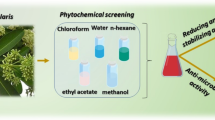
Abstract
Methanolic extraction (ME) of terminalia bentzoe leaves and its aqueous (QF) and n-butanol (nBF) fractions are investigated as sustainable green compounds for both anticorrosion and antimicrobial activity. Extracted and fractionated phytochemicals and their functional groups are detected by GC-MS and FTIR. According to the electrochemical results, ME, QF and nBF samples are considered as anodic inhibitors that mainly inhibit the anodic dissolution of copper (Cu) in 0.6 M NaCl solution. Their inhibition efficiencies increase by increasing their concentrations up to 60 ppm, and the highest value of inhibition efficiency is found to be 94.4% for nBF. The presence of the protective adsorbed layer from the extracted and fractionated phytochemical compounds onto the Cu surface was supported by FESEM analyses and theoretical. On the other hand, exploring the antimicrobial activity of ME, QF and nBF samples against Staphylococcus aureus, Bacillus cereus, Listeria monocytogenes, Escherichia coli, Salmonella typhi, Shigella boydii, Aspergillus niger and Candida albicans was made by using modified Kirby-Bauer disc diffusion technique. Data indicated good resistance against a variety of bacterial infections for the studied green compounds.
Similar content being viewed by others
Introduction
The extensive use of copper and its alloys in a variety of industrial applications, including decorations, electronics, building, industrial equipment, and transportation gave them an opportunity in many studies1,2. However, because of the use of high chloride ions in some aggressive conditions, particularly in a marine environment, copper can severely corrode3. Thus, copper corrosion protection has drawn the attention and several efforts have been devoted to develop an eco-friendly and effective copper corrosion inhibitors, including imidazoles, triazoles, and some schiff bases with N, O, S, P, polar functional groups, and/or conjugated double bonds4,5,6,7. On the other hand, plant extracts have received much attention, because they have anti-microbial and anti-corrosion properties. These properties can be explained, due to richness in bioactive compounds. The phytochemicals included in plant extracts have electron-rich regions and functional groups that enable them to adsorb powerfully on metal surfaces with very low concentrations. These phytochemicals contain tannins, phenolics, organic acids, amino acids, alkaloids, and flavonoids, which can be employed to prevent metallic components from corroding8,9. Many efforts have been occured recently to report on plant extracts’ ability to suppress metal corrosion growth10,11,12,13,14. Many studies show the inhibition effects of various plant extracts against the corrosion of Cu in acidic15,16,17,18,19,20 and saline solutions21,22,23,24,25,26,27.
Terminalia bentzoe is one of the medical plants that classified as the second largest genus in the Combretaceae, which is widespread in Egypt and other sub-tropical and tropical regions of the world. It has been reported that the plant extract of Terminalia is commonly rich in phenolics, flavonoids, alkaloids, triterpenoids, tannins and other compounds28. Because of these phytoconstituents, most of Terminalia species have multiple biological, pharmacological and medicinal activities29,30,31,32,33,34,35.
The main aim of this study is to examine the usage of the methanolic Terminalia bentzoe extract and its aqueous and n-butanol fractions as anti-corrosion and anti-microbial properties. Thus, the present work studies the extraction (methanolic), fractionation (aqueous and n-butanol) and elucidation of the Terminalia bentzoe leaves, and investigates their extracted and fractionated phytochemicals samples as sustainable green inhibitors against Cu corrosion in saline solution, using electrochemical measurements, surface analysis, and Monte Carlo (MC) simulation. In addition, modified Kirby-Bauer disc diffusion technique is used to inspect their effect as antimicrobial compounds against Staphylococcus aureus, Bacillus cereus, Listeria monocytogenes, Escherichia coli, Salmonella typhi, Shigella boydii, Aspergillus niger and Candida albicans.
Experimental
Materials and chemicals
Collected Terminalia bentzoe leaves from Giza Zoo, Cairo, Egypt and identified at the Ministry of Agriculture, Egypt was used in the study. Sodium chloride, methanol, n-Butanol were purchased from PIOCHEM laboratory chemicals Co., Egypt. The working electrode was made from Cu metal and the stock solutions of the studied compounds were prepared using distilled water.
Extraction, fractionation and chemical constituents
Fresh leaves were washed 3 times with 10 L of distilled water, then dried at room temperature for 3 days, and grounded for 4 h prior to extraction. 710 g of dried and grounded sample were thoroughly extracted with 10 L of 70% methanol at room temperature with constant shaking. 43 g of the whole extract was obtained after the vaporization of the solvent under reduced pressure. Initially, 38 g of the total extract is dissolved in 150 mL distilled water and then extracted with 150 mL of n-Butanol. Finally, the mixed extractions were dried under vacuum to produce 5.7 g sample from n-Butanol fraction and 12.2 g sample from aqueous fraction. Extraction and fractionation were graphically represented in Fig. 1.
