Abstract
Chrysotile asbestos, a well-established carcinogen, is known to induce various malignant cancers, contributing to an estimated annual death toll of 107,000 individuals due to asbestos-related diseases. While cell and animal models play a crucial role in elucidating the carcinogenic mechanisms of asbestos, existing models are plagued by prolonged experimental durations. In this investigation, we established a novel NIH/3T3 cell model of asbestos-induced malignant transformation using 3D culture techniques, followed by the development of a corresponding mouse model via orthotopic transplantation. Subsequent administration of the HIF-1α inhibitor PX-478 to both models allowed for the assessment of model reliability through the observation of malignant phenotypes and associated protein alterations.
Similar content being viewed by others
Introduction
Chrysotile is classified as a Group I carcinogen by the International Agency for Research on Cancer (IARC)1. It has been reported to be associated with malignant tumors, pleural abnormalities, and pulmonary fibrosis2,3,4,5,6,7,8,9,10,11,12,13. Chrysotile exposure at work harms approximately 125 million individuals worldwide, and diseases linked to this mineral account for approximately 107,000 worker deaths each year14,15,16,17,18,19,20,21. Among them, the most important cause of occupational lung cancer death is asbestos exposure22. The carcinogenic mechanism of asbestos has been shown to involve multiple pathways such as physical damage, chronic inflammation, and reactive oxygen species (ROS) mediated genomic instability. However, there is still a key scientific gap in the dynamic regulatory network that causes lesions under long-term low-dose exposure23. There are very few models for this part, which urgently need to be explored.
For a long time, cancer research, especially the study of toxin-induced carcinogenesis, such as asbestos exposure, has mainly relied on two-dimensional (2D) cell culture systems. However, these traditional 2D models lack physiologically relevant cell-cell and cell-extracellular matrix (ECM) interactions, which makes them seriously inadequate in simulating real physiological environments. This further causes non-physiological deviations in drug responses and metabolic activities, and cannot accurately reflect drug metabolism and efficacy in vivo. At the same time, gene expression patterns are dysregulated due to being out of the real microenvironment, and signal pathway activation is abnormal, which greatly limits the accuracy of disease mechanism research and clinical translation value. Based on this, current 2D cell models can no longer meet the needs of accurately describing and simulating the rich environment and complex processes observed in vivo24. In recent years, three-dimensional (3D) culture systems have significantly improved the physiological relevance of carcinogenic mechanism research by reconstructing ECM stiffness and oxygen gradient distribution. For example, 3D collagen scaffolds can simulate the characteristics of asbestos-induced interstitial fibrosis and enhance cell invasiveness through the YAP/TAZ signaling pathway, providing new ideas for the construction of chronic carcinogenic models25. 3D culture has been increasingly developed in recent years and has been used in various aspects of tumor research, such as tumor progression, tumor microenvironment, gene and protein expression, cancer-promoting signaling pathways, and drug resistance26,27. However, a chronic exposure model of chrysotile based on a 3D microenvironment has not yet been systematically established28.
In vitro studies have the advantages of convenience, short time and repeatability, but they cannot completely replicate the in vivo cell growth microenvironment. Various organs and systems of the body play an important role in the occurrence and development of cancer. In the study of carcinogenic pathways, in vivo experiments show incomparable advantages over in vitro studies. Therefore, even though there are problems such as high cost, ethical issues and significant species differences29, animal models are still widely used in current research. At present, there are four common methods for constructing mouse cancer models: chemically induced models, cell line-derived xenograft (CDX) models, patient-derived xenograft (PDX) models and genetically engineered mouse models (GEMM)30. Chemically induced models refer to experimental tumor models induced by chemical carcinogens, which mimic the occurrence of human cancer from the beginning of the carcinogenic process30. However, the main disadvantage of this method is that the modeling cycle is extremely long, requiring 30–50 weeks31. The cell line-derived xenograft (CDX) model refers to a xenograft model produced by subcutaneously injecting cancer cell lines into immunodeficient mice32. The establishment of this model is simple and time-saving, but after long-term in vitro culture, the biological behavior and tumor heterogeneity of the tumor cell line are very different from the original tumor tissue33. The existing mouse models used in the study of asbestos carcinogenicity are mainly based on the first two methods. Establishing an efficient, time-saving and effective animal model is a problem that needs to be solved at present.
Hypoxia-inducible factor-1 α (HIF-1α) is a key transcription factor for cancer progression and targeted cancer therapy34, and, it has been shown to play a role in the mechanism of asbestos carcinogenesis35. The role of HIF-1α varies depending on the presence or absence of oxygen. In an aerobic environment, HIF-1α is completely inactivated and destroyed by the ubiquitin proteasome pathway (UPP). Conversely, it escapes destruction and enters the cell nucleus, where it then upregulates many genes involved in cancer progression. Overexpressed HIF-1α and downstream genes support cancer progression through various mechanisms, including angiogenesis, cell proliferation and survival, metabolic reprogramming, invasion and metastasis, cancer stem cell maintenance, induction of genetic instability, and therapeutic resistance34,36. There is evidence that asbestos activates HIF-1α by inducing reactive oxygen species and hypoxic stress37. Therefore, the development and progression of asbestos-related cancers can be modulated by targeting HIF-1α and its downstream signaling molecules. In this regard, HIF-1α inhibitors are classified as drugs that regulate HIF-1α at the gene, mRNA, and protein levels, and are used as an effective approach for cancer therapy.
