyme extract protein amounts up to 200 µg. The Michaelis constant (Km) for the substrate 5,10-methylene THF was determined by varying its concentration between 5 and 400 µmol/L using the GraphPad Prism software program, ver. 10 (GraphPad Software, Boston, MA, USA).
LC-MS/MS analyses
-
5-methyl THF standards and internal standards.
The 5-methyl THF standard was obtained from Cayman (Ann Arbor, MI, USA). A 5-methyl THF standard solution was prepared by dissolving the powder in dimethyl sulfoxide (DMSO) at 1 mg/mL and then diluting it to a final concentration of 100 µg/mL. The diluted 5-methyl THF standard solution was stored at − 80 °C. The 5-methyl THF internal standard, 5-methyltetrahydrofolate acid-(glutamic acid-13C5) −99 atom % 13C, was purchased from Merck KGaA. The internal standard stocks were prepared by dissolving the powder in DMSO at a concentration of 1 mg/mL and diluting it to a final concentration of 100 µg/mL. The diluted internal standard solution was stored at − 80 °C.
-
LC-MS/MS instrumentation.
The reaction product 5-methyl THF was analyzed using an LC-MS/MS system consisting of a Nexera X2 series ultra-high-performance liquid chromatograph (UHPLC) coupled to an LCMS-8060 triple quadrupole mass spectrometer (Shimadzu, Kyoto, Japan).
The LC-MS/MS parameters are listed in Table 3. Chromatographic separation was achieved using a Kinetex C18 LC column (2.6 μm, 100 × 2.1 mm) (Phenomenex, Torrance, CA, USA). Gradient elution was performed at a flow rate of 0.3 mL/min, using 0.1% formic acid in water as mobile phase A and acetonitrile as mobile phase B. Elution started with 95% mobile phase A for 0.33 min, followed by a gradual increase in mobile phase B to 95% over 2.70 min. The mobile phase was returned to its initial composition over the next 2.30 min, resulting in a total analysis duration of 5 min per injection. An injection volume of 2 µL was used for the sample extract. The column oven and autosampler temperatures were set at 40 °C and 5 °C, respectively. Positive electrospray ionization was used for detection. Multiple reaction monitoring (MRM) was performed for the analysis. Nitrogen gas (purity > 97%) was used as the heating and drying gas, and argon (purity > 99.99%) was used as the collision-induced dissociation gas. The protonated molecular ions were selected as precursor ions. Instrument optimization was performed to set the voltage values and select the m/z of the product ions, as described in a previous study2. Each sample was diluted 100-fold or 1000-fold with 5 g/L ascorbic acid, and the 5-methyl-THF internal standard was added to a final concentration of 100 ng/mL.
The LabSolutions LCMS software program ver. 5.118 (Shimadzu, Kyoto, Japan) was used for the system control and data acquisition. The analytes were quantified using the peak area ratio (analyte/internal standard) and least-squares linear regression with a 1/x weighting function.
Validation of methods
The feasibility of using LC-MS/MS to measure 5-methyl THF was validated by analyzing QC specimens using Bioanalytical Method Validation guidelines35,36. QC samples were prepared to confirm the precision and accuracy of the LC-MS/MS. These samples were prepared by spiking 5-methyl THF standard solutions into a neat diluent (neat 5 g/L ascorbic acid solution) and a blank sample at four concentration levels: high QC sample (25 ng/mL), middle QC sample (2.5 ng/mL), low QC sample (0.25 ng/mL), and limit of quantification (LOQ) QC sample (0.1 ng/mL). Intra- and inter-day assay variability was assessed by analyzing each QC sample five times per day for three days.
The accuracy of the method was expressed as the percentage relative error (RE%), calculated as follows:
RE% = (mean measured concentration − nominal concentration)/nominal concentration × 100.
The precision of the method was evaluated by calculating the percentage coefficient of variation (CV%).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Rosenblatt, D. S. & Fenton, W. A. Inherited disorders of folate and cobalamin transport and metabolism in The Metabolic and Molecular Bases of Inherited Disease (ed Scriver, C. R.) 3897–3907 (McGraw-Hill, (2001).
Nandania, J., Kokkonen, M., Euro, L. & Velagapudi, V. Simultaneous measurement of folate cycle intermediates in different biological matrices using liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 1092, 168–178. https://doi.org/10.1016/j.jchromb.2018.06.008 (2018).
Fan, J. et al. Quantitative flux analysis reveals folate-dependent NADPH production. Nature 510, 298–302. https://doi.org/10.1038/nature13236 (2014).
Surtees, R., Leonard, J. & Austin, S. Association of demyelination with deficiency of cerebrospinal-fluid S-adenosylmethionine in inborn errors of methyl-transfer pathway. Lancet 338, 1550–1554. 10.1016/0140–6736(91)92373-a (1991).