The chemical composition of methanol extract (ME), aqueous fraction (QF) and n-butanol fraction (nBF) samples were performed using Trace GC-TSQ mass spectrometer (Thermo Scientific, Austin, TX, USA) with a direct capillary column TG–5MS (30 m × 0.25 mm × 0.25 μm film thickness). Initially, the temperature of column oven was adjusted at 50 °C, and then raised by 5 °C /min to 250 °C to be held for 2 min. Finally, the temperature raised to 300 °C by 30 °C /min and held for 2 min. The injector and MS transfer line temperatures were kept at 270 and 260 °C respectively. Helium was used as a carrier gas at a constant flow rate of 1.0 mL/min. The solvent delay was 4 min, and 1 µl of diluted samples are automatically injected by Auto sampler AS1300 coupled with GC in the split mode. Over the range of m/z 50–650 in full scan mode, EI mass spectra were collected at ionization voltages of 70 eV. The ion source temperature was fixed at 200 °C. Identification of the components was done by comparing their mass spectra with those of WILEY 09 and NIST 14 mass spectral database. Infrared measurements were passed out using a Bruker optics VERTEX 70 spectrophotometer, Germany.
Electrochemical experiments
Electrochemical tests in the absence and presence of ME, QF and nBF samples in 0.6 M NaCl were performed in a typical three-electrode configuration cell consisting of Cu (1.0 cm2) as working electrode (WE), saturated Ag/AgCl as reference electrode (RE), and counter electrode (CE) from Pt wire using Origalys OGS 200 potentiostat/galvanostat, France.
Potentiodynamic polarization curves were adjusted at a potential range between − 0.3 and 0.1 V (vs. Ag/AgCl) with scan rate of 1 mVs− 1. Electrochemical impedance spectroscopy (EIS) experiments were implemented at EOCP with AC voltage amplitude of 10 mV and a scanning frequency of 100 kHz to 0.1 Hz. The electrochemical measurements were recorded and fitted by OrigaMaster-5 software. Before each experiment, Cu working electrode was polished with fine grade emery paper (1200P then 2500P), cleaned, rinsed with distilled H2O, and lastly dried before each experiment.
Surface analysis
Morphological structure for the Cu samples after 3 days of immersion in 0.6 M NaCl in the absence and presence of 60 ppm of each of ME, QF and nBF samples were analyzed using scanning electron microscope JEOL-JSM-5600, Japan.
Monte Carlo simulation
Monte Carlo (MC) simulation was employed to determine the adsorption energy (ΔEads) of ME, QF and nBF samples on Cu box with a size of 10 nm (containing 994 atoms of copper) using Materials Studio 7 package36. The copper unit cell adopted a face-centered cubic structure with a length of 4.022 Å, possessing space group symmetry Fm-3 m, and crystal class m-3 m. The structural optimization is performed using the Condensed-Phase Optimized Molecular Potential for Atomistic Simulation Studies (COMPASS II) force field37,38. The convergence criteria for energy, force, stress, and displacement are set to 2.0 × 10− 5 kcal·mol− 1, 0.001 kcal·mol− 1·Å−1, 0.001 GPa, and 1.0 × 10− 5 Å, respectively. Both Ewald and atom-based methods were employed to handle electrostatic and van der Waals forces. The optimization process was executed using the Forcite Module. The MC simulation utilized the Adsorption Locator Module, based on simulated annealing with 10 temperature cycles and 100,000 Monte Carlo steps per cycle. The maximum and final temperatures were set to 1.0 × 105 K and 100 K, respectively. The Adsorption Locator Module utilizes the Metropolis MC method to seek the lowest-energy adsorption configurations. The construction of ME, QF and nBF samples to investigate their adsorption on Cu surface was designed according to the percentage of their phytochemical compounds derived from GC-MS analysis as presented in Table 1.
Antimicrobial activity
Bacteria testing
Gram-positive bacteria (Staphylococcus aureus, Bacillus cereus, and Listeria monocytogenes) and Gram-negative bacteria (Escherichia coli, Salmonella typhi, and Shigella boydii) were cultivated in nutritious broth for 24 h before testing for antibacterial activity. Aspergillus niger and Candida albicans were cultivated in potato dextrose broth for 48 h before using for antifungal activity. Using a modified Kirby-Bauer disc diffusion technique, the widths of the inhibition zones were determined in millimeters to determine the antibacterial activity of the examined samples39,40. Filter discs impregnated with water served as a negative control for antimicrobial activity, while Gentamycin (antibacterial agent) and nystatin (antifungal) standard discs served as positive control.
Determination of the minimum Inhibition concentrations (MICs)
Terminalia bentzoe extract and its fractions revealed significant antimicrobial activity in antimicrobial susceptibility tests. Using dimethyl sulfoxide (DMSO) as a solvent, the condensed extract was utilized to generate a stock solution of 10 mg/mL. The solution was diluted to achieve concentrations ranging from 3.12 to 50 mg/mL.
