Abstract
This study examined the relationship between the neutrophil-to-albumin ratio (NPAR) and both all-cause and cardiovascular mortality in U.S. patients with cardiovascular disease (CVD) and abnormal glucose metabolism, using NHANES data from 1999 to 2018. Restricted cubic spline analysis identified a significant nonlinear association between NPAR and mortality (p < 0.001). Cox regression results showed that patients in the highest NPAR group (T3, ≥ 15.8) had higher risks of all-cause (HR 1.75, 95% CI 1.50–2.04) and cardiovascular mortality (HR 2.03, 95% CI 1.53–2.68) compared to the lowest group (T1, < 13.5), both with p < 0.0001. Kaplan–Meier survival curves confirmed greater mortality in the T3 group. Mediation analysis found that renal function, measured by eGFR, accounted for 14.49% of the effect on all-cause mortality and 13.38% on cardiovascular mortality. Among the 3163 participants, 1342 experienced all-cause deaths and 462 cardiovascular deaths. This study demonstrated a significant correlation of high NPAR and increased mortality in patients with abnormal glucose metabolism and CVD, suggesting that NPAR may represent a reliable predictor of mortality risk in this population, and emphasizing the importance of both inflammation and renal function monitoring.
Similar content being viewed by others
Introduction
With an aging global population, the incidence and mortality rates of cardiovascular disease (CVD) have risen significantly worldwide1. CVD not only significantly affects the quality of life of patients, but also puts enormous economic pressure on the global health system. Numerous studies demonstrate that increased human lifespan has contributed to CVD becoming one of the leading causes of mortality globally, particularly among middle-aged and older individuals2. Recent epidemiological data exhibit a significant increase in both crude and CVD-specific mortality across numerous countries, posing a growing public health challenge1.
Glucose metabolism abnormalities are common among individuals with CVD and are strongly correlated with negative clinical outcomes3,4 . Identifying remaining risk factors is critical for effectively reducing mortality risk, especially for those with diabetes and prediabetes.
Research has demonstrated that traditional inflammatory markers, such as CRP and leukocyte counts, are closely associated with the risk of cardiovascular events5,6. However, NPAR, a novel composite marker incorporating neutrophil and albumin ratios, may offer supplementary information for cardiovascular risk assessment7. Neutrophils contribute to regulating responses to acute injury, autoimmune processes, and chronic inflammation8; whereas, albumin levels typically demonstrate an inverse relationship with oxidative stress and inflammation9. Recent studies indicate that NPAR is strongly correlated with NAFLD10, depression11, and chronic kidney disease12 and it is also significantly correlated with the prevalence of cardiovascular disease (CVD)13. Additionally, emerging evidence suggests that NPAR has increasingly utilized in assessing disease risk and prognosis14.
Novel methodologies that has found its application in clinical trials is mediation analysis15; it allows the breaking down of total effects into direct effects and mediated effects, allowing researchers to assess the effectiveness of the interventions from a broader perspective. In relation to cardiovascular diseases, renal function (e.g., eGFR)16 is considered a highly important prognostic factor and is assumed to act as a mediator in the link between NPAR and cardiovascular disease mortality. While prior work has examined the prognostic value of NPAR in broader diabetic or hypertensive populations17,18, our study is to focus specifically on individuals with coexisting CVD and abnormal glucose metabolism (AGM)—a particularly high-risk subgroup. Moreover, we incorporated mediation analysis to investigate the mechanistic role of eGFR in this relationship, offering novel insight into how inflammation-related renal dysfunction may contribute to mortality.
Therefore, this study aims to evaluate the association between NPAR and both all-cause and cardiovascular mortality in U.S. adults with CVD and AGM, using data from NHANES 1999–2018.