The main objectives of this study were to establish a novel in vitro three-dimensional co-culture model of chrysotile-induced malignant transformation of mouse embryonic fibroblasts and to use these transformed cells to establish a mouse orthotopic lung cancer model. To validate the biological relevance and predictive power of this newly established in vitro-in vivo model system, we used the HIF-1α inhibitor PX-478. This choice was based on evidence linking HIF-1α pathway dysregulation to asbestos-related carcinogenesis. That is, changes in HIF-1 expression can affect the malignant phenotype of tumor cells (including proliferation, migration, invasion, etc.). Ultimately, the inhibitory effects of PX-478 observed in our model recapitulated key aspects of the drug response associated with HIF-1α inhibition documented in previous in vivo studies and clinical observations. Overall, this comprehensive model system provides a reliable platform for studying the carcinogenicity of chrysotile and facilitates future mechanistic studies of mineral particle- and fiber-induced lung cancer.
Materials and methods
Cell culture and transfection
The Mouse Embryonic Fibroblast Cell line NIH/3T3 was obtained from the National Collection of the Authenticated Cell Cultures (China). Cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) at 37 °C in a humidified incubator with 5% CO2. Cells were passaged every 2–3 days using 0.25% trypsin-EDTA.
A 24-well plate was seeded with 5 × 104 cells in each well. A puromycin killing curve was constructed by adding the prepared screening medium containing 0, 3, 6, 9, 12, and 15 µg/ml puromycin after an overnight culture in an incubator with a constant temperature. The screening medium was then prepared by choosing the proper puromycin concentration based on this information. Three duplicates of each batch of cells were sown in a 24-well plate. After an overnight culture in an incubator set at a constant temperature, the transfection medium was added. This was made using complete medium, lentivirus dilution, and Polybrene solution at a ratio of 7:2:1. After 12 h, we threw away the infected media and used ordinary medium in its stead. The successfully transfected cells were screened after 32 h of growth, and the screened cells were then grown, cultured, and properly frozen. The screening medium was then changed.
Asbestos preparation
The standard chrysotile asbestos of the International Union for Cancer Control (UICC) was donated by the Japan Mineral Fiber Association. When it was used, 5 mg chrysotile fiber was suspended in 1 ml PBS to prepare 5 mg/ml stock suspension, which was ultrasonically treated in ice water to become a uniform suspension. Ultrasonic conditions: 180 W, 10 s on, 5 s off, a total of 30 cycles38. Sterilized by autoclaving after ultrasound.
Establishment of carcinogenic model of chrysotile asbestos under 3D condition
The matrix gel was equilibrated at 4 °C overnight before initiating malignant transformation. Cells were dissociated using 0.25% trypsin-EDTA upon reaching 70–80% confluency, followed by homogenization into a single-cell suspension. The cells were resuspended in a complete culture medium to ensure that the cell density was set at 1 × 106 cells/mL. After mixing the cell suspension and matrix gel at a 1:1 ratio, 200 µL of the mixture was plated to a 24-well plate. To prevent solidification, all experiments were performed on ice. After 30–45 min of incubation at 37 °C, 500 µL of culture medium was added to each well to complete the plate. After the cells were injected for 24 h, the culture medium was discarded, diluted chrysotile fibers were added to the transformed cell group, and the control group was treated with an equal amount of PBS, ensuring that each group had three parallel samples. Transfer to regular culture media and continue cultivation after 48 h. After seven days, the medium was changed. Chrysotile fibers should be administered for 48 h before switching to a fresh culture medium. The medium was changed after 7 more days. After seven more days, the cells were collected for a total of twenty-one days. NIH/3T3 cells underwent malignant transformation after three rounds of toxicity, and the cells were extracted.
Cell proliferation assay
Cells were seeded into 96-well plates at a density of 2,000 cells per well and cultured under standard conditions (37 °C, 5% CO2) for 24 h. Then, we added Cell Counting Kit-8 reagent (CCK8, Dojindo, Japan) and incubated the plate for 2 h in a cell culture incubator. We then measured the absorbance at 450 nm using a microplate reader (Thermo Scientific, USA).
Soft agar clone formation experiment
Anchorage-independent growth was tested as follows: a base layer of 1.2% agar in medium (DMEM plus 20% FBS and 2 × antibiotics) was plated in six-well plates and allowed to solidify. Next, wells were overlaid with 200 cells per well in a 0.6% agar. The plates were incubated at 37 °C, 5% CO2 for 10–14 days and checked every 2–3 days for colony formation. Colonies were counted in the entire dish by using a microscope.
Cell cycle and apoptosis analysis
The cells were collected and then harvested and stained according to the manufacturer’s instructions. For cell cycle detection, a Cell Cycle Staining Kit (Beyotime, China) was used, whereas for apoptosis detection, an Annexin V-FITC Apoptosis Kit (Beyotime, China) was used. After staining, the cells were analysed and counted using flow cytometer (BD FACSCalibur, USA).
Wound healing assay
NIH/3T3 were inoculated and cultured in 6-well plates. Using the ruler as a reference, cell layer was created a wound with a 10 µL pipette. After cultured with serum-free medium for 0 h, 12 h, 24 h and 48 h, cell wound area was photographed and the migration distance was counted by detecting wound area at 0 h, 12 h, 24 h and 48 h.
Transwell assay
The migration and invasion abilities of cells were detected by Transwell assay. In brief, 6 × 105 cells were seeded into the upper chamber (8 μm pore size, 24-well) of the transwell apparatus in serum-free medium, and medium supplemented with 10% FBS was added to the bottom chamber. After 24–48 h, the cells on the upper surface that did not pass through the 8-µm pore-size polycarbonate filter were removed using a moistened cotton swab; the cells migrating to the lower membrane surface were fixed in 100% methanol for 20 min, stained with 0.1% crystal violet for 20 min, and counted under a microscope (Nikon). The invasion assay was performed as described in the migration assay, except that the upper chamber was precoated with 50 µl of a matrigel solution.