Results and discussions
Structural elucidation of of ME, QF and nBF samples
GC-MS analysis of ME, QF and nBF samples are performed using Trace GC-TSQ mass spectrometer, the retention time (RT), concentrations (% peak area) and molecular formula for the main phytochemicals presented in the studied samples are presented in Table 1.
Functional groups of the phytochemical active compounds presented in the studied ME, QF, and nBF samples are indicated using FTIR analysis as shown in Fig. 2. From the figure, it can be concluded that there is no drastic changes in the band positions, but some changes in the band intensities can be observed. The variation of the band intensities indicates the consistent percentages in the studied samples. Hence, the increase of transmittance means the decrease of the consistent construction. The infrared characteristic absorption bands and the transmittance for stretching and bending vibrations for C-H band modes, -COO- band modes, C-C band modes, CO-NH band modes and their assignment are summarized in Tables 2, 3, 4, 5 and 6).
Corrosion studies
Potentiodynamic polarization (PDP) measurements
PDP measurements are performed for Cu in 0.6 M NaCl solution in the absence and presence of various concentrations from 20 to 100 ppm of nBF, ME, and QF samples from – 0.3 to 0.1 V (vs. Ag/AgCl) with scan rate of 1 mVs− 1 at ambient temperature. The electrochemical polarization parameters are calculated and tabulated in Table 7. The inhibition efficiency (IE%) is estimated using the following equations41.
Where θ represents the surface coverage, \(\:{i}_{corr}^{o}\:\)and \(\:{i}_{corr}\) denote the corrosion current densities for the blank solution and the studied compounds, respectively.
Figure 3a–c represents the PDP of Cu in 0.6 M NaCl blank (free from inhibitors) and with different concentrations of ME, QF and nBF samples at ambient temperature. Results of Table 7 indicate that, the addition of different concentrations (20–100 ppm) of ME, QF, and nBF samples to 0.6 M NaCl solution shifts the corrosion potential (Ecorr) of Cu to more noble values, which agrees with the open circuit measurements, and reduces its corrosion current densities (Icorr) compared to the blank solution, indicating that the ME, QF and nBF samples are considered as anodic inhibitors that mainly inhibit the anodic dissolution of Cu. This effect is interpreted bases of the adsorption of the phytochemical compounds in ME, QF and nBF samples on the anodic site of the Cu surface which forms a barrier adsorbed layer that blocks the active anodic sites in Cu surface. On the other hand, the values of the calculated IE% of the studied compounds increase with increasing their concentrations with the following order: QF ˂ ME ˂ nBF samples, and the maximum value is found to be 94.4% for 60 ppm of nBF. This order can be correlated to the orientation of the adsorbed phytochemical active compound on Cu surface, which will be discussed in MC simulation section. The mechanism of the electrochemical anodic dissolution of Cu and cathodic reduction of O2 in saline solution is summarized as follows42,43:
Anodic dissolution of copper.
-
(I)
$${\text{Cu }} \leftrightarrow {\text{ Cu}}^{ + } + {\text{ e }}^-$$(2)$${\text{Cu}}^{ + } + {\text{ 2Cl}}^{ - } \leftrightarrow {\text{CuCl}}_{{\text{2}}} ^{ - }$$(3)
-
(II)
$${\text{Cu }} + {\text{ Cl}}^{ - } \leftrightarrow {\text{ CuCl }} + {\text{ e}}^{ - }$$(4)$${\text{CuCl}} + {\text{ Cl}}^{ - } \leftrightarrow {\text{CuCl}}_{{\text{2}}} ^{ - }$$(5)$${\text{2 CuCl}}_{{\text{2}}} - {\text{ }} + {\text{ OH}}^{ - } \leftrightarrow {\text{Cu}}_{{\text{2}}} {\text{O }} + {\text{ 4Cl}}^{ - } + {\text{ H}}^{ + }$$(6)
Cathodic reduction of oxygen.
Reactions (2–3 & 4–5) are two possible cases of the initial electro-dissolution of copper. However, the presence of ME, QF and nBF molecules replace the adsorbed chloride ions which are originally adsorbed at the metal/solution interface and form a protective adsorbed layer that slows down copper dissolution.