Methods
The design and population of the study
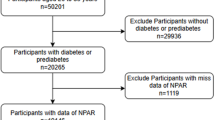
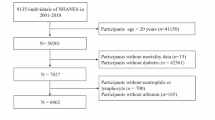
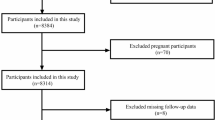
The National Health and Nutrition Examination Survey (NHANES) is a crucial program designed to evaluate the health and nutritional well-being of adults and children in the United States. Overseen by the Centers for Disease Control and Prevention (CDC), NHANES operates under a protocol reviewed and approved by the NCHS Research Ethics Review Board, guaranteeing participant rights through informed consent. Data from this study are available to the public on the official NHANES website (https://www.cdc.gov/nchs/nhanes/index.html), and this study utilized NHANES data between 1999 and 2018. Diabetes was identified utilizing the ADA’s diagnostic criteria, which include self-reported diagnosis, current use of insulin or oral hypoglycemic medication, a fasting blood glucose FBG level of 126 mg/dL or higher, or a glycated hemoglobin HbA1c level of 6.5% or higher17. Prediabetes was determined based on self-reported prediabetes, an FBG level between 100 mg/dL and 125 mg/dL, or an HbA1c level between 5.7% and 6.4%17. CVD diagnosis was determined through self-reported physician diagnoses collected during standardized individual interviews utilizing a medical status questionnaire. Participants were asked, "Have you been informed by a medical professional on your having congestive heart failure, coronary heart disease, angina, myocardial infarction, or stroke?" confirmative responses to any of these questions indicated a CVD diagnosis. A total of 4,295 adults aged 20 to 85 years were included based on the following inclusion criteria: a confirmed diagnosis of cardiovascular disease (CVD) and either diabetes or prediabetes. Participants were excluded if they had missing NPAR data (n = 326), were pregnant (n = 1), had a history of cancer (n = 801), or lacked sufficient follow-up data (n = 4). After applying these exclusion criteria, the final analysis included 3,163 eligible participants (Fig. 1).
Assessment of covariates
Data on relevant population and health information were collected, including factors such as age, sex, race/ethnicity, education background, household income, smoking habit, and medical history. Body mass index (BMI) was computed by dividing weight in kilograms by the square of height in meters. Race/ethnicity was categorized as White, Black, Mexican American, or other. Education level was categorized as less than high school completion, high school graduate or equivalent, or completion of post-secondary education. Smoking habit was recorded as never smoker, former smoker, or current smoker. Drinking behavior was classified into five categories: heavy drinkers (defined as consuming 3 or more drinks on a daily basis for women and 4 or more drinks on a daily basis for men, or binge drinking five times or more per month); moderate drinkers (defined as consuming 2 or more drinks on a daily basis for women and 3 or more drinks on a daily basis for men, or binge drinking two times or more per month); mild drinkers (those not meeting the criteria for heavy or moderate drinking); and non-drinkers or individuals with a history of alcohol abuse. Clinical indicators included blood glucose, glycosylated hemoglobin (HbA1c), triglycerides (TG), total cholesterol (TC), LDL cholesterol (LDL-C), HDL cholesterol (HDL-C), serum creatinine (Scr), estimated glomerular filtration rate (eGFR), lactate dehydrogenase (LDH), uric acid (UA), albumin (Alb), aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin (TBil), and gamma-glutamyltransferase (GGT), all of which were measured in the "NHANES laboratory."
Assessment of NPAR
Blood samples were collected, processed, and sent to NHANES for analysis. An extensive explanation of the procedures that were adopted in the laboratory is located at the NHANES site. The complete blood count was conducted utilizing the Beckman-Coulter method. Then, the NPAR was calculated from the same blood sample as the ratio of neutrophil percentage to albumin: (neutrophil percentage (%) ÷ albumin (g/dL))18. For NPAR tertile analysis, participants were grouped and categorized in three NPAR tertiles (T1, T2, T3), wherein T1 being the reference group.
Ascertainment of mortality
Mortality information had been sourced through linking the cohort database to national mortality indices. Crude mortality rate comprised any documented cause of death, while CVD mortality was categorized according to the International Statistical Classification of Diseases and Related Health Problems, Tenth Edition (ICD-10)19. The specific codes employed for this classification ranged from I00 to I09, I11, I13, I20 to I51, and I60 to I69. To maintain the accuracy of mortality data, a probabilistic matching algorithm was employed to associate the data with participant IDs.