Hiraoka, M. & Kagawa, Y. Genetic polymorphisms and folate status. Congenit Anom. 57, 142–149. https://doi.org/10.1111/cga.12232 (2017).
Thomas, M. A. & Rosenblatt, D. S. Severe methylenetetrahydrofolate reductase deficiency in MTHFR Polymorphisms and Disease (ed Rozen, R.) 41–53 (Landes Bioscience, (2005).
Huemer, M. et al. Guidelines for diagnosis and management of the cobalamin-related remethylation disorders cblc, cbld, cble, cblf, cblg, CblJ and MTHFR deficiency. J. Inherit. Metab. Dis. 40, 21–48. https://doi.org/10.1007/s10545-016-9991-4 (2017).
Prasad, A. N., Rupar, C. A. & Prasad, C. Methylenetetrahydrofolate reductase (MTHFR) deficiency and infantile epilepsy. Brain Dev. 33, 758–769. https://doi.org/10.1016/j.braindev.2011.05.014 (2011).
Gales, A. et al. Adolescence/adult onset MTHFR deficiency May manifest as isolated and treatable distinct neuro-psychiatric syndromes. Orphanet J. Rare Dis. 13 https://doi.org/10.1186/s13023-018-0767-9 (2018).
Tsuji, M. et al. 5,10-Methylenetetrahydrofolate reductase deficiency with progressive polyneuropathy in an infant. Brain Dev. 33, 521–524. https://doi.org/10.1016/j.braindev.2010.08.013 (2011).
Iida, S. et al. Rapidly progressive psychotic symptoms triggered by infection in a patient with methylenetetrahydrofolate reductase deficiency: a case report. BMC Neurol. 17 https://doi.org/10.1186/s12883-017-0827-0 (2017).
Arai, M. & Osaka, H. Acute leukoencephalopathy possibly induced by phenytoin intoxication in an adult patient with methylenetetrahydrofolate reductase deficiency. Epilepsia 52, e58–61. https://doi.org/10.1111/j.1528-1167.2011.03064.x (2011).
Marelli, C. et al. Clinical and molecular characterization of adult patients with late-onset MTHFR deficiency. J. Inherit. Metab. Dis. 44, 777–786. https://doi.org/10.1002/jimd.12323 (2021).
Tamura, A. et al. Posterior-predominant leukoencephalopathy which was caused by methylenetetrahydrofolate reductase deficiency and successfully treated with folic acid. Rinsho Shinkeigaku. 54, 200–206. https://doi.org/10.5692/clinicalneurol.54.200 (2014).
Diekman, E. F., de Koning, T. J., Verhoeven-Duif, N. M., Rovers, M. M. & van Hasselt, P. M. Survival and psychomotor development with early betaine treatment in patients with severe methylenetetrahydrofolate reductase deficiency. JAMA Neurol. 71, 188–194. https://doi.org/10.1001/jamaneurol.2013.4915 (2014).
Froese, D. S. et al. Mutation update and review of severe methylenetetrahydrofolate reductase deficiency. Hum. Mutat. 37, 427–438. https://doi.org/10.1002/humu.22970 (2016).
Weile, J. et al. Shifting landscapes of human MTHFR missense-variant effects. Am. J. Hum. Genet. 108, 1283–1300. https://doi.org/10.1016/j.ajhg.2021.05.009 (2021).
Rosenblatt, D. S. & Erbe, R. W. Methylenetetrahydrofolate reductase in cultured human cells. I. Growtha and metabolic studies. Pediatr. Res. 11, 1137–1141. https://doi.org/10.1203/00006450-197711000-00004 (1977).
Suormala, T., Gamse, G. & Fowler, B. 5,10-Methylenetetrahydrofolate reductase (MTHFR) assay in the forward direction: residual activity in MTHFR deficiency. Clin. Chem. 48, 835–843. https://doi.org/10.1093/clinchem/48.6.835 (2002).
Matthews, R. G. & Baugh, C. M. Interactions of pig liver methylenetetrahydrofolate reductase with methylenetetrahydropteroylpolyglutamate substrates and with dihydropteroylpolyglutamate inhibitors. Biochemistry. 19, 2040–; (2045). https://doi.org/10.1021/bi00551a005 (1980).
Savojardo, C., Babbi, G., Baldazzi, D., Martelli, P. L. & Casadio, R. A glance into MTHFR deficiency at a molecular level. Int. J. Mol. Sci. 23, 167. https://doi.org/10.3390/ijms23010167 (2021).
Froese, D. S. et al. Structural basis for the regulation of human 5,10-methylenetetrahydrofolate reductase by phosphorylation and S-adenosylmethionine Inhibition. Nat. Commun. 9, 2261. https://doi.org/10.1038/s41467-018-04735-2 (2018).
Zhang, X. K. et al. Multiplex enzyme assay screening of dried blood spots for lysosomal storage disorders by using tandem mass spectrometry. Clin. Chem. 54, 1725–1728. https://doi.org/10.1373/clinchem.2008.104711 (2008).