Electrochemical impedance spectroscopy measurements
Nyquist plots of Cu in 0.6 M NaCl solution at Eocp in the absence and presence of different concentrations of ME, QF and nBF samples are represented in Fig. 4. Nyquist spectra obtained consist of one depressed capacitive loop (Fig. 4a–c); the depression of the semicircle signifies on the inhomogeneity of the surface44. The diameter of the capacitive loop increased in the presence of the studied green compounds compared to the blank solution indicating their inhibitive effect of Cu corrosion. The high frequency capacitive loop can be recognized to the redox of Cu to Cu+ reaction, reflecting on the rate-determining phase in the charge transfer reaction during the corrosion process. The semicircle shapes for the uninhibited and inhibited copper are similar, which suggests the same electrochemical behavior. In addition, increasing the concentrations of these green compounds leads to increasing the diameters of the capacitive semicircles, because of changing the Cu/NaCl interface structure by substituting water molecules, producing barrier protective insulating layers adsorbed on the Cu surface. On the otherwise lowering the local dielectric constant and/or increasing the thickness of the adsorption barrier protection layer impede the charge transfer process across the interface.
The Bode plots in Fig. 4d–f show one phase with narrow maximum for copper in 0.6 M NaCl without inhibitors (Blank) indicating less protective layer on the copper surface despite the maximum broaden by adding the inhibitor and increasing its concentration indicating the formation of more protective film45,46. The results of the blank were fitted by equivalent circuit shown in Fig. 4g that consists of two parallel simple circuits in series with the solution resistance (Rs); the first one involves a resistance for the polarization (R1) which included the charge transfer resistance through the interface of copper surface and the solution (Rct), the resistance of the diffused layer (Rd), and the resistance of any corrosion products accumulated at the surface47,48. R1 was in parallel with the constant phase element (CPE), Q. The second circuit consists of the resistance (R2) and the double layer capacitance (C) of the oxide film formed on the copper surface. A diffusion process occurs on the copper surface that represented by a Warburg impedance, Zw, combined to R2C combination for the for copper ions diffusion through corrosion49. The presence of Warburg impedance indicated that the corrosion process is controlled by both the charge transfer and the diffusion.
The experimental results of the inhibited copper was fitted by an equivalent circuit (Fig. 4h) which is like the one used for the blank but the combination R2C is replaced by R2Q2 for the adsorbed layer of the extract molecules without the presence of the diffusion. The impedance of the phase constant Q can be described by both the modulus of CPE, Yo, and the parameter of the phase shift deviation, n, as follows;50,51.
ω, and j is the angular frequency, and the imaginary number, respectively. The fitted parameters for both inhibited and uninhibited copper are given in Table 8. R2 increases by increasing the inhibitor concentration to a certain concentration which is 60 ppm for nBF and ME and 20 ppm for QF. With the increase in the resistance, a decrease in Y occurs, which indicates an increase in the adsorbed layer thickness. In addition, the presence of inhibitors results in increasing the value of n which means increasing the surface homogeneity as a result of the inhibitor molecules’ adsorption on the active sites52.
The results for impedance measurements agree with those observed from the polarization measurements which indicate the ability of the three extracts to decrease the corrosion of copper in NaCl solution.
Time of immersion effect on the corrosion behaviour
Time of immersion effect on the corrosion behavior of copper was tracked through the relation between the open-circuit potentials (Eocp values) and the time for 3 days in 0.6 M NaCl solution free from (blank) and contains 60 ppm of ME, QF and nBF samples and showed in Fig. 5a. After 240 min of immersion in the inhibited solution, the steady state potential nearly was grasped; however, in the uninhibited solution, it took more than 1000 min to reach. The potential of steady state is shifted to either more negative or more positive in the presence of the inhibitors depending on the type of the extract and its composition.
Open circuit measurements (a), Nyquist plots (b) for copper after 3 days of immersion in 0.6 M NaCl in the absence and presence of 60 ppm of nBF, ME, and QF, (c, d) Nyquist plots for copper at different time of immersion in 0.6 M NaCl solution, and 0.6 M NaCl solution containing 60 ppm of nBF, respectively.
The value of open-circuit potential for the uninhibited copper after long time (3 days) of immersion in 0.6 M NaCl is – 280 mV, however the Eocp values for inhibited samples by ME, QF and nBF samples are − 242, -157, and − 115, respectively. It is noted that the values of Eocp for the inhibited samples are less negative than the uninhibited sample which confirmed the ability of the extracts to inhibit the corrosion of copper in chloride medium. This performance can be explained by the adsorption through heteroatoms and active substituents (OH, SH and NH2) that present on the compounds of extracts (inhibitor molecules) at the surface active sites of copper53.
In addition, the impedance measurements are performed for copper immersed in 0.6 M NaCl without and with 60 ppm of nBF, ME, and QF samples for 3 days. The Nyquist plots are presented in Fig. 5b, the plots show that the diameter of semicircle for nBF is larger than the semicircle diameter of the blank without and with ME and QF samples indicating high resistance to corrosion and more protection. The plots are fitted to the same equivalent circuits (Fig. 4g, h) and the fitted parameters are listed in Table 9. It is noted from the values in Table 9 that all the studied samples inhibited copper corrosion even after long immersion in solution. Figure 5c & d shows that the resistance of copper decreases with time in the blank solution, however it increases with increasing time in the presence of nBF sample indicating more adsorption of nBF phytochemicals on the copper surface which blocks the active sites and reduces the dissolution of copper5. The molecular structure of the phytochemicals present in the studied samples plays a role in increasing the molecules adsorption and exchanging charges with copper surface to form new bonds54,55. In addition, the direction of the molecules and their structure in space are giving the facility to form strong bonds, as the parallel orientation is favored56. The substituent difference in the molecules moieties is also an important parameter, as shown from Table 1, nBF sample has high content of 9-Octadecenamide (Z) as observed from GC-MS analysis which contains both -O and -N functional groups in addition to the presence of E, E,Z-1,3,12-Nonadecatriene-5,14-diol with its -OH groups that increases the facility to be adsorbed on the copper surface.