Statistical analysis
R software (version 4.4.1; https://www.r-project.org) facilitated all statistical analyses. Considering the complex sampling design of the National Health and Nutrition Examination Survey, analyses accounted for sample weights, clustering, and stratification, crucial for accurate interpretation of NHANES data. Participants were categorized into three cohorts based on NPAR tertiles (T1–T3). Continuous variables are reported as means ± standard deviations (SD), while categorical variables are presented as counts and percentages. Baseline characteristics were compared across NPAR tertile cohorts through one-way analysis of variance for continuous variables and Pearson’s chi-square test for categorical variables. Missing covariate data were handled with multiple imputation approach utilizing the “mi” R package. Crude and CVD mortality rates were determined for each NPAR tertile group throughout follow-up. To evaluate the independent prognostic significance of NPAR, multivariate Cox proportional hazards regression models were developed with three levels of covariate adjustment. Model 1 was unadjusted; Model 2 was adjusted for age, race, and sex; and Model 3 further accounted for body mass index, smoking habit, alcohol consumption, educational attainment, hypertension, and household income-to-poverty ratio. These variables were selected based on prior literature and clinical guidelines, given their strong associations with systemic inflammation, renal function, and cardiovascular outcomes in patients with cardiometabolic diseases17,22. This progressive modeling approach aimed to control for potential confounders while preserving model stability and statistical power. The correlation of NPAR and mortality was explored utilizing Cox proportional hazards models incorporating restricted cubic splines and penalized spline methods for smooth curve fitting. Cumulative survival differences across NPAR cohorts were compared utilizing weighted Kaplan–Meier curves and the log-rank test. Hazard ratios (HRs) and 95% confidence intervals (CIs) for the correlation of NPAR levels and both crude and CVD mortality risk were calculated utilizing multivariate Cox proportional hazards regression models.
To explore the mediating role of renal function (eGFR) in the relationship between NPAR and mortality, the distribution-of-the-product (DTP) method was employed to analyze the mediating effect. Statistical significance of the mediating effect was assessed utilizing 95% confidence intervals (CIs), calculated with the R mediation software package. The mediating effect was considered statistically significant if the CI did not contain zero. All statistical analyses were performed utilizing R version 4.4.1 software, and two-sided p-values less than 0.05 were considered statistically significant.
Results
Baseline characteristics of study participants
Table 1 presents the baseline characteristics of the study participants (n = 3163), stratified by NPAR tertiles. The mean age of the participants was 65.04 years, with 56.18% being male. The mean NPAR among participants was 14.73 ± 2.93. The laboratory characteristics at baseline, stratified by NPAR tertiles, are demonstrated in Table 2. Participants with higher NPAR were more to be older, obese, and have a higher representation of males compared to those in the lowest tertile. In addition, family income-poverty ratio, marital status, and race were significantly correlated with the NPAR index. Among patients with abnormal glucose metabolism, a significant difference in the distribution was observed across the NPAR cohorts (p-value < 0.0001), with a higher proportion of diabetic patients present in the group with the higher NPAR index. Significant differences in biochemical indices were also observed among the three cohorts presented in Table 2, with participants in the highest tertile exhibiting higher levels of HbA1c, BUN, Scr, and UA, and lower levels of eGFR, LDL, TC, TG, Albumin, and AST compared to those in the first tertile.
Crude and CVD mortality with tertiles of NPAR levels and survival analyses
Table 3 details crude and CVD mortality (1342 and 462 mortalities, respectively) during follow-up. Three Cox regression models analyzed the independent relationship between NPAR levels and mortality risk. After adjusting for age, sex, race, BMI, smoking, alcohol consumption, education, hypertension, and the household income-to-poverty ratio (model 3), the risk ratio for crude mortality rate comparing high and low NPAR tertiles was 1.75(95%CI 1.50–2.04). Likewise, the risk ratio for CVD mortality was 2.03(95% CI 1.53–2.68). This positive association with mortality remained statistically significant for both outcomes (trend p-value < 0.0001).
