Abstract
The genus Alcea is an herbaceous, annual and perennial plant of the Malvaceae family, which is rich in flavonoids, phenolic acids, coumarins, triterpenes, alkaloids, polysaccharides (mucilage), pectins, scopoletin, asparagine, tannins, steroids, proteins and minerals. The genus Alcea has various biological properties, including antimicrobial, anti-inflammatory, antioxidant, anti-estrogenic, enzyme inhibitory and immunomodulatory effects. In this study, agro-morphological, phytochemical and proximate composition variability among six Iranian species of the plant was studied to find adequate samples for further use in breeding and exploitation programs. For this purpose, we evaluated six species of the genus Alcea in terms of functional traits, phenolic compounds, anthocyanin content, mucilage, minerals, and proximate compositions, including A. kurdica, A. tehranica, A. koelzii, A. lavateriflora, A. rechingeri, A. xanthochlora. The highest coefficient of variation was obtained for rutin, chlorogenic acid, antioxidant activity, flower dry weight. A. rechingeri species indicated the maximum (1220 g/plant) and A. xanthochlora species revealed the minimum (200 g/plant) dry flower weight. The species A. lavateriflora (32.91%), A. tehranica (23.11%), and A. rechingeri (16.40%) had the highest mucilage content. The anthocyanin content in different species varied from 11.47 (in A. koelzii) to 30.39 (in A. tehranica) mg C3G/g DW. Maximum content of total phenol (48.42 mg GAE/g DW), total flavonoid (34.32 mg RE/g DW), and antioxidant activity, were observed in A. tehranica species. Chlorogenic acid, rutin, kaempferol, and apigenin were detected as the major phenolic compounds using high-performance liquid chromatography (HPLC). The analyzed species exhibited variations in carbohydrate (42.44 to 58.62 g/100 DW), fat (1.30 to 2.53 g/100 DW), protein (6.06 to 11.30 g/100 DW), and fiber (13.34 to 28.64 g/100 DW) contents. The extraordinary diversity in terms of the studied parameters indicates the ability of different Alcea species to be selected and used in breeding, cultivation, and production programs.
Similar content being viewed by others
Introduction
Plants have a high aesthetic and increase the visual beauty of the landscape. They are used as functional properties and they have an important biodiversity and food source, particularly for rural communities. They are rich for human health-promoting substances and have excellent medicinal value1,2,3.
Nowadays, the trend towards consuming natural products is expanding. The use of products derived from these compounds in the pharmaceutical, food, cosmetic, and dietary supplement industries and their biological effects on the human body has been widely recognized4,5,6.
The genus Alcea, widely scattered throughout different areas of the country, is a main component of this botanical richness. The genus Alcea belongs to the family Malvaceae, which has about 70 annual and perennial herbaceous species distributed in Asia, Europe, and America7. The genus has undergone marked species radiation in Iran including 34 endemic species, and numerous taxa are naturally scattered across west and southwest Iran. Given its striking appearance and geometry, Alcea could be easily distinguished from other genera; the flowers are either solitary or arranged in racemes or fascicles on an unbranched upright stem rising to over 2 m at a fast rate. This genus is also characterized by having long notched petals ranging in color from white and yellow to pink and purple. The leaves usually appear on long petioles and are often lobed or toothed, and adorn the whole parts of the herbage. From an industrial and medicinal point of view, Alcea is among the most important genera recognized in the family Malvaceae8. Alcea species contain starch, pectin, sucrose, mucilage, saccharides, flavonoids, steroids, triterpenoids, coumarins, and minerals including calcium, magnesium, chromium, potassium, nickel, sodium, copper, and amino acids including threonine, serine, glutamine, proline, glycine, cysteine, asparagines and alanine9,10,11,12. This plant has various biological properties, including antimicrobial, anti-inflammatory, anti-estrogenic, antitussive, antioxidant, immunomodulatory, sedative, diuretic, antipyretic, and emollient properties11,13,14. In traditional medicine, this plant is used to treat a variety of diseases, including blood clotting, muscle contraction, tooth and bone weakness, cataracts, stomach ulcers, kidney stones, cough, and respiratory problems, skin burns, mild gastritis, inflammation, ulcers, abscesses, and constipation13,15.
In addition to its medicinal properties, it is also widely used in the food industry. Today, one of the concerns of the food, cosmetic and health industries is the replacement and use of natural colors instead of synthetic colors, and anthocyanins are the main candidates for this16. Colorful flowers of the genus Alcea, with high levels of anthocyanins (a group of natural pigments), are used as a rich source of natural food colors8. Marshmallow flowers and roots are also rich in polysaccharide hydrocolloids such as mucilage. Mucilages are a group of biological macromolecules that have physicochemical properties as emulsifiers, thickeners, friction reducers, suspension agents, stabilizers, hydrocolloidal laxatives, and water retainers in various pharmaceutical and food industries7.