Surface morphology
To confirm the effect of the extracts on the corrosion of copper in 0.6 M NaCl, the morphology of the four surfaces of copper after 3 days immersion in NaCl solution without (Blank) and with 60 ppm of each of nBF, ME and QF samples are studied as presented in Fig. 6a–d. The surface of copper immersed in NaCl shows the formation of an oxide film with big pores and the precipitation of some crystals from the chloride solution (Fig. 6a), however the inhibited copper surface with 60 ppm nBF sample appears homogenously without pores indicating the formation of protective adsorbed film on Cu surface as shown in Fig. 6b57. The inhibited copper surface by 60 ppm ME shown in Fig. 6c shows the formation of thin film where the scratched lines appear with the presence of tiny pores, however, the surface inhibited by 60 ppm QF in Fig. 6d shows a thick film with larger pore size than the ME inhibited surface. The morphology results confirm that the order by which the samples studied decrease the copper corrosion is nBF > ME > QF which consistent with the polarization and impedance results.
MC calculations
MC simulations are applied to understand the interaction between ME, QF and nBF samples with Cu surface. Figure 7a–c shows the lowest-energy structures of the interaction of the studied green samples with Cu surface, while the calculated adsorption energies (∆Eads) are summarized in Table 10. It is notice from the figure that, the presence of heteroatoms (O and N) significantly affects the adsorption pattern on Cu plane. This leads to interactions between the backbone of the inhibitor molecule and the surface atoms. Also, the values of the calculated ∆Eads for all studied samples exhibit a notably high degree of adsorption to Cu surface, signified by their elevated ∆Eads values and follows the following order ∆Eads ( nBF) > ∆Eads (ME) > ∆Eads (QF). This intensive interaction results in the formation of a protective adsorbed layer on Cu surface, effectively acting as a barrier to impede further corrosion. As demonstrated from the figure, the observed order in the calculated values of ΔEads can be correlated to the orientation of the predominant phytochemical compounds of the studied samples on Cu surface. The parallel orientation of 9-octadecenamide compound observed in nBF, facilitates its stronger interaction with Cu surface, leading to its higher adsorption energy, compared to the perpendicular orientation in QF.
Finally, the mechanism of inhibition of these molecules can be suggested to be due to the electrostatic adsorption of the partially negative oxygen atoms of the inhibitor molecules on the positively charged sites Cu2+, or the coordination of these positive ions with the heteroatoms such as N or O. These interactions can slow down the copper dissolution and shield the aggressive ions interaction (Cl−) resulting in forming a more protective adsorbed layer on the copper surface.
Antimicrobial activity
Anti-microbial activity of ME, QF and nBF samples are tested against against Staphylococcus aureus, Bacillus cereus, Listeria monocytogenes, Escherichia coli, Salmonella typhi, Shigella boydii, Aspergillus niger and Candida albicans. Results show low inhibition zone with all bacteria except Shigella and Candiaa. QF and nBF samples show highest antimicrobial activity against Shigella 26 and 25 cm inhibition zone, respectively, and Candida with 18 and 21 cm inhibition zone, respectively. Whereas the lower activities against Shigella and Candida are recorded by ME sample with 16 and 12 cm inhibition zone, respectively. Increasing the dilution of the studied green compounds leading to the decrease in their antimicrobial activities against Shigella and Candida. The antimicrobial and the minimum inhibition concentrations data are recorded in Tables 11 and 12 and illustrated in Fig. 8.
Conclusion
In this study, extracted and fractionated phytochemicals of Terminalia bentzoe leaves (ME, QF, nBF) are elucidated and investigated as sustainable green inhibitors against Cu corrosion in saline solution, as well as their antimicrobial activity against a variety of bacterial infections. According to the electrochemical measurements, the compounds studied revealed as efficient anodic inhibitors for Cu in 0.6 M NaCl solution, with 94.4% maximum value of inhibition efficiency for 60 ppm of nBF sample. The FESEM analysis indicated that the inhibited surface with nBF appears homogenous without pores indicating the formation of adsorbed protective film on the surface. MC simulations confirm the adsorption of the protective layers onto the Cu surface with different orientation of the predominant phytochemical compound of the extract and its fractions. Also, the studied green compounds show good resistance against a variety of bacterial infections.