Nonlinear association of NPAR with all- cause mortality and CVD-cause mortality
Prior multivariate analyses indicated a nonlinear relationship between baseline NPAR and both crude and cardiovascular disease mortality. To clarify this relationship, we employed restricted cubic spline (“RCS”) fitting, generating the adjusted smoothed curves depicted in Fig. 2. Figure 2A presents the nonlinear relationship between NPAR and crude mortality rate, and Fig. 2B displays the corresponding association for CVD mortality. The risk of crude mortality rate increased sharply as NPAR values approached 12.45923, while the risk of CVD mortality demonstrated a significant increase beginning at an NPAR of 11.00435. Above these thresholds, further increases in NPAR were correlated with much greater risks of both crude and CVD mortality. Subgroup analyses, stratifying CVD patients by diabetes mellitus (“DM”) and prediabetes mellitus (“PreDM”) status, confirmed a significant nonlinear correlation of NPAR and both crude and CVD mortality in those with CVD and diabetes(Fig. 3). This relationship was particularly significant among the diabetic patients (P < 0.001), where higher NPAR values corresponded with a significantly increased risk of both outcomes. A similar, albeit weaker, yet still statistically significant (P < 0.001) nonlinear relationship was observed between NPAR and mortality among the prediabetic cohort.
Dose–response relationships of NPAR level with the probability of crude mortality rate (A) and CVD mortality (B) in CVD patients with Impaired glucose metabolism or A nonlinear correlation of NPAR level with both crude and CVD-cause mortality was identified (P < 0.001). Solid and dashed lines represent the estimated values and their corresponding 95% confidence intervals, respectively. Covariates included age, sex, ethnicity, education level, poverty income, and number of people with diabetes or pre-diabetes. Additional adjustments included poverty income ratio, marital status, smoking habit, and body mass index.
Dose–response relationships of NPAR level with the probability of crude mortality rate (A) and CVD mortality (B) in CVD patients with diabetes and crude mortality rate (C) and CVD mortality (D) in CVD patients with pre-DM. A nonlinear correlation of NPAR level with both crude and CVD-cause mortality was identified (P < 0.001). Solid and dashed lines represent the estimated values and their corresponding 95% confidence intervals, respectively. Covariates included age, sex, ethnicity, education level, poverty income, and number of people with diabetes or pre-diabetes. Additional adjustments included poverty income ratio, marital status, smoking habit, and body mass index.
Survival analysis
The NPAR level analysis included 3163 adults (mean (standard error) age 65.04 ± 0.27years; 1347 females (weighted 43.82%) and 1816 males (weighted 56.18%)). The median follow-up time was 76 months (interquartile range = 85 months), 1,342 crude mortalities and 462 cardiovascular mortalities were observed. Figure 4 displays Kaplan–Meier survival curves from the start of follow-up. Unadjusted log-rank tests indicated significantly higher crude mortality rate (Fig. 4A) and cardiovascular mortality (Fig. 4B) in the T3 group compared with the T1 and T2 cohorts (P < 0.001).
Role of eGFR in the associations of NPAR with crude mortality rate and CVD mortality
Mediation analysis indicated that eGFR levels significantly mediated the relationship between NPAR and mortality (Fig. 5). After adjusting for age, sex, race, household income-to-poverty ratio, marital status, education, BMI, alcohol consumption, smoking habit, and hypertension, eGFR mediated 14.49% (Table 4) of the total effect of NPAR on crude mortality rate (P < 0.001). Similarly, eGFR mediated 13. 38% (P < 0.001) of the total effect of NPAR on cardiovascular mortality (Table 5).
The mediating effect of eGFR on the relationship between NPAR and all-cause mortality (A) and cardiovascular mortality (B). ACME (Averaged Causal Mediation Effects); ADE (Average Direct Effect); Prop. Mediated (Proportion Mediated). eGFR, Estimated Glomerular Filtration Rat. Adjusted: age, sex, race, poverty-income ratio, marital, educational level, bmi, alcohol intake, smoke, Hypertension.