Western blotting
The protein of cells was obtained using the RIPA cell lysate (Beyotime), and the lung tissue was thoroughly ground in liquid nitrogen, lysed, centrifuged, and proteins were extracted. The Bicinchoninic Acid Assay (BCA) method was used to measure the protein concentration, and the absorbance of each well at 562 nm was measured using a microplate reader (Thermo Scientific, USA). After protein pretreatment, western blotting was performed to detect the protein expression. The primary antibody diluents were prepared at a 1:1000 ratio. The prepared antibody diluent was added to an incubation box containing polyvinylidene fluoride (PVDF) membrane, and sealed overnight at 4 °C or on a shaking table at room temperature for 60 min. The dilution solution of the first antibody was retrieved, the membrane was placed in Tris-buffered saline with Tween-20 (TBST) solution, washed at room temperature on a shaking table for 10 min, washed three times, diluted with the secondary antibody at a dilution ratio of 1:5000, prepared the secondary antibody incubation solution, and incubated at room temperature on a shaking table for 60 min. After incubation with the secondary antibody, the PVFD membrane was cleaned three times on a shaking table with TBST solution for 10 min each. Solution A and solution B were mixed in a 1:1 ratio in the ultra-sensitive ECL chemiluminescence reagent kit (Beyotime, China), take 1mL and evenly applied near the band of the PVDF film, and developed within 10 min. ImageJ plugin version measures band protein content.
Animal experiments
All methods were carried out in accordance with relevant guidelines and regulations set forth by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International and are reported by ARRIVE guidelines. The Institutional Animal Care and Use Committee (IACUC) of Hangzhou Medical College reviewed and approved all experiments before their initiation (IACUC, ZJCLA Protocol No. ZJCLA-IACUC-20020084; Approval Date (3/8/22). Nude mice were obtained from the Hangzhou Medical College Experimental Animal Center (Hangzhou, China). All animals were housed under controlled room temperature (22 ± 1 °C), with a 12:12 h light-dark cycle, and fed a standard rodent diet and water. All animals received humane care according to the institutional animal care guidelines approved by the Experimental Animal Ethics Committee of Hangzhou Medical College. Five mice were selected, and the first part of chrysotile malignant transformed NIH/3T3 cells were injected into the lungs; The behavior, weight and signs of mice were observed regularly. After 4 weeks, 1% pentobarbital sodium solution was injected intraperitoneally for anesthesia, and then they were euthanized by cervical dislocation. All mice were taken for pathological examination to determine the tumor and metastasis.
In the study of the effects of PX-478 inhibitors, there were 12 mice in the control group and 12 mice in the experimental group, and the first part of NIH/3T3 cells with malignant transformation of chrysotile were injected into the lungs. This part of the mice was euthanized in the same way as above after 4 weeks. Samples were stored at − 80 °C until processing.
Mouse live imaging
Prior to luciferase substrate administration, mice were weighed to calculate the injection volume. A dose of 200 µL luciferin sodium salt solution (15 mg/mL in PBS) per 20 g body weight was administered via intraperitoneal injection. After injecting the luciferase substrate for 5 min, anesthesia was induced with isoflurane gas. After anesthesia, a small animal live-imaging device was used for live imaging, and the fluorescence intensity in the body was measured. After the mice wake up, they are placed back in the cage and undergo live imaging every seven days. When the mice died after the fourth imaging session, they were euthanized. The lung tissue of the mice was cut open, half was soaked in 4% paraformaldehyde for fixation, and the other half was quickly frozen in liquid nitrogen and transferred to a − 80 °C freezer for storage.
Statistical analysis
In this study, the data are presented as the mean ± standard deviation. ANOVA was used for analysis and testing among multiple groups, and the obtained data followed a normal distribution. When the P-value was less than 0.05, there was a difference between the groups, and the results were statistically significant.
Results
Construction of malignant transformation cell model by NIH/3T3 cells induced by low concentration chrysotile asbestos
Various concentrations of asbestos were utilized to induce short-term toxicity in NIH/3T3 cells cultured in both 2D and 3D environments for 48 h. Cell proliferation decreased progressively with increasing asbestos concentration. At 0.625 µg/cm2, cell proliferation rates were 94.2% in 2D and 97.2% in 3D, while at 1.25 µg/cm2, rates were 86.4% in 2D and 93.4% in 3D, Fig. 1A. A concentration of 0.625 µg/cm2 was selected for constructing a 3D cell model (designated as 3D-Asb for the chrysotile exposure group and 3D-CT for the control group) based on the comparative effects of different asbestos concentrations on cell proliferation in the two conditions. Following induction with low concentrations of chrysotile in 3D conditions, a soft agar clone formation assay was conducted to assess the establishment of a malignant transformation cell model. The findings demonstrated that the 3D-Asb group exhibited anchorage-independent growth, confirming the successful construction of the malignant transformation cell model, with a significantly higher clone formation rate compared to the 3D-CT group (P < 0.01), Fig. 1B. Subsequently, tumor phenotype experiments were performed on the malignant transformation cell model to further validate its malignancy. Figure 1C illustrates a substantial decrease in apoptosis in the 3D-Asb group in comparison to the 3D-CT group (P < 0.01), indicating apoptosis resistance. There was no significant change in cell proliferation ability (Fig. 1D). Figure 1E shows an increased proportion of 3D-Asb cells in the G1 phase and a shortened proportion in the S + M phase compared to control cells, indicating that the proportion of asbestos-transformed cells blocked in the G1 phase is increased. Additionally, the 3D-Asb group displayed enhanced wound healing (P < 0.01), Transwell migration (P < 0.01), and Transwell invasion (P < 0.001) capabilities relative to the 3D-CT group (Fig. 1F, G and H), suggesting the acquisition of a malignant phenotype by chrysotile-exposed cells.
