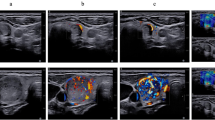
Abstract
Thyroid nodules are common, as they are found in up to 8% of adults. These nodules are becoming increasingly prevalent, possibly because of the increased use of ultrasound imaging. These nodules are mostly benign but remain a source of concern for both physicians and patients due to the possibility of malignant transformation. The aim of this study was to assess the ability and usefulness of thyroid function tests in predicting ultrasound and FNA outcomes of thyroid nodules and therefore confirm or rule out the malignant potential of these nodules. In this case‒control, prospective study, one hundred patients who presented to the outpatient clinic from March 2022 to June 2024 for evaluation of thyroid nodules were included. Follow-up protocols included medical history review, physical examination, thyroid function tests, ultrasound imaging examination and fine needle aspiration if indicated. Data were analyzed using SPSS 25, and a p value less than 0.05 indicated statistical significance. 40% of patients who had thyroid nodules had normal thyroid function tests, 28% had subclinical hyperthyroidism, 22% had subclinical hypothyroidism, 8% had overt hypothyroidism according to laboratory findings, and 2% had overt hyperthyroidism according to laboratory findings. A higher-than-normal BMI was significantly associated with TIRADS 2 nodules (95% C. I 0.40–0.97, p value < 0.0001), and a low BMI was significantly associated with TIRADS 4 nodules (95% C. I 0.08–0.53, p value 0.04). High levels of TSH were significantly associated with TIRADS 2 nodules (95% C. I 0.52_1.11, p value < 0.0001), and low levels of TSH, despite normal T3 and T4 levels, were correlated with TIRADS 4 nodules (95% C. I -1.10_0.9, p value < 0.0001). Low levels of TSH, despite normal T3 and T4 levels, and cytological findings were associated with Bethesda 2 nodules, which are benign follicular neoplasms (95% c. I 1.08–1.44, p value < 0.0001. Most patients who had thyroid nodules had normal thyroid function tests; however, low TSH levels were significantly associated with ultrasound evidence of TIRAD 4 nodules and Bethesda 2 nodules according to FNA, and high TSH levels were significantly associated with ultrasound evidence of TIRADS 2 nodules.
Similar content being viewed by others
Introduction
The thyroid gland is a butterfly shaped gland in front of the neck that produces L-thyroxine and triiodothyronine1.The thyroid gland, consisting of two connected lobes, is one of the largest endocrine glands in the human body, weighing 20–30 g in adults. Thyroid lesions are often found on the gland, with a prevalence of 4–7% by palpation and 20–60% by ultrasound(mostly discovered incidentally). Most of them are asymptomatic, and thyroid hormone secretion is normal2. Most of them are benign, but 7–15% are malignant. The increase in prevalence is due mostly to increased detection and increased availability of ultrasound as part of cancer screening programs3. the increased prevalence of thyroid nodules may also be due to risk factors such as iodine deficiency, exposure to radiation, autoimmune thyroid disease, and genetic predisposition.
Thyroid nodules are associated with a range of benign and malignant conditions that may have an indolent course or very aggressive behavior4. It is important to evaluate all thyroid nodules to identify those that are clinically significant and require follow-up or intervention.