Ethics approval
Not applicable.
Data availability
All data generated or analysed during this study are included in this published article.
References
Lin, M-C., Wang, Y., Wang, R. & Zhang, X. The synergetic effect of Tannic acid as adhesion promoter in electrodeposition of polypyrrole on copper for corrosion protection. Mater. Chem. Phys. 294, 126991 (2023).
Fathi, A. M. & Mandour, H. S. Electrosynthesized conducting poly (1, 5-diaminonaphthalene) as a corrosion inhibitor for copper. Polym. Bull. 77 (6), 3305–3324 (2020).
Kozlica, D. K., Kokalj, A. & Milošev, I. Synergistic effect of 2-mercaptobenzimidazole and octylphosphonic acid as corrosion inhibitors for copper and aluminium–An electrochemical, XPS, FTIR and DFT study. Corros. Scienc. 182, 109082 (2021).
Qiang, Y., Zhang, S., Yan, S., Zou, X. & Chen, S. Three Indazole derivatives as corrosion inhibitors of copper in a neutral chloride solution. Corros. Sci. 126, 295–304 (2017).
Fathi, A. M. et al. Evaluation of the Inhibition effect of novel cyclohepta [b] pyridine derivatives for copper corrosion and theoretical calculations. J. Phys. Org. Chem. 35(3), e4297 (2022).
Mandour, H. S., Abdel-Karim, A. M. & Fathi, A. M. Inhibition efficiency of copper corrosion in a neutral chloride solution by barbituric and thiobarbituric acids. Portugaliae Electrochim. Acta. 39 (2), 85–103 (2021).
Chen, S. et al. Synthesis and surface characterization of self-assembled monolayers of thiazoles incorporating hydrocarbon and fluorocarbon chains on copper substrates. Appl. Surf. Sci. 456, 25–36 (2018).
Holla, B. R., Mahesh, R., Manjunath, H. & Anjanapura, V. R. Plant extracts as green corrosion inhibitors for different kinds of steel: a review. Heliyon. 10(2), e33748 (2024).
Salmasifar, A., Edraki, M., Alibakhshi, E., Ramezanzadeh, B. & Bahlakeh, G. Combined electrochemical/surface investigations and computer modeling of the aquatic artichoke extract molecules corrosion Inhibition properties on the mild steel surface immersed in the acidic medium. J. Mol. Liq. 327, 114856 (2021).
Wang, Q. et al. Evaluation of Ficus Tikoua leaves extract as an eco-friendly corrosion inhibitor for carbon steel in HCl media. Bioelectrochemistry 128, 49–55 (2019).
Tan, B. et al. Papaya leaves extract as a novel eco-friendly corrosion inhibitor for Cu in H2SO4 medium. J. Colloid Interface Sci. 582, 918–931 (2021).
Luo, Z-G. et al. Modified nano-lignin as a novel biomass-derived corrosion inhibitor for enhanced corrosion resistance of carbon steel. Corros. Sci. 227, 111705 (2024).
Wang, Q. et al. Insight into the anti–corrosion performance of Artemisia argyi leaves extract as eco–friendly corrosion inhibitor for carbon steel in HCl medium. Sustainable Chem. Pharm. 27, 100710 (2022).
Medupin, R. O., Ukoba, K. O., Yoro, K. O. & Jen, T-C. Sustainable approach for corrosion control in mild steel using plant-based inhibitors: a review. Mater. Today Sustain. 22, 100373 (2023).
Chauhan, J. S. & Gupta, D. The anti-corrosive characteristics of Betalains (Bougainvillea flower extract) (2015).
Sharma, A., Varshney, A. K. & Varshney, S. Sesmum indicum oil as a potential inhibitor for the corrosion of copper in acidic environment. Adv. Sci. Lett. 22 (11), 3824–3826 (2016).
Maibulangu, B., Ibrahim, M. & Akinola, L. Inhibitory effect of African pumpkin (Momordica balsamina Linn.) leaf extract on copper corrosion in acidic media. J. Appl. Sci. Environ. Manage. 21 (6), 1067–1071 (2017).
Ahmed, R. K. & Zhang, S. Bee pollen extract as an eco-friendly corrosion inhibitor for pure copper in hydrochloric acid. J. Mol. Liq. 316, 113849 (2020).
Jmiai, A. et al. Application of Zizyphus Lotuse-pulp of jujube extract as green and promising corrosion inhibitor for copper in acidic medium. J. Mol. Liq. 268, 102–113 (2018).
Büyüksağiş, A. & Dilek, M. The use of papaver somniferum L. Plant extract as corrosion inhibitor (2019).
Fallavena, T., Antonow, M. & Gonçalves, R. S. Caffeine as non-toxic corrosion inhibitor for copper in aqueous solutions of potassium nitrate. Appl. Surf. Sci. 253 (2), 566–571 (2006).