Under 3D conditions, asbestos induces malignant transformation in NIH/3T3 cells. (A) Cytotoxicity of chrysotile asbestos in 2D and 3D conditions (* indicates P < 0.05, *** indicates P < 0.001). Malignant phenotypes of 3D-CT and 3D-Asb cells, including (B) cellular ability to form clones (** indicates P < 0.01), (C) apoptosis rate (** indicates P < 0.01), (D) proliferative capacity, (E) cell cycle profiling (** denotes P < 0.01), (F) migration capacity (** denotes P < 0.01), and (G) Transwell invasion (** denotes P < 0.01) and (H) migration capacity (*** denotes P < 0.001) were partially different.
Establishment of nude mouse transformed cell lung cancer model
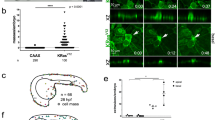
Following the successful establishment of the cell model, an animal model was subsequently developed. Nude mice were inoculated with malignant transformed cells labeled with fluorescent proteins and intraperitoneally administered with luciferase substrate for in vivo imaging on days 7, 14, and 21 post-injection. Imaging results revealed conspicuous fluorescence in the lungs of the mice on day 7, with a progressive increase in lung fluorescence intensity on days 14 and 21. The fluorescence intensity in the mouse lungs exhibited a gradual rise over time, as depicted in Fig. 3A and C. Subsequent to the third imaging session, one mouse expired, prompting the euthanization of the remaining mice. Comparative analysis unveiled notable alterations in the morphology of the mouse lung tissue, characterized by adhesions to the chest wall and the presence of irregular tumors in both lungs, accompanied by the development of translucent fish-like tumors.
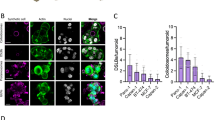
Effect of HIF-1α inhibition on the malignant phenotype of malignantly transformed cells and changes in the expression of EMT-related proteins
After 24 h of inhibitor treatment, the cell proliferation capacity of the PX-478-20µM group significantly decreased compared to the control group (P < 0.05). Subsequently, following 48 h of inhibitor treatment, both the PX-478-10µ M and PX-478-20 µM groups exhibited a significant reduction in cell proliferation compared to the control group (P < 0.001), as depicted in Fig. 2A and B. The results of the scratch test indicated a notable decrease in the relative migration rates of the PX-478 inhibitor groups at concentrations of 5 µM, 10 µM, and 20 µM compared to the control group at 12 h, 24 h, and 36 h (P < 0.05), as illustrated in Fig. 2C. Furthermore, the Transwell invasion assay demonstrated a reduction in the number of invasive cells in the PX-478 inhibitor groups at concentrations of 5 µM, 10 µM, and 20 µM compared to the control group (P < 0.001), with a decrease in invasive cells corresponding to an increase in PX-478 concentration (Fig. 2D). The Transwell migration assay results revealed a decrease in the number of migrating cells in the PX-478 inhibitor groups at concentrations of 5 µM, 10 µM, and 20 µM compared to the control group (P < 0.001), with a decrease in migrating cells corresponding to an increase in PX-478 inhibitor concentration, as shown in Fig. 2E. Various concentrations of PX-478 were administered to the cells in the 3D-Asb group. After 24 and 48 h of treatment, total cell protein was extracted, and EMT-related proteins were quantitatively analyzed using Western Blot. Following 24 h of inhibitor treatment, the N-Cadherin protein expression in the cells across all concentration groups remained relatively stable (Fig. 2F), while the TWIST protein expression decreased in the PX-478-20 µM group compared to the control group(Fig. 2G). After 48 h of inhibitor treatment, the TWIST protein expression in the PX-478-20 µM group decreased compared to the control group. Across the three PX-478 concentration groups, the expression of TWIST protein exhibited a decreasing trend with increasing inhibitor concentration. Furthermore, there were no significant changes in Vimentin protein expression in malignantly transformed cells after 24 and 48 h of inhibitor treatment (Fig. 2H).
The effect of the PX-478 on the malignant phenotype of 3D-Asb cells. Effects of different concentrations of PX-478 on cell proliferation after (A) 24 h of treatment, and (B) 48 h of treatment (*P < 0.05, ***P < 0.001). and on (C) migration (*P < 0.05, **P < 0.01, ***P < 0.001) and (D) Transwell invasion (*** indicates P < 0.001), (E) Transwell migration (*** indicates P < 0.001) ability. Also, the effect of PX-478 on the expression of EMT-associated proteins (F) N-Cadherin, (G) TWIST (* indicates P < 0.05), and (H) Vimentin.