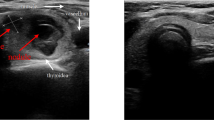
Initial assessments include a detailed review of the patient’s family history to reveal a history of thyroid or other malignancies; a physical examination of the thyroid gland; clinical thyroid tests to confirm or rule out cervical lymphadenopathy; laboratory thyroid function tests, mainly TSH, T3, and T4; and neck ultrasound imaging to ascertain the European Thyroid Imaging Reporting Data System (TIRAD) classification5. The TIRADS classification system categorizes thyroid nodules on the basis of their risk of malignancy, with scores ranging from 1 to 5: TIRADS 1 corresponds to a normal gland; TIRADS 2 corresponds to a benign nodule (with a 0% risk of malignancy); TIRADS 3 corresponds to a highly probable benign nodule (< 5% risk of malignancy); TIRADS 4 (5 to 80% risk of malignancy); and TIRADS 5 (> 80% risk of malignancy) corresponds to a suspicious nodule (TIRADS 4 A, 4B and 4 C correspond to nodules with a low, intermediate and moderate suspicion for malignancy, respectively; and TIRADS 5 corresponds to a high suspicion for malignancy), with the risk of malignancy ranging from 5 to 80%. Color Doppler data were not used because of its poor reproducibility, as well as operator and device dependency and variability6,7. In 2016, the American Association of Clinical Endocrinologists recommended ultrasound and FNAB of nodules > 0.5 cm with suspicious ultrasound features and of nodules > 1.0 cm with other specific conditions, while nodules < 5 mm should be monitored. According to the Ultrasound Consensus Statement, the Society of Radiologists reported that microcalcifications and a solid component were ultrasound features associated with thyroid cancer8,9. Fine needle aspiration for cytopathology by an expert pathologist can aid in diagnosing malignant thyroid growth that requires surgery, thus reducing the incidence of unnecessary surgery and its associated morbidities. In view of this, the National Cancer Institute hosted a meeting in 2007 in Bethesda, MD, USA, introducing the Bethesda System for Reporting Thyroid Cytopathology. The reporting system divides nodules into 6 different categories, with each having a different risk of malignancy and a proper action plan10,11.
In patients with higher Bethesda categories (IV and V), The choice between total thyroidectomy (TT) and subtotal thyroidectomy (STT) remains debated. Some studies suggest that total thyroidectomy may carry a slightly higher risk of early complications, but offers more comprehensive disease control in malignancy cases (11). Additionally, surgical tools such as the Harmonic Scalpel (HS) and LigaSure (LS) have comparable safety profiles in thyroidectomy, though HS may provide superior hemostasis, particularly in patients with thyroid carcinoma12.
Aim
The aim of this study was to assess the ability and usefulness of thyroid function tests in predicting ultrasound and FNA outcomes of thyroid nodules and therefore confirm or rule out the malignant potential of these nodules.
Patients and methods
Study design
In this prospective observational cohort analysis, 100 patients who attended private outpatient clinics from March 2022 to June 2024 and had incidental findings of thyroid nodules were included. All of them were clinically euthyroid.
Data interpretation
A detailed review of the patient’s medical and family history was conducted to ascertain a history of malignancy, as were a physical examination of the neck and body, including clinical thyroid assessment, thyroid gland examination to confirm or rule out cervical lymphadenopathy, and ultrasound assessment of the thyroid nodule to ascertain the TIRADS classification using high performance ultrasound device (Ge Logiq E9; GE, solingen, Germany) with multifrequency probe (Ge 9 L-D linear with 2.0 to 9.0 MHz). The features of the TIRADS scoring system are illustrated in Table 1. Patients with suspicious sonographic features were scheduled for FNA, which was performed after the patient was informed about the procedure and provided informed consent.
Statistical analysis
To evaluate the correlations between thyroid function test results, TI-RADS score and the Bethesda score, data were collected and analyzed. Categorical variables were presented as numbers and percentages, whereas continuous data were presented as means and standard deviations. Chi-square tests were used to evaluate the associations between categorical variables, and Student’s t tests were used to compare two groups. A p value less than 0.05 indicated statistical significance.
Results
Patient characteristics
One hundred patients were included in the study, 75% of whom were female and 25% of whom were male. The mean age of the patients was 52.9+−12.713. The age group ranged from 15 to 75 years, and the most common age group was 55–64 years (53%, mean 61.016–2.6044), followed by 35–44 years (17%, mean 29.37+−3.159) and 45–54 years (16%, mean 48.68+−7.029). 21% of the patients were hypertensive, whereas 12% of the patients were diabetic. 25% of the patients were smokers. Regarding body weight, 35% of the patients had a BMI less than 18, whereas 24%.
of the patients had a higher-than-normal BMI, as shown in Table 1.