Emad, M. & Al-Rasheedi, M. Nigella sativa and natural honey as corrosion inhibitors for copper in cooling water systems. J. Mater. Environ. Sci. 6, 201–206 (2015).
Oukhrib, R. et al. The inhibitory effect of saffron extract (Crocus sativus., L) on copper corrosion in seawater. Chem. Sci. Rev. Lett. 4 (13), 241–251 (2015).
Rahal, C. et al. Olive leaf extract as natural corrosion inhibitor for pure copper in 0.5 M NaCl solution: a study by voltammetry around OCP. J. Electroanal. Chem. 769, 53–61 (2016).
Kaswan, P. et al. Phytochemicals/Plant Extracts in Corrosion Prevention: Comparison with Organic Inhibitors. Phytochemistry in Corrosion Sciencep. 37–60 (CRC, 2024).
Messaoudi, H. et al. Surface analysis and adsorption behavior of caffeine as an environmentally friendly corrosion inhibitor at the copper/aqueous chloride solution interface. J. Adhes. Sci. Technol. 34 (20), 2216–2244 (2020).
Oubahou, M. et al. Exploring sustainable corrosion Inhibition of copper in saline environment: an examination of Hydroquinazolinones via experimental and Ab initio DFT simulations. Arab. J. Chem. 17 (5), 105716 (2024).
El-Rafie, H. M. & Hamed, M. A. A. Antioxidant and anti-inflammatory activities of silver nanoparticles biosynthesized from aqueous leaves extracts of four Terminalia species. Adv. Nat. Sci. NanoSci. NanoTechnol. 5 (3), 035008 (2014).
Rummun, N. et al. Terminalia bentzoe, a mascarene endemic plant, inhibits human hepatocellular carcinoma cells growth in vitro via G0/G1 phase cell cycle arrest. Pharmaceuticals 13 (10), 303 (2020).
Das, G. et al. Plants of the genus terminalia: an insight on its biological potentials, pre-clinical and clinical studies. Front. Pharmacol. 11, 561248 (2020).
El-Rafie, H., Mohammed, R., Hamed, M., Ibrahim, G. & Abou Zeid, A. Phytochemical and biological studies of total ethanol and petroleum ether extracts of Terminalia bentzoe (L.) leaves. Int. J. Pharmacognosy Phytochemical Res. 8 (4), 392–603 (2016).
Manosroi, A. et al. Biological activities of phenolic compounds and triterpenoids from the galls of Terminalia chebula. Chem. Biodivers. 10 (8), 1448–1463 (2013).
Fahmy, N., Al-Sayed, E. & Singab, A. Genus terminalia: a phytochemical and biological review. Montin Species Med. Aromat. Plants. 4 (5), 1–22 (2015).
Khan, Z. H., Faruquee, H. M. & Shaik, M. M. Phytochemistry and pharmacological potential of Terminalia Arjuna L. Med. Plant. Res. 3 (10), 70–77 (2013).
Bag, A., Bhattacharyya, S. & Chattopadhyay, R. The development of Terminalia chebula Retz.(Combretaceae) in clinical research. Asian Pac. J. Trop. Biomed. 3, 244–252 (2013).
Banjare, M. K. & Tandon, D. Explicit Modeling of Corrosion Inhibition. Computational Modelling and Simulations for Designing of Corrosion Inhibitorsp. 227–240 (Elsevier, 2023).
Assadollahzadeh, B. & Schwerdtfeger, P. A systematic search for minimum structures of small gold clusters Aun (n = 2–20) and their electronic properties. J. Chem. Phys. 131(6), 064306 (2009).
Shao, Q. & Hall, C. K. Allosteric effects of gold nanoparticles on human serum albumin. Nanoscale 9 (1), 380–390 (2017).
Narayanankutty, A. Natural products as PI3K/Akt inhibitors: implications in preventing hepatocellular carcinoma. Curr. Mol. Pharmacol. 14 (5), 760–769 (2021).
Narayanankutty, A. Pharmacological potentials and nutritional values of tropical and subtropical fruits of india: emphasis on their anticancer bioactive components. Recent Pat. Anti-cancer Drug Discov. 17 (2), 124–135 (2022).
Fathi, A. M., Anouar, E. H., Ahmed, A. O. & Hegab, M. I. Electrochemical, molecular dynamics, density functional theory, and corrosion Inhibition studies of some Chromeno-oxadithiin and Chromeno disulfide derivatives for mild steel in 3.5% NaCl. J. Solid State Electrochem. 27 (12), 3539–3555 (2023).
Chen, S. & Zhang, D. Study of corrosion behavior of copper in 3.5 wt% NaCl solution containing extracellular polymeric substances of an aerotolerant sulphate-reducing bacteria. Corros. Sci. 136, 275–284 (2018).