Effects of PX-478 treatment on fluorescence intensity of in vivo imaging and expression of EMT-related proteins in nude mice
Fluorescent protein-labeled malignant transformed cells were first injected into nude mice, followed by PX-478 injection for 5 consecutive days. In vivo fluorescence imaging was performed on the 7th, 14th, 21st and 28th days after the intrapulmonary injection. Obvious fluorescence signals appeared in the chest of nude mice. Over time, the intensity of the fluorescence signal increased and the range became larger. On the 28th day, the fluorescence intensity of the lungs of nude mice was significantly higher than that of the previous three imagings, indicating that asbestos-induced malignant transformed cells survived and proliferated and migrated in the lungs of mice (Fig. 3F). The weight of mice was measured and recorded every two days, and the weight changes of the two groups of mice were not obvious (Fig. 3E). After 28 days, the mice were killed, and it was seen that the morphology of their lungs had changed significantly, the lung tissue had lost its original morphology, and solid tumors had grown. When the lungs were peeled off from the chest wall, it was seen that the deformed lungs were severely adhered to the chest wall, and some tumors invaded the chest wall tissue deeply and were tightly adhered to the ribs (Fig. 3D). The results of in vivo fluorescence imaging of the two groups of mice showed that the fluorescence range of the mouse lungs gradually increased. The fluorescence intensity and range of variation of the three groups of mice were small on the 7th, 14th and 21st days. On the 28th day, the fluorescence intensity of the mice increased significantly (Fig. 3G).
In vivo experiments in mice. After intra-lung injection of fluorescently labeled 3D-Asb cells, (A) in vivo fluorescence imaging of nude mice was performed to observe (B, D) the morphology of lung tissue in nude mice. (C) Evaluation of the total fluorescence intensity in the lungs of mice on days 7, 14 and 21 after injection. (E)Changes in body weight of nude mice, and (F) changes in total fluorescence intensity in lungs were monitored. (G) Four in vivo fluorescence imaging maps of control and PX-478 group mice and changes in fluorescence intensity. (H) The fluorescence intensity profiles were recorded on days 7, 14, 21 and 28 after intrapulmonary injection of fluorescent markers in both groups of mice, respectively (** indicates P < 0.01, *** indicates P < 0.001). (I) The expression of N-calmodulin, waveform protein, and TWIST protein was examined in the lung tissues of mice after the mice were executed.
After statistical analysis of the fluorescence intensity of the mice at the four time points, it can be seen that on the 7th day, there was no significant difference in the fluorescence between the two groups of mice (Fig. 3H). On the 14th day, the fluorescence imaging of the living mice showed that the fluorescence intensity of the PX-478 group of mice was lower than that of the control group, and the difference was statistically significant. The results of in vivo fluorescence imaging of the mice on the 21st day showed that the fluorescence intensity of the lungs of the PX-478 group of mice was lower than that of the control group, and the difference was statistically significant. After in vivo imaging of the mice on the 28th day, it can be seen that compared with the control group mice, the fluorescence intensity of the PX-478 group of mice decreased, and the difference was statistically significant. There was no significant change in the expression of N-Cadherin protein in the lung tissue of mice, the expression of TWIST protein in mice showed a downward trend, which was not statistically significant, and the expression of Vimentin protein showed a downward trend, which was not statistically significant (Fig. 3I).
Discussion
Chrysotile can induce malignant transformation of NIH/3T3 cells under 3D conditions
Compared with traditional 2D culture, 3D culture models can translate research results into in vivo applications because they simulate natural physiological properties and conditions39,40,41. This novel approach makes it possible to bridge the gap between in vitro and in vivo models through new and alternative biotechnologies, such as the use of hydrogels that can simulate extracellular matrix (ECM) behavior and growth factor activity42. Our CCK-8 assay showed that the survival rate of cells cultured under 2D conditions was significantly lower than that of 3D cultured cells exposed to the same concentration of asbestos. This is because the response of cells to toxicants in 3D systems is different from that in 2D systems43. Cells in 3D systems are less susceptible to toxicants, while 2D cells are the opposite. Cells form a three-dimensional structure with sufficient intercellular connections in the culture medium, which more effectively restores the cell microenvironment seen in vivo44. In this special environment, the range of cell development is expanded, and the area of contact with toxicants is also correspondingly enlarged.
Under normal circumstances, human body inhales asbestos multiple times. We used multiple asbestos exposure methods in the construction of 3D cell models to simulate the way human body is exposed to asbestos. In the human body, asbestos fibers that reach the pleura will be retained for a long time45,46, and cause long-term inflammation due to the frustration of phagocytosis. At the same time, the carcinogenicity of asbestos has a significant dose-response relationship with the equivalent fiber cumulative exposure index (CEI) of asbestos. High levels of asbestos exposure may lead to a significant increase in the risk of malignant tumors such as pleural mesothelioma47, esophageal cancer48, and lung cancer49, as well as an increase in mortality50,51. A cohort study showed that the relative risk of pleural and peritoneal mesothelioma, lung cancer, and colorectal cancer associated with asbestos exposure was related to the intensity of exposure (or average exposure level, AEL) after adjusting for age as a time-dependent variable and sex52. In a population-based asbestos exposure study, we found that the risk of death from mesothelioma increased with cumulative asbestos exposure53. Based on the above evidence, the 3D cell model we designed is in line with human reality and has a certain degree of reliability.
In traditional studies of asbestos carcinogenic mechanisms, 2D cell models have always played an important role. This study applied the 3D culture method to the construction of an asbestos carcinogenic mechanism model for the first time. Through multi-stage chrysotile exposure, a chrysotile-induced NIH/3T3 malignant transformation cell model was successfully established. Malignantly transformed NIH/3T3 cells have a certain degree of malignancy, as well as enhanced migration, invasion, and anti-apoptosis capabilities.