Discussion
This study evaluated the correlation of NPAR and crude and cardiovascular mortality in patients with CVD and abnormal glucose metabolism. Abnormal glucose metabolism, including diabetes and prediabetes, is generally correlated with an increased risk of mortality in CVD patients. This increased risk is closely related to the combined effects of systemic inflammation and metabolic dysfunction21. Analysis of health screening data from 3,163 participants in the NHANES database between 1998 and 2018 indicated a significant, nonlinear correlation of high NPAR levels and both crude and cardiovascular mortality. Patients with abnormal glucose metabolism, particularly those with diabetes and prediabetes, demonstrate a greater probability of developing renal impairment due to the combined influence of chronic inflammation and metabolic dysfunction, which thereby elevates their risk of mortality. This further strengthens NPAR’s potential as a robust, independent predictor of adverse survival outcomes. Mediation analysis indicated that eGFR is a significant mediator in the relationship between NPAR and mortality. This suggests that NPAR may be a crucial prognostic indicator for CVD patients with abnormal glucose metabolism, facilitating the identification of high-risk patients and informing the development of personalized intervention strategies.
Studies have also evaluated the relationship between NPAR and mortality in diverse populations. For instance, a significant positive correlation between NPAR and crude mortality rate has been observed in heart failure patients20. Among individuals with chronic obstructive pulmonary disease (COPD), NPAR predicted an increased risk of mortality and proved superior to other hematologic inflammatory biomarkers in forecasting 5-year crude mortality rate10. In addition, an analysis of 8,990 U.S. adults with hypertension demonstrated a significant correlation of high NPAR levels and increased risks of crude and CVD mortality, independent of potential confounders21. These results consistently highlight a strong correlation of NPAR and mortality risk, presumably as NPAR reflects underlying systemic inflammation and immune activity. While the predictive utility of NPAR has been extensively confirmed across various cohorts, analyses in CVD populations represented by abnormal glucose metabolism are scarce. By closely analyzing this high-risk group, this study offers novel insights into the prognostic significance of NPAR in patients with both abnormal glucose metabolism and CVD, thus filling a critical gap in the existing literature.
The association between NPAR and cardiovascular mortality may be explained by the synergistic pathophysiological roles of its components—neutrophils and serum albumin—both of which reflect underlying inflammatory and nutritional states that are critical in cardiovascular disease (CVD) progression. While neutrophils mainly reflect the pro-inflammatory component of NPAR, the albumin level contributes another crucial dimension—nutritional and antioxidative status23,24. Neutrophils, as key effectors of innate immunity, participate in the initiation and amplification of systemic inflammation25. Elevated neutrophil counts have been implicated in the activation of endothelial cells, release of reactive oxygen species (ROS), and the promotion of pro-thrombotic states, which contribute to atherosclerotic plaque formation and instability26,27. In diabetic or prediabetic populations, chronic hyperglycemia may further potentiate neutrophil adhesion and dysfunction, compounding vascular injury28.In parallel, serum albumin is widely recognized as both a nutritional and anti-inflammatory biomarker. Beyond its function in maintaining oncotic pressure, albumin binds and neutralizes free radicals and pro-inflammatory molecules, exhibiting antioxidant and protective vascular effects. Hypoalbuminemia is independently associated with oxidative stress, endothelial dysfunction, increased vascular permeability, and cardiac cachexia in patients with CVD28. Additionally, low albumin levels have been linked to impaired drug binding, delayed wound healing, and immune dysregulation, all of which may worsen long-term outcomes in high-risk populations29,30,31. Therefore, a higher NPAR—reflecting both heightened neutrophilic inflammation and reduced albumin-mediated antioxidant capacity—may serve as a composite indicator of immune-metabolic dysregulation, associated with poor cardiovascular prognosis. Several cohort studies have validated this hypothesis, showing consistent associations between elevated NPAR and increased all-cause and CVD mortality in patients with chronic comorbidities, including diabetes17, chronic kidney disease12, and heart failure20.