Kear, G., Barker, B. & Walsh, F. Electrochemical corrosion of unalloyed copper in chloride media––a critical review. Corros. Sci. 46 (1), 109–135 (2004).
Hanza, A. P., Naderi, R., Kowsari, E. & Sayebani, M. Corrosion behavior of mild steel in H2SO4 solution with 1, 4-di [1′-methylene-3′-methyl imidazolium bromide]-benzene as an ionic liquid. Corros. Sci. 107, 96–106 (2016).
Ismail, K. M. & Badawy, W. Electrochemical and XPS investigations of Cobalt in KOH solutions. J. Appl. Electrochem. 30, 1303–1311 (2000).
Ismail, K. M., Fathi, A. M. & Badawy, W. A. The influence of Ni content on the stability of copper—nickel alloys in alkaline sulphate solutions. J. Appl. Electrochem. 34, 823–831 (2004).
Hosseinpour Rokni, M., Naderi, R., Soleimani, M., Kowsari, E. & Pourfath, M. J. C. S. Indirect interactions between the ionic liquid and Cu surface in 0.5 M HCl: a novel mechanism explaining cathodic corrosion inhibition. Corros. Sci. 216, 111100 (2023).
Solomon, M. M. et al. Tailoring Poly (2-ethyl-2-oxazoline) towards effective mitigation of chloride-induced dissolution of S235JR steel: the synergistic contributions of potassium iodide and Myristyl Trimethylammonium bromide. J. Mol. Liq. 396, 123935 (2024).
Qiang, Y., Zhang, S. & Wang, L. Understanding the adsorption and anticorrosive mechanism of DNA inhibitor for copper in sulfuric acid. Appl. Surf. Sci. 492, 228–238 (2019).
Fathi, A. M., Mandour, H. S. & Elkarim, A. M. The inhibiting effect of Non toxic 4-Amino antipyrine and 4, 6-Dimethyl-1H-pyrazolo [3, 4-b] pyridin-3-amine on mild steel corrosion in sulphuric acid. IJES 11, 5580–5595 (2016).
Zhang, Z., Chen, S., Li, Y., Li, S. & Wang, L. A study of the Inhibition of iron corrosion by imidazole and its derivatives self-assembled films. Corros. Sci. 51 (2), 291–300 (2009).
Luo, X. et al. Corrosion Inhibition of mild steel in simulated seawater solution by a green eco-friendly mixture of glucomannan (GL) and bisquaternary ammonium salt (BQAS). Corros. Sci. 125, 139–151 (2017).
Verma, C., Ebenso, E., Bahadur, I., Obot, I. & Quraishi, M. 5-(Phenylthio)-3H-pyrrole-4-carbonitriles as effective corrosion inhibitors for mild steel in 1 M hcl: experimental and theoretical investigation. J. Mol. Liq. 212, 209–218 (2015).
Quadri, T. W. et al. Chromeno-carbonitriles as corrosion inhibitors for mild steel in acidic solution: electrochemical, surface and computational studies. RSC Adv. 11 (4), 2462–2475 (2021).
Udhayakala, P., Samuel, A., Rajendiran, T. & Gunasekaran, S. Theoretical assessment of corrosion Inhibition performance of some pyridazine derivatives on mild steel. J. Chem. Pharmac Res. 5, 142–153 (2013).
Subramanian, R. & Lakshminarayanan, V. Effect of adsorption of some Azoles on copper passivation in alkaline medium. Corros. Sci. 44 (3), 535–554 (2002).
Mishra, A. et al. Chemical, electrochemical and computational studies of newly synthesized novel and environmental friendly heterocyclic compounds as corrosion inhibitors for mild steel in acidic medium. J. Bio-and Tribo-Corrosion. 4 (3), 1–20 (2018).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
N. A. El-Sawy: Preparation, analysis and writing draftA. M. Fathi: Conceptualization, methodology, validation, investigation, Formal analysis, Funding acquisition, Writing – review & editing.S. K. Ali: Conceptualization, validation, investigation, Formal analysis, Funding acquisition, Writing – review & editingD. A. Abdelrheem: Conceptualization, methodology, validation, investigation, Formal analysis, Funding acquisition, Writing – review & editing M. M. Hegab: Conceptualization, methodology, validation, investigation, Formal analysis, Funding acquisition, Writing – review & editing M. M. El-Deeb: Conceptualization, methodology, validation, investigation, Formal analysis, Funding acquisition, Writing – review & editing .
Corresponding authors
Ethics declarations
Consent for publication
The authors agree to the journal’s policy.
Consent to participate
The authors agree to the journal’s policy.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Sawy, N.A., Fathi, A.M., Ali, S.K. et al. Anticorrosion and antimicrobial performance of extracted and fractionated phytochemicals of terminalia bentzoe leaves. Sci Rep 15, 24626 (2025). https://doi.org/10.1038/s41598-025-08585-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-08585-z