Malignant transformed cells can successfully establish a mouse lung cancer model
In order to study the direct carcinogenic effect of chrysotile asbestos, the traditional method of inducing lung cancer in mice mainly involves inhaling or dripping chrysotile directly into the animal’s trachea54, inducing lung tissue tumor formation. The required exposure time is prolonged, the technical requirements for operation are high, and the degree of tumor development is difficult to judge55. We used cells constructed by a 3D system that is closer to the in vivo growth mode as cell-derived transplants, and successfully completed the establishment of a mouse model in only about 4 weeks, greatly shortening the modeling cycle. We performed orthotopic transplantation by intrapulmonary injection to form tumors in the mouse lungs, making the type of tumor studied more specific.
The animal model we constructed not only has a short experimental cycle, but also can well reflect the characteristics of asbestos-induced lung cancer. The orthotopic transplantation of transformed cells by intrapulmonary injection increased the implantation rate and made the tumor site simulated by the mouse model clearer and more specific. We used lentivirus to label the cells, so that the growth of tumors in the mouse model could be observed in stages. The results showed that after real-time imaging, obvious fluorescence appeared in the lungs of the model mice, and the fluorescence range and intensity of the lung tissue gradually expanded and increased over time. After three weeks, tumor development was observed in the lungs of all model animals. Compared with existing models, the newly established model has the advantages of being able to track malignant transformed cells, shortening modeling time, and enhancing model reproducibility. Therefore, the animal model we established is not only suitable for the study of asbestos carcinogenic mechanism, but also provides a reference for the study of carcinogenic mechanism of other poisons.
PX-478 plays a certain role in two asbestos carcinogenic models
To further verify the robustness and external applicability of the model, we used the HIF-1α inhibitor PX-478. The results of cell experiments showed that PX-478 could significantly inhibit the proliferation, migration and invasion of malignant transformed cells. In animal experiments, the intervention of PX-478 significantly reduced tumor volume, inhibited mediastinal metastasis, and prolonged the survival of mice56. In other studies, PX-478 has antitumor activity against a variety of human tumor xenografts (including non-small cell lung cancer, colon cancer, prostate cancer, etc.) in immunodeficient mice, and is positively correlated with HIF-1α levels57. PX-478 was originally identified by screening compounds that reduce cellular HIF-1α levels, and it significantly inhibits tumors, shortens tumor growth cycles, and cures some animals58. Therefore, the effect of PX-478 on our model is consistent with existing factual results.
Further analysis found that in both models, PX-478 could specifically downregulate the expression of TWIST protein, even though the results were not significant in the mouse model. As a core regulator of EMT, TWIST promotes tumor progression by inhibiting cell apoptosis and senescence and inducing treatment resistance59,60,61,62. In this study, the reduction of TWIST was closely associated with the inhibition of cell malignant phenotype, the reduction of tumor metastasis in mice, and the extension of survival, suggesting that PX-478 may exert anti-tumor effects by targeting the EMT pathway. In addition, in vivo imaging showed that the fluorescence intensity of lung tissue in the PX-478 group of mice was significantly lower than that in the control group, which is consistent with the results of Su et al. in their study of prostate cancer63. It is worth noting that despite the inhibition of TWIST expression, the levels of N-cadherin and vimentin proteins did not change in both models.
Conclusion
In this study, we constructed two models at the same time, including a cell model and a mouse model. Among them, the cell model uses a 3D culture system to simulate the way asbestos fibers enter the human body through multi-stage low-concentration asbestos poisoning. This modeling form is reliable. On the basis of the cell model, we use fluorescent labeling to make the cells self-labeled. The nude mouse model obtained by in-vitro implantation into the mouse body can intuitively and regularly observe the modeling situation, tumor size, tumor formation rate, etc. In addition, our nude mouse model has a short modeling time, a high correlation with the poison, and can be observed in real time. Based on the above advantages, our model can play an important role in the future asbestos carcinogenic mechanism, and at the same time, it also provides a reference for the carcinogenic model of other poisons.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
IARC monographs on the evaluation of the carcinogenic risk of chemicals to man: asbestos. IARC monographs on the evaluation of the carcinogenic risk of chemicals to man, Vol. 14, 1–106 (1977).
Scherpereel, A. et al. ERS/ESTS/EACTS/ESTRO guidelines for the management of malignant pleural mesothelioma. Eur. Respir. J. 55(6), 1900953 (2020).
Broaddus, V. C. et al. Non-neoplastic and neoplastic pleural endpoints following fiber exposure. J. Toxicol. Environ. Health B 14(1–4), 153–178 (2011).
Case, B. W. et al. Applying definitions of asbestos to environmental and low-dose exposure levels and health effects, particularly malignant mesothelioma. J. Toxicol. Environ. Health B 14(1–4), 3–39 (2011).
Huang, S. X. L. et al. Role of mutagenicity in asbestos fiber-induced carcinogenicity and other diseases. J. Toxicol. Environ. Health B 14(1–4), 179–245 (2011).
Mossman, B. T. et al. Pulmonary endpoints (lung carcinomas and asbestosis) following inhalation exposure to asbestos. J. Toxicol. Environ. Health B 14(1–4), 76–121 (2011).
Institute of Medicine (US) and National Research Council (US) Committee for the Review of the NIOSH Research Roadmap on Asbestos Fibers and Other Elongate Mineral Particles. A Review of the NIOSH Roadmap for Research on Asbestos Fibers and Other Elongate Mineral Particles (National Academies Press (US), 2009).
Lanphear, B. P. & Buncher, C. R. Latent period for malignant mesothelioma of occupational origin. J. Occup. Med. 34(7), 718–721 (1992).
Lemen, R. A. Mesothelioma from asbestos exposures: Epidemiologic patterns and impact in the united States. J. Toxicol. Environ. Health B 19(5–6), 250–265 (2016).