Thyroid function test profile in patients with thyroid nodules
Laboratory analysis of the patients’ thyroid status revealed that 40% had normal thyroid function; 28% had subclinical hyperthyroidism, as evidenced by low TSH levels, normal free T3 and free T4 levels; 22% had subclinical hypothyroidism, as evidenced by high TSH levels and normal free T3 and T4 levels; 8% had overt hypothyroidism according to laboratory findings; and 2% had overt hyperthyroidism according to laboratory findings, as illustrated in Figure 1.
TI-RADS scores of thyroid nodules
41% of the patients had TIRADS 3 nodules, 32% had TIRADS 4 nodules, 24% had TIRADS 2 nodules, and only 3% had TIRADS 5 nodules, as shown in Figure 2.
FNA cytological results
Nodules that were suspicious on ultrasound subjected to fine needle aspiration for cytological analysis. Thirty-five patients were scheduled for FNA. Twenty-one (60%) of them had a Bethesda score of 2, 9 (25.7%) had a Bethesda score of 3, 4 (11.5%) had a Bethesda score of 4, and only 1 (2.8%) had a Bethesda score of 5, as shown in Figure 3.
Correlations between BMI and the TIRADS score
A significant association was found between patients who had a higher-than-normal BMI and TIRADS 2 nodules (95% C. I 0.40–0.97, p value < 0.0001), and a significant association between a low BMI and TIRADS 4 nodules (95% C. I 0.08–0.53, p value 0.04) was noted. This is illustrated in Table 2.
Correlations between thyroid function test results and TI-RADS classification
A significant association between high TSH levels (overt or subclinical hypothyroidism) and TIRADS 2 nodules (95% C. I 0.52_1.11, p value < 0.0001) was noted, as was a significant association between low TSH levels, normal T3 and T4 and TIRADS 4 nodules (95% C. I −1.10_0.9, p value < 0.0001), as shown in Table 3.
Correlations between thyroid function test results and the Bethesda score
There was a significant association between low TSH levels, normal T3 and T4 levels and cytological findings indicating Bethesda 2 nodules, which are benign follicular neoplasms (95% c. I 1.08–1.44, p value < 0.0001. This is shown in Table 4.
Discussion
Thyroid nodules are challenging to diagnose because of the need to determine malignancy and avoid unnecessary surgical interventions if the nodule is proven to be benign. The aim of this study was to investigate and elucidate the relationships between TSH, T4, and T3 levels and malignant thyroid nodules as easily achieved predictors.
This study revealed a significant correlation among high TSH levels, TIRADS 2 nodules and a high BMI. This contradicts the findings of a previous study by Fiore et al., who reported that high levels of TSH are associated with a greater risk of thyroid malignancy and that lower levels of TSH are associated with a lower risk of papillary thyroid cancer (PTC). This study revealed that ongoing TSH stimulation can change slowly differentiated thyroid carcinoma into rapidly growing malignancies12.
Differentiated thyroid tumors express TSHR on their plasma membranes. Thyroid-stimulating hormone increases adenylate cyclase activity, leading to increased cAMP production and cell growth via TSHR in vitro. However, elevated TSH levels may be linked to poor thyroid hormone synthesis due to chronic iodine deficiency, which leads to the development of thyroid colloid cysts that are benign in nature. Moreover, the relationship between Hashimoto’s thyroiditis and follicular cell carcinoma of the thyroid remains controversial. Hashimoto’s thyroiditis is regarded by some as a premalignant lesion for which thyroidectomy is definitely indicated13. Notably, in iodine-deficient areas, chronic iodine deficiency increases the frequency of thyroid nodularity and autonomy in older people. On the basis of these data, Fiore et al. proposed that the elevated TSH levels in cases of PTC are not caused by increased thyrotropin in thyroid cancer patients but are largely due to the decrease in serum TSH levels in those with nodular goiters. However, a radionuclide thyroid scan was not conducted to verify thyroid autonomy in these patients14.