Our findings not only reinforce these observations but also add mechanistic support by incorporating eGFR as a mediator, suggesting a possible inflammation-mediated renal dysfunction pathway through which NPAR exerts its deleterious effects on survival. Despite these prior findings17, few studies have investigated the intermediate biological pathways through which NPAR affects mortality, particularly in high-risk cardiometabolic populations. Our mediation analysis indicated that NPAR levels were correlated with increased crude and cardiovascular mortality through a reduction in eGFR, especially among individuals with CVD and abnormal glucose metabolism, suggesting potential mechanisms. Individuals with abnormal glucose metabolism, particularly those with diabetes and prediabetes, frequently present with chronic inflammation and renal impairment, increasing their susceptibility to renal function decline and higher mortality risk. Extensive research demonstrates the prevalence of low-grade chronic inflammation in CVD and other chronic conditions, including chronic kidney disease (CKD). Inflammation plays a crucial role in CKD progression, with neutrophils possibly exacerbating renal impairment by increasing the inflammatory response32. Simultaneously, hypoalbuminemia is a known risk factor for CKD progression, potentially contributing to further renal function decline through the combined effects of malnutrition and systemic inflammation33. In individuals with CVD and abnormal glucose metabolism, high NPAR levels signal a serious inflammatory state and compromised renal function. Declining eGFR not only magnifies the effect of NPAR on mortality risk but also strengthens this relationship through feedback mechanisms. Our mediation analysis demonstrated that eGFR explained 14.49% of the mediating effect of NPAR on crude mortality rate and 13.38% on cardiovascular mortality. This strong mediating influence was particularly evident in patients with diabetes and prediabetes, emphasizing the critical importance of renal function management in these vulnerable populations. Therefore, managing renal function, particularly by slowing or reversing eGFR decline, may be a vital strategy for reducing mortality risk in individuals with CVD and abnormal glucose metabolism. Further research should explore whether interventions targeting renal function can effectively lessen the effect of NPAR on mortality.
The primary strength of this study is reflected by its extensive sample size and protracted follow-up period, contributing to reliable results and a robust statistical analysis. Multiple statistical methods controlled for confounding factors, strengthening the study’s conclusions. Moreover, this study represents the first systematic evaluation of the long-term relationship between NPAR and crude and cardiovascular mortality in patients with abnormal glucose metabolism and CVD. The sample weighting procedure enhances the generalizability of the findings to a broader U.S. population. Nevertheless, this study is subject to limitations. As a single-center observational study, it cannot determine causality. While multivariate adjustment lessened the effect of confounding factors, residual confounders may remain. Finally, this study analyzed only the prognostic value of baseline NPAR, highlighting the need for additional research to study whether changes in NPAR during follow-up similarly forecast mortality.
Conclusion
In conclusion, the extensive sample size and protracted follow-up period of this study indicated a nonlinear correlation of NPAR and both crude and cardiovascular mortality in patients with abnormal glucose metabolism and CVD. The study also verified the mediating role of eGFR in the relationship between NPAR and mortality. These results suggest that NPAR represents a reliable predictor of mortality risk in patients with abnormal glucose metabolism and CVD. By observing early changes in renal function, clinicians can better evaluate patient risk and apply specific interventions to enhance prognosis.
Data availability
This study utilized data from a publicly available database, which can be accessed at https://wwwn.cdc.gov/nchs/nhanes/Default.aspx. For further research inquiries or access to additional data, requests can be made directly to the authors.
References
Benjamin, E. J. et al. Heart disease and stroke statistics—2019 update: A report from the American heart association. Circulation 139, e56–e528. https://doi.org/10.1161/CIR.0000000000000659 (2019).
Dong, C. et al. Cardiovascular disease burden attributable to dietary risk factors from 1990 to 2019: A systematic analysis of the Global burden of disease study. Nutr. Metab. Cardiovasc. Dis. 32, 897–907. https://doi.org/10.1016/j.numecd.2021.11.012 (2022).
Poznyak, A. V., Litvinova, L., Poggio, P., Sukhorukov, V. N. & Orekhov, A. N. Effect of glucose levels on cardiovascular risk. Cells 11, 3034. https://doi.org/10.3390/cells11193034 (2022).
Cosentino, F. et al. 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart. J. 41, 255–323. https://doi.org/10.1093/eurheartj/ehz828 (2020).
Ridker, P. M. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation 107, 363–369. https://doi.org/10.1161/01.CIR.0000053730.47739.3C (2003).