Bononi, A. et al. Latest developments in our understanding of the pathogenesis of mesothelioma and the design of targeted therapies. Expert Rev. Respir. Med. 9(5), 633–654 (2015).
Creaney, J. & Robinson, B. W. S. Malignant mesothelioma biomarkers: From discovery to use in clinical practice for diagnosis, monitoring, screening, and treatment. Chest 152(1), 143–149 (2017).
Fennell, D. A. et al. Advances in the systemic therapy of malignant pleural mesothelioma. Nat. Clin. Pract. Oncol. 5(3), 136–147 (2008).
Peto, J. et al. The European mesothelioma epidemic. Br. J. Cancer 79(3–4), 666–672 (1999).
Kazan-Allen, L. The international ban asbestos secretariat. Int. J. Occup. Environ. Health 6(2), 164 (2000).
Chen, T., Sun, X. M. & Wu, L. High time for complete ban on asbestos use in developing countries. JAMA Oncol. 5(6), 779 (2019).
Olsen, N. J. et al. Increasing incidence of malignant mesothelioma after exposure to asbestos during home maintenance and renovation. Med. J. Aust. 195(5), 271–274 (2011).
Wei, B. et al. Concentrations of asbestos fibers and metals in drinking water caused by natural crocidolite asbestos in the soil from a rural area. Environ. Monit. Assess. 185(4), 3013–3022 (2013).
Baumann, F. et al. The presence of asbestos in the natural environment is likely related to mesothelioma in young individuals and women from Southern Nevada. J. Thoracic Oncol. 10(5), 731–737 (2015).
Carbone, M. et al. Erionite exposure in North Dakota and Turkish villages with mesothelioma. Proc. Natl. Acad. Sci. U.S.A. 108(33), 13618–13623 (2011).
Arachi, D. et al. Development of the national asbestos profile to eliminate asbestos-related diseases in 195 countries. Int. J. Environ. Res. Public Health 18(4), 1804 (2021).
Aryal, A. & Morley, C. Call for a global ban policy on and scientific management of asbestos to eliminate asbestos-related diseases. J. Public Health Policy 41(3), 279–285 (2020).
Markowitz, S. B. Lung cancer screening in asbestos-exposed populations. Int. J. Environ. Res. Public Health 19(5), 2688 (2022).
Cortesi, E. In Vitro Studies on Chemical Carcinogenesis in BALB/c 3T3 Cells[M]//The Use of Human Cells for the Evaluation of Risk from Physical and Chemical Agents 767–771 (Springer, 1983).
Wang, H. et al. 3D cell culture models: Drug pharmacokinetics, safety assessment, and regulatory consideration. Clin. Transl. Sci. 14(5), 1659–1680 (2021).
Wang, M. et al. Effect of three-dimensional ECM stiffness on cancer cell migration through regulating cell volume homeostasis. Biochem. Biophys. Res. Commun. 528(3), 459–465 (2020).
Kandir, S., Animal Models for Cancer Research, Pathak, S., Banerjee, A. & Bisgin, A. The choice of the right model system. In Handbook of Animal Models and its Uses in Cancer Research. 1–16 (Springer Nature, Singapore, 2022).
Jubelin, C. et al. Three-dimensional in vitro culture models in oncology research. Cell Biosci. 12(1), 155 (2022).
Habanjar, O. et al. 3D cell culture systems: Tumor application, advantages, and disadvantages. Int. J. Mol. Sci. 22(22), 12200 (2021).
Zhou, R. et al. Vascularised organoids: Recent advances and applications in cancer research. Clin. Transl. Med. 15(3), e70258 (2025).
Li, Z. et al. Application of animal models in Cancer research: Recent progress and future Prospects. Cancer Manage. Res. 13, 2455–2475 (2021).
De Minicis, S. et al. Liver carcinogenesis: Rodent models of hepatocarcinoma and cholangiocarcinoma. Dig. Liver Dis. 45(6): 450–459. (2013).
Brennecke, P. et al. CXCR4 antibody treatment suppresses metastatic spread to the lung of intratibial human osteosarcoma xenografts in mice. Clin. Exp. Metastasis 31(3), 339–349 (2014).
Ye, F. et al. Genetic profiling reveals an alarming rate of cross-contamination among human cell lines used in China. FASEB J. 29(10), 4268–4272 (2015).
Semenza, G. L. Defining the role of Hypoxia-Inducible factor 1 in cancer biology and Therapeutics. Oncogene 29(5), 625–634 (2010).
Kim, M. C., Cui, F. J. & Kim, Y. Hydrogen peroxide promotes epithelial to mesenchymal transition and stemness in human malignant mesothelioma Cells. Asian Pac. J. Cancer Prev. 14(6), 3625–3630 (2013).
Balamurugan, K. HIF-1 at the crossroads of hypoxia, inflammation, and cancer. Int. J. Cancer 138(5), 1058–1066 (2016).
Wu, H. et al. Mitochondrial dysfunction promotes the transition of precursor to terminally exhausted T cells through HIF-1α-mediated glycolytic reprogramming. Nat. Commun. 14(1), 6858 (2023).
Ju, L. et al. miR-30d is related to asbestos exposure and inhibits migration and invasion in NCI-H2452 cells. FEBS Open. Bio. 7(10), 1469–1479 (2017).
Ravi, M. et al. 3D cell culture systems: Advantages and applications. J. Cell. Physiol. 230(1), 16–26 (2015).
Antoni, D. et al. Three-dimensional cell culture: A breakthrough in vivo. Int. J. Mol. Sci. 16(3), 5517–5527 (2015).