The results revealed a significant association between low TSH, low TIRADS-4 score, and low BMI and benign features identified by cytology via the Bethesda score. These findings support the results of other studies in that low TSH levels is an indicator of benignity in most cases. Therefore, the next step in the evaluation of a patient with a low TSH level would be an iodine-123 (123-I) or pertechnetate scintigraphy scan to exclude the possibility of an autonomously functioning nodule. Hyperfunctioning thyroid nodules are almost always benign and generally do not require further cytologic investigation15,16, but a nonfunctioning or “cold” nodule in a patient with low TSH levels may indicate malignant potential. Significant age-dependent development of thyroid autonomy (TSH concentration less than 0.4 mU/ml) was observed in BTND patients but not in PTC patients. When patients were considered separately, according to their clinical diagnosis, in patients with benign thyroid disease, the prevalence of thyroid autonomy was significantly greater in those with multinodular goiter, in which thyroid autonomy seems to play a protective role against the development of papillary thyroid cancer, and the prevalence of PTC in patients with a TSH concentration less than 0.4 mU/ml was significantly lower than that in patients with no evidence of thyroid autonomy (P value 0.0001). On the other hand, in S/I, the frequency of PTC in patients with thyroid autonomy was lower (5.3%) than that in those with normal TSH levels. Recent studies investigated the possible association between serum TSH concentration and thyroid cancer. TSH was found to be an independent predictor of malignancy in thyroid nodules17,18,18. These findings imply that PTC patients with MNG may have a distinct genetic background and that certain mutations could enable PTC to grow even in patients with low serum TSH levels. In our study group, the prevalence of PTC in patients with a serum TSH concentration > 0.4 mU/ml was significantly lower than that in patients with no evidence of thyroid autonomy in MNG, but our data did not allow us to draw a definitive conclusion in patients with S/I nodules. We hypothesize that the development of thyroid autonomy, by reducing TSH levels, reduces the probability that mutated oncogenes may cause clinically detectable cancer. The most common mutations in patients with papillary carcinomas are point mutations of the BRAF gene and RET/PTC rearrangements. These genetic changes are present in more than 70% of patients with papillary carcinomas, and they seldom occur together in the same tumor. Therefore, it can be hypothesized that the development of thyroid autonomy, which lowers TSH levels, might serve as a form of ‘self-treatment,’ particularly in patients residing in iodine-deficient regions such as those included in this study. This phenomenon may be less relevant in iodine-sufficient areas19,20.
The study revealed a significant association between low TSH levels (with normal T3 and T4) and cytological findings consistent with Bethesda II nodules, which are considered benign follicular lesions (95% CI: 1.08–1.44, p < 0.0001). This may suggest that some Bethesda II nodules exhibit subclinical hyperthyroid patterns due to autonomous function. These findings are consistent with several previous studies21,23,23 which found that benign nodules (Bethesda II) were more likely to present with suppressed TSH, whereas higher TSH levels were associated with Bethesda III–V score. These findings support the hypothesis that TSH suppression may serve as a biochemical marker of benignity in certain nodules. cytological evaluation alone cannot provide definitive diagnosis and postoperative histopathological confirmation following surgery remains the gold standard, particularly in indeterminate cases24,26,26.
Conclusion
Most patients who had thyroid nodules had normal thyroid function tests; however, low TSH levels were significantly associated with ultrasound TIRAD 4 nodules and FNA Bathesda scores of 2, and high TSH levels were significantly associated with ultrasound TIRADS 2 nodules.
Recommendations
-
1)
Thyroid nodules should be assessed via laboratory, ultrasound and pathological methods for full analysis.
-
2)
Most of the nodules have a benign course, which should be taken into account to prevent unnecessary surgical interventions.