Pearson, T. A. et al. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: a statement for healthcare professionals from the centers for disease control and prevention and the American heart association. Circulation 107, 499–511. https://doi.org/10.1161/01.CIR.0000052939.59093.45 (2003).
Kurkiewicz, K., Gąsior, M. & Szyguła-Jurkiewicz, B. E. Markers of malnutrition, inflammation and tissue remodeling are associated with one-year outcomes in patients with advanced heart failure. Pol. Arch. Intern. Med. 133, 16411. https://doi.org/10.20452/pamw.16411 (2023).
Dinauer, M. C. Primary immune deficiencies with defects in neutrophil function. Hematology 2016, 43–50. https://doi.org/10.1182/asheducation-2016.1.43 (2016).
Jiao, S. et al. The role of neutrophil percentage to albumin ratio in predicting 1-year mortality in elderly patients with hip fracture and external validation. Front. Immunol. 14, 1223464. https://doi.org/10.3389/fimmu.2023.1223464 (2023).
Liu, C. F. & Chien, L. W. Predictive role of neutrophil-percentage-to-albumin ratio (NPAR) in nonalcoholic fatty liver disease and advanced liver fibrosis in nondiabetic US adults: Evidence from NHANES 2017–2018. Nutrients 15, 1892. https://doi.org/10.3390/nu15081892 (2023).
Wang, L., Liu, L., Liu, X. & Yang, L. The association between neutrophil percentage-to-albumin ratio (NPAR) and depression among US adults: A cross-sectional study. Sci. Rep. 14, 21880. https://doi.org/10.1038/s41598-024-71488-y (2024).
Li, J., Xiang, T., Chen, X. & Fu, P. Neutrophil-percentage-to-albumin ratio is associated with chronic kidney disease: Evidence from NHANES 2009–2018. PLoS ONE 19, e0307466. https://doi.org/10.1371/journal.pone.0307466 (2024).
Wang, R., Tao, W., Chen, H., Ma, T. & Cheng, X. Investigating nonlinear associations between neutrophil percentage to albumin ratio and cardiovascular disease: A nationally representative cross-sectional study. Sci. Rep. 14, 23632. https://doi.org/10.1038/s41598-024-75111-y (2024).
Cui, H., Ding, X., Li, W., Chen, H. & Li, H. The neutrophil percentage to albumin ratio as a new predictor of in-hospital mortality in patients with ST-segment elevation myocardial infarction. Med. Sci. Monit. 25, 7845–7852. https://doi.org/10.12659/MSM.917987 (2019).
Rijnhart, J. J. M. et al. Mediation analysis methods used in observational research: A scoping review and recommendations. BMC Med. Res. Methodol. 21, 226. https://doi.org/10.1186/s12874-021-01426-3 (2021).
Manjunath, G. et al. Level of kidney function as a risk factor for cardiovascular outcomes in the elderly. Kidney Int. 63, 1121–1129. https://doi.org/10.1046/j.1523-1755.2003.00838.x (2003).
Ji, H. et al. Neutrophil percentage to albumin ratio predicts cardiovascular and all-cause mortality in diabetes and pre diabetes patients. Sci. Rep. 15, 10075. https://doi.org/10.1038/s41598-025-93558-5 (2025).
Liu, Z. et al. Associations of neutrophil-percentage-to-albumin ratio level with all-cause mortality and cardiovascular disease-cause mortality among patients with hypertension: Evidence from NHANES 1999–2010. Front. Cardiovasc. Med. 11, 1397422. https://doi.org/10.3389/fcvm.2024.1397422 (2024).
ElSayed, N. A. et al. 2. Classification and diagnosis of diabetes: Standards of care in diabetes—2023. Diabetes Care 46, S19–S40. https://doi.org/10.2337/dc23-S002 (2023).
Wu, C. C., Wu, C. H., Lee, C. H. & Cheng, C. I. Association between neutrophil percentage-to-albumin ratio (NPAR), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and long-term mortality in community-dwelling adults with heart failure: Evidence from US NHANES 2005–2016. BMC Cardiovasc. Disord. 23, 312. https://doi.org/10.1186/s12872-023-03316-6 (2023).