Delarosa-Rodriguez, R. et al. Accuracy diagnosis improvement of Fabry disease from dried blood spots: enzyme activity, lyso-Gb3 accumulation and GLA gene sequencing. Clin. Genet. 99, 761–771. https://doi.org/10.1111/cge.13936 (2021).
Schittmayer, M., Birner-Gruenberger, R. & Zamboni, N. Quantification of cellular folate species by LC-MS after stabilization by derivatization. Anal. Chem. 90, 7349–7356. https://doi.org/10.1021/acs.analchem.8b00650 (2018).
Huang, L., Zhang, J., Hayakawa, T. & Tsuge, H. Assays of methylenetetrahydrofolate reductase and methionine synthase activities by monitoring 5-methyltetrahydrofolate and tetrahydrofolate using high-performance liquid chromatography with fluorescence detection. Anal. Biochem. 299, 253–259. https://doi.org/10.1006/abio.2001.5421 (2001).
Tonetti, C. et al. Relations between molecular and biological abnormalities in 11 families from siblings affected with methylenetetrahydrofolate reductase deficiency. Eur. J. Pediatr. 162, 466–475. https://doi.org/10.1046/j.1365-2141.2002.03876.x (2003).
Burda, P. et al. Insights into severe 5,10-methylenetetrahydrofolate reductase deficiency: molecular genetic and enzymatic characterization of 76 patients. Hum Mutat. 36, 611–621. https://doi.org/10.1002/humu.22779 (2015).
Dolgin, E. The most popular genes in the human genome. Nature 551, 427–431. https://doi.org/10.1038/d41586-017-07291-9 (2017).
Liew, S. C. & Gupta, E. D. Methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism: epidemiology, metabolism and the associated diseases. Eur. J. Med. Genet. 58, 1–10. https://doi.org/10.1016/j.ejmg.2014.10.004 (2015).
Levin, B. L. & Varga, E. M. T. H. F. R. Addressing genetic counseling dilemmas using Evidence-Based literature. J. Genet. Couns. 25, 901–911. https://doi.org/10.1007/s10897-016-9956-7 (2016).
van der Put, N. M. et al. A second common mutation in the methylenetetrahydrofolate reductase gene: an additional risk factor for neural-tube defects? Am. J. Hum. Genet. 62, 1044–1051. https://doi.org/10.1086/301825 (1998).
Hanson, N. Q., Aras, O., Yang, F. & Tsai, M. Y. C677T and A1298C polymorphisms of the methylenetetrahydrofolate reductase gene: incidence and effect of combined genotypes on plasma fasting and post-methionine load homocysteine in vascular disease. Clin. Chem. 47, 661–666. https://doi.org/10.1093/clinchem/47.4.661 (2001).
Rummel, T. et al. Intermediate hyperhomocysteinaemia and compound heterozygosity for the common variant c.677C > T and a MTHFR gene mutation. J. Inherit. Metab. Dis. 30, 401. https://doi.org/10.1007/s10545-007-0445-x (2007).
Committee for Medicinal Products for Human Use (CHMP). European Mediciens Agency. Guideline on bioanalytical method validation. (2011). https://www.ema.europa.eu/en/homepage
U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Center for Veterinary Medicine (CVM). Bioanalytical Method Validation Guidance for Industry. (2018). https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioanalytical-method-validation-guidance-industry
Acknowledgements
We thank Dr. Asako Tamura (Mie University Hospital, Mie, Japan), Dr. Masataka Nakamura (Kansai Medical University Hospital, Osaka, Japan), Dr. Megumi Tsuji (Kanagawa Children’s Medical Center, Kanagawa, Japan), and Dr. Satoko Kumada (Tokyo Metropolitan Neurological Hospital, Tokyo, Japan) for their help with the collection of fibroblasts from individuals with MTHFR deficiency.
Funding
This research was supported by a grant from the Project for Health Research on Infants, Children, Adolescents, and Young Adults from the Agency of Medical Research and Development, a grant from the Japan Agency for Medical Research and Development (grant numbers im0210625h0001, 17ek0109270s0301, 21ek0109511h0001, 22ek0109511h0002, and 23ek0109511h0003), and the MEXT Project for the Establishment of Research and Clinical Trial Hubs for Gene Therapy and Rare Diseases to H.O.
Author information
Authors and Affiliations
Contributions
H.O. designed the study. H.O. and E.J. supervised this study. K.S., M.Y., S.A., H.S., and G.T. performed experiments. K.S. drafted the original manuscript. H.O. and E.J. reviewed and edited the manuscript. All authors have reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sunoki, K., Watanabe, M., Aoki, S. et al. Development of a novel liquid chromatography-tandem mass spectrometry based enzymatic assay of 5,10-methylenetetrahydrofolate reductase. Sci Rep 15, 22674 (2025). https://doi.org/10.1038/s41598-025-07926-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-07926-2