Limitations
This study has several limitations. First, the small number of patients included affects the statistical power and limits the generalizability of the results to a broader population. Second, the assessment of the nodules was based on subjective interpretation by ultrasound and microscopy, which can introduce observer bias and variability, potentially affecting the accuracy of nodule characterization. Third, a radionuclide thyroid scan (which is the standard method to confirm functional thyroid autonomy) was not performed due to high costs in the private sector and lack of availability in the public sector. Additionally, the size of the thyroid nodules may have influenced the thyroid function tests, as larger nodules can be associated with hormonal changes. This factor was not specifically analyzed and may have impacted the interpretation of the hormonal data. Future studies are recommended to include nodule size stratification to better understand its relationship with thyroid function and to enhance the precision of risk assessment.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due to patients privacy issues but are available from the corresponding author on reasonable request.
References
Wada, N. et al. Lymph node metastasis from 259 papillary thyroid microcarcinomas: frequency, pattern of occurrence and recurrence, and optimal strategy for neck dissection. Ann. Surg. 237, 399–407. https://doi.org/10.1097/01.SLA.0000055273.58908.19 (2003).
Mulita, F., Anjum, F. & Thyroid, A. Apr 27. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 32965923. (2023).
Tomimori, E. K., Bisi, H., Medeiros-Neto, G. & Camargo, R. Y. A. Avaliação ultra-sonográfica Dos Nódulos tireóideos: comparação com exame citológico e histopatológico. Arquivos Brasileiros De Endocrinologia Metabologia. 48, 105–113. https://doi.org/10.1590/S0004-27302004000100012 (2004).
Yan, Y. et al. Risk factors associated with the prevalence of thyroid nodules in adults in Northeast china: a cross- sectional population- based study. BMJ Open. 13, e069390. https://doi.org/10.1136/bmjopen-2022-069390 (2023).
Shimura, H. et al. Distinct diagnostic criteria for ultrasonographic examination of papillary thyroid carcinoma: a multicenter study. Thyroid 15, 251–258. https://doi.org/10.1089/thy.2005.15.251 (2005).
Nam-Goong, I. S. et al. Ultrasonography-guided fine-needle aspiration of thyroid incidentaloma: correlation with pathological findings. Clin. Endocrinol. 60, 21–28. https://doi.org/10.1046/j.1365-2265.2003.01912.x (2004).
Rago, T. et al. Role of conventional ultrasonography and color flow-doppler sonography in predicting malignancy in ‘cold’ thyroid nodules. Eur. J. Endocrinol. 138, 41–46. https://doi.org/10.1530/eje.0.1380041 (1998).
Cappelli, C. et al. Thyroid nodule shape suggests malignancy. Eur. J. Endocrinol. 155, 27–31. https://doi.org/10.1530/eje.1.02177 (2006).
Kim, E. K. et al. New sonographic criteria for recommending fine-needle aspiration biopsy of nonpalpable solid nodules of the thyroid. AJR Am. J. Roentgenol. 178, 687–691. https://doi.org/10.2214/ajr.178.3.1780687 (2002).
Gharib, H. et al. American association of clinical endocrimologists, American college of endocrinology, and associazione Medici endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules-2016 update. Endocr. Pract. 22(Suppl 1), 1–60. https://doi.org/10.4158/EP161208.GL (2016).
Park, J. Y. et al. A comprehensive assessment of the harms of Fine-Needle aspiration biopsy for thyroid nodules: A systematic review. Endocrinol. Metab. (Seoul). 38(1), 104–116. https://doi.org/10.3803/EnM.2023.1669 (2023).
Mulita, F., Verras, G. I. & Dafnomili, V. D. Thyroidectomy for the Management of Differentiated Thyroid Carcinoma and their Outcome on Early Postoperative Complications: A 6-year Single-Centre Retrospective Study. Chirurgia (Bucur). ;117(5):556–562. (2022). https://doi.org/10.21614/chirurgia.2736. PMID: 36318685.
Mulita, F., Theofanis, G. & Verras, G. I. Comparison of postoperative bleeding using harmonic scalpel and LigaSure in thyroid surgery: a 15-year single-centre retrospective study. Med Glas (Zenica). ;20(2). (2023). https://doi.org/10.17392/1629-23. PMID: 37585298.
Bongiovanni, M., Spitale, A., Faquin, W. C., Mazzucchelli, L. & Baloch, Z. W. The Bethesda system for reporting thyroid cytopathology: a meta-analysis. Acta Cytol. 56(04), 333–339 (2012).