Horning, J. L. et al. 3-D tumor model for in vitro evaluation of anticancer drugs. Mol. Pharm. 5(5), 849–862 (2008).
Cacciamali, A., Villa, R. & Dotti, S. 3D cell cultures: evolution of an ancient tool for new applications. Front. Physiol. 13, 836480 (2022).
Wang, H., Xu, T. & Yin, D. Emerging trends in the methodology of environmental toxicology: 3D cell culture and its applications. Sci. Total Environ. 857, 159501 (2023).
Sun, M. et al. 3D cell culture—Can it be as popular as 2D cell Culture?. Adv. NanoBiomed. Res. 1(5), 2000066 (2021).
Dörger, M. et al. Differential responses of rat alveolar and peritoneal macrophages to Man-Made vitreous fibers in vitro. Environ. Res. 85(3), 207–214 (2001).
Schinwald, A. & Donaldson, K. Use of back-scatter electron signals to visualise cell/nanowires interactions in vitro and in vivo; frustrated phagocytosis of long fibres in macrophages and compartmentalisation in mesothelial cells in vivo. Part. Fibre Toxicol. 9, 34 (2012).
Berge, L. A. M. et al. Exposure to fibres and risk of pleural mesothelioma in the Norwegian offshore petroleum workers cohort. Occup. Environ. Med. 81(7), 331–338 (2024).
Li, B., Tang, S. P. & Wang, K. Z. Esophagus cancer and occupational exposure to asbestos: Results from a meta-analysis of epidemiology studies. Dis. Esophagus 29(5), 421–428 (2016).
Metintas, M., Ak, G. & Metintas, S. Environmental asbestos exposure and lung cancer. Lung Cancer (Amsterdam Netherlands) 194, 107850 (2024).
Clin, B. et al. Asbestos exposure, pleural plaques and digestive cancers. BMC Public. Health 25(1), 686 (2025).
Huh, D. A. et al. Air pollution and survival in patients with malignant mesothelioma and asbestos-related lung cancer: A follow-up study of 1591 patients in South Korea. Environ. Health Glob. Access Sci. Source 23(1), 56 (2024).
Clin, B. et al. Cancer incidence within a cohort occupationally exposed to asbestos: A study of dose–response relationships. Occup. Environ. Med. 68(11), 832–836 (2011).
Otte, N. et al. Asbestos surveillance program Aachen (ASPA): Cancer mortality among asbestos exposed power industry workers. Lung Cancer (Amsterdam Netherlands) 195, 107899 (2024).
Okazaki, Y. Asbestos-induced mesothelial injury and carcinogenesis: Involvement of iron and reactive oxygen species. Pathol. Int. 72(2), 83–95 (2022).
Kadariya, Y. et al. Modeling malignant mesothelioma in genetically engineered mice. Curr. Protocols 5(1), e70086 (2025).
Jacoby, J. J. et al. Treatment with HIF-1alpha antagonist PX-478 inhibits progression and spread of orthotopic human small cell lung cancer and lung adenocarcinoma in mice. J. Thoracic Oncol. 5(7), 940–949 (2010).
Welsh, S. et al. Antitumor activity and pharmacodynamic properties of PX-478, an inhibitor of hypoxia-inducible factor-1alpha. Mol. Cancer Ther. 3(3), 233–244 (2004).
Koh, M. Y. et al. Molecular mechanisms for the activity of PX-478, an antitumor inhibitor of the hypoxia-inducible factor-1α. Mol. Cancer Ther. 7(1), 90–100 (2008).
Kress, W. et al. Saethre-Chotzen syndrome caused by TWIST 1 gene mutations: Functional differentiation from Muenke coronal synostosis syndrome. Eur. J. Hum. Genetics: EJHG. 14(1), 39–48 (2006).
Yin, G. et al. Constitutive proteasomal degradation of TWIST-1 in epithelial-ovarian cancer stem cells impacts differentiation and metastatic potential. Oncogene 32(1), 39–49 (2013).
Norozi, F. et al. Twist as a new prognostic marker in hematological malignancies. Clin. Transl. Oncol. 18(2), 113–124 (2016).
Yuen, H. F. et al. TWIST modulates prostate cancer cell-mediated bone cell activity and is upregulated by osteogenic induction. Carcinogenesis 29(8), 1509–1518 (2008).
Palayoor, S. T. et al. PX-478, an inhibitor of hypoxia-inducible factor-1alpha, enhances radiosensitivity of prostate carcinoma cells. Int. J. Cancer 123(10), 2430–2437 (2008).
Acknowledgements
Sincerely thank the Japan Mineral Fiber Association for the kind donation of chrysotile asbestos fibers.
Funding
This work was supported by Natural Science Foundation of Zhejiang Province (LGD21C040008).
Author information
Authors and Affiliations
Contributions
Y.G. designed the study; Y.G., W.Y., F.Z. wrote the manuscript; Y.G., W.Y., L.Z. collected and analyzed the data, and wrote the manuscript; R.L., Q.W., M.Z., H.X., Y.Z. analyzed the data; F.Z. and L.Z. designed and supervised the study and provided financial support.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Nude mice were obtained from the Hangzhou Medical College Experimental Animal Center (Hangzhou, China). All animals received humane care according to the institutional animal care guidelines approved by the Experimental Animal Ethics Committee of Hangzhou Medical College (ZJCLA-IACUC-20020084).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gao, Y., Yu, W., Li, R. et al. Dual phase modeling of Chrysotile carcinogenesis from 3D cell transformation to orthotopic tumors. Sci Rep 16, 2199 (2026). https://doi.org/10.1038/s41598-025-08158-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-08158-0