Eisfeld, J. International statistical classification of diseases and related health problems. TSQ. 1, 107–110. https://doi.org/10.1215/23289252-2399740 (2014).
Zhang, Q., Xiao, S., Jiao, X. & Shen, Y. The triglyceride-glucose index is a predictor for cardiovascular and all-cause mortality in CVD patients with diabetes or pre-diabetes: Evidence from NHANES 2001–2018. Cardiovasc. Diabetol. 22, 279. https://doi.org/10.1186/s12933-023-02030-z (2023).
Chien, S. C. et al. Critical appraisal of the role of serum albumin in cardiovascular disease. Biomark Res. 5, 31. https://doi.org/10.1186/s40364-017-0111-x (2017).
Belinskaia, D. A., Voronina, P. A. & Goncharov, N. V. Integrative role of albumin: Evolutionary, biochemical and pathophysiological aspects. J Evol Biochem Phys 57, 1419–1448. https://doi.org/10.1134/S002209302106020X (2021).
Mantovani, A. et al. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 11, 519–531. https://doi.org/10.1038/nri3024 (2011).
Moris, D. et al. The role of reactive oxygen species in the pathophysiology of cardiovascular diseases and the clinical significance of myocardial redox. Ann. Transl. Med. 5, 326–326 (2017).
Silvestre-Roig, C. et al. Neutrophils as regulators of cardiovascular inflammation. Nature reviews. Cardiology 17(6), 327–340 (2020).
Giri, B. et al. Chronic hyperglycemia mediated physiological alteration and metabolic distortion leads to organ dysfunction, infection, cancer progression and other pathophysiological consequences: An update on glucose toxicity. Biomed. Pharmacother. 107, 306–328. https://doi.org/10.1016/j.biopha.2018.07.157 (2018).
Yang, F., Zhang, Y. & Liang, H. Interactive association of drugs binding to human serum albumin. IJMS 15, 3580–3595. https://doi.org/10.3390/ijms15033580 (2014).
V., S. K., Prakash, D. G. & Pottendla, V. K. Preoperative serum albumin level as a predictor of surgical complications after emergency abdominal surgery. Int Surg J 6, 361–364. https://doi.org/10.18203/2349-2902.isj20190383
De Simone, G., Di Masi, A. & Ascenzi, P. Serum albumin: A multifaced enzyme. IJMS 22, 10086. https://doi.org/10.3390/ijms221810086 (2021).
Liang, Z. et al. Identification of shared gene signatures and molecular mechanisms between chronic kidney disease and ulcerative colitis. Front. Immunol. 14, 1078310. https://doi.org/10.3389/fimmu.2023.1078310 (2023).
Zhang, X. et al. Risk factors for progression of CKD with and without diabetes. J. Diabetes Res. 2022, 9613062 (2022).
Acknowledgements
We sincerely thank everyone who contributed to this article, especially the NHANES staff for their dedication. We are also very grateful to Jing Zhang for his valuable work on the nhanesR package and webpage, which has greatly simplified our use of the NHANES database.
Funding
This work was supported by Development Fund of Affiliated Hospital of Xuzhou Medical University (grant number XYFC22020005).
Author information
Authors and Affiliations
Contributions
JX. L.: Data curation, formal analysis, investigation, methodology, software, visualization, and writing; KL. F.: Data curation, formal analysis, investigation, methodology, software, visualization, and writing, Supervision, conceptualization, review & editing; MY. Y: Review, supervision & validation; X. Z.: Resources, project administration, and review & editing; R. H.: Review, supervision & validation; Y. Z.: Review, supervision & validation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The study was conducted in accordance with the Declaration of Helsinki and approved by the National Center for Health Statistics (NCHS) Research Ethics Review Board (ERB) Approval.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, J., Yang, M., Zhang, X. et al. Neutrophil to albumin ratio predicts cardiovascular and all cause mortality in CVD patients with abnormal glucose metabolism. Sci Rep 15, 21976 (2025). https://doi.org/10.1038/s41598-025-08130-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-08130-y