Fiore, E. et al. Lower levels of TSH are associated with a lower risk of papillary thyroid cancer in patients with thyroid nodular disease: thyroid autonomy May play a protective role. Endocr. Relat. Cancer. 16, 1251–1260 (2009).
Wang, F. et al. Strong association of high urinary iodine with thyroid nodule and papillary thyroid cancer. Tumour Biol: J. Int. Soc. Oncodev Biol. Med. 35(11), 11375–11379 (2014).
Ignjatovic, V. D., Matovic, M. D., Vukomanovic, V. R., Jankovic, S. M. & Džodić, R. R. Is there a link between Hashimoto’s thyroiditis and primary hyperparathyroidism? A study of serum parathormone and anti-TPO antibodies in 2267 patients. Hell J. Nucl. Med. 16(2), 86–90 (2013 May-Aug). PMID: 23865082.
Meller, J. & Becker, W. The continuing importance of thyroid scintigraphy in the era of high-resolution ultrasound. Eur. J. Nucl. Med. Mol. Imaging. 29(Suppl 2), S425–S438 (2002).
Boelaert, K. et al. Serum Thyrotropin concentration as a novel predictor of malignancy in thyroid nodules investigated by fine-needle aspiration. J. Clin. Endocrinol. Metab. 91(11), 4295–4301 (2006).
Haymart, M. R. et al. Higher serum TSH in thyroid cancer patients occurs independent of age and correlates with extrathyroidal extension. Clin. Endocrinol. (Oxf). 71(3), 434–439 (2009).
Haymart, M. R. et al. Higher serum thyroid stimulating hormone level in thyroid nodule patients is associated with greater risks of differentiated thyroid cancer and advanced tumor stage. J. Clin. Endocrinol. Metab. 93(3), 809–814 (2008).
Ciampi, R. & Nikiforov, Y. E. RET/PTC rearrangements RAF mutations in thyroid tumorigenesis. Endocrinology 148, 936–941 (2007).
Mazzaferri, E. L. An overview of the management of papillary and follicular thyroid carcinoma. Thyroid 9, 421–427 (1999).
Rocha, J. T. Q. et al. Bethesda category III thyroid nodules: descriptive cytological aspects of a series. Surg. Exp. Pathol. 6, 16. https://doi.org/10.1186/s42047-023-00141-1 (2023).
Alaraifi, A. K., Alessa, M., Hijazi, L. O., Alayed, A. M. & Alsalem, A. A. TSH level as a risk factor of thyroid malignancy for nodules in euthyroid patients. Acta Otorhinolaryngol. Ital. 43(3), 183–188. https://doi.org/10.14639/0392-100X-N2288 (2023). PMID: 37204842; PMCID: PMC10198371.
Mulita, F. et al. Patient outcomes following surgical management of thyroid nodules classified as Bethesda category III (AUS/FLUS). Endokrynol Pol. 72(2), 143–144. https://doi.org/10.5603/EP.a2021.0018 (2021).
Mulita, F. et al. Cancer rate of Bethesda category II thyroid nodules. Med Glas (Zenica). Published online February 1, 2022. https://doi.org/10.17392/1413-21
Author information
Authors and Affiliations
Contributions
Sama Atta Gitti Conceptualization, data collection, statistical analysis, writing Saman SarKo Baha Al-den Writing of introduction and discussion.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Medical Ethics Committee of the Alkindy College of Medicine. Informed consent was obtained from all participants prior to their inclusion in the study. Participants were informed about the purpose of the study, the procedures involved, and their right to withdraw at any time without penalty. All personal information collected was anonymized to ensure the privacy of the participants.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gitti, S.A., Baha Al-den, S.S. Thyroid function test profile in patients who have thyroid nodule and its correlation with ultrasound TIRAD scoring system and cytological results. Sci Rep 15, 21851 (2025). https://doi.org/10.1038/s41598-025-08156-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-08156-2