Today, most of the raw materials of the Alcea are harvested from natural habitats and are used in the domestic markets of Iran or exported abroad. Increased exploitation of nature and excessive consumption of medicinal plants cause rapid erosion of natural ecosystems and habitats and the loss of medicinal species17. Cultivation and domestication are one of the most important approaches to the protection of medicinal plants. Domestication of medicinal plants provides easy access to plant raw materials and reduces the pressure of collection on the natural environment18. The most important and first step in domesticating medicinal plants is to carefully examine the various phytochemical, genetic, and physiological aspects, as well as the potential for producing genotypes of the species in question. Therefore, studying the genetic diversity of existing plants based on morphological, functional, and phytochemical characteristics can provide breeders with basic and essential information for domestication, cultivation, and breeding19.
Given the importance of Alcea as a native plant species in the country and its place and value in the pharmaceutical, food, cosmetic, and health industries, domestication, creation of high-quality and homogeneous varieties and extensive cultivation of these plants seem important and necessary. Therefore, this study was conducted to investigate the diversity of functional, morphological, and phytochemical traits (especially anthocyanin, mucilage, and phenolic compounds content) of different species of the genus Alcea as the first step towards the cultivation and domestication of this plant. Related industries require homogeneous and high-quality plants in terms of phytochemistry, and on the other hand, to create a profitable agricultural production system, varieties with a high percentage of active ingredients and high flower yield are considered. Therefore, this research helped to identify the diversity existing between different marshmallow genotypes and provides the possibility of achieving genotypes with desirable traits and high yield and introducing superior genotypes for cultivation and domestication.
Materials and methods
Plant materials and experimental design
Seeds of different species were collected from different regions of Iran (Table 1) and were cultivated in trays measuring 61 × 40 mm with a depth of 76 mm, composed of coco peat and perlite in a 1:1 ratio. Prof. Ali Sonboli identified the samples, and voucher specimens were deposited at the Shahid Beheshti University herbarium (Table 1). The authors confirm that the necessary permissions to collect and cultivate the samples have been obtained. In addition, the present study complies with the IUCN Policy Statement on Research Involving Species at Risk of Extinction and the Convention on the Trade in Endangered Species of Wild Fauna and Flora. After 25 days (in May), the seedlings were transferred to the main field (Shahid Beheshti University research farm, Tehran) and planted in a randomized complete block design with five replications. Irrigation (once a week) and weeding were regularly done during the growing season. The soil and climatic characteristics of the planting site are presented in Table 2.
Assessment of agro-morphological parameters
Morphological parameters such as plant height, Number of lateral branches, number of flowers per bush and flower diameter were recorded at the full flowering stage using a ruler and digital caliper. The harvested flowers were then transported to the laboratory and dried at room temperature. The dry weight of the flowers was then assessed using a digital scale.
Extraction and yield of mucilage
Flower samples were ground and subsequently combined with distilled water to extract mucilage using the hot water method as described by Husain et al.20. The samples were immersed in distilled water at a weight-to-volume ratio of 1:100 and boiled for 20 min. After cooling to room temperature, the water-soluble mucilage was filtered from the plant debris using a Buchner funnel with vacuum assistance and standard filter paper. The volume of the resulting solution was concentrated by evaporating water with a rotary evaporator (RV10, IKA, Germany) at 70 °C until it reduced to approximately 40 mL. To precipitate the mucilage, 1.5 times the volume of the solution of 96% ethanol was added. This mixture of water, ethanol, and mucilage was then stored in a refrigerator at 4 °C for 4 h. Following this period, the mucilage was isolated using a vacuum funnel and filter, and then dried in an oven at 40 °C for 24 h. The resulting dried mucilage was ground, and sifted through a no. 80 sieve, and kept in desiccators for future use. The yield percentage was calculated by dividing the weight of the dried mucilage by the weight of the dry plant material, multiplied by 100, as follows:
Percentage Yield = (Weight of dried mucilage obtained / Weight of dry plant material) × 100.
Extraction
To determine the bioactive compound, 0.5 g of the flower was finely ground and subsequently combined with 20 ml of 80% methanol. The extraction was performed using a sonicator (SingenHtw Elmasonic-D 78224; Elma Germany) at 40 ºC for a duration of 20 min. Following extraction, the mixture was centrifuged in a refrigerated centrifuge (R5702; Eppendorf) at 3000 rpm for 10 min, after which the supernatants were stored in a refrigerator for further analysis.
Evaluation of total phenol content (TPC) and flavonoid content (TFC(
Total phenolic compounds turned into quantified the usage of the approach described by Singleton et al.,21, which includes by the Folin-Ciocalteu reagent. In summary, 25 µl methanolic extract (1000 ppm) and 125 µl Folin-Ciocalteu reagent, 100 µl sodium carbonate (7.5%) were taken in a test tube. The final volume was made up of 6 ml of distilled water. The solution was stored for 30 min in the dark. The absorbance was recorded at 765 nm using a spectrophotometer (Bio-Tek Instruments, Inc., USA). The results are expressed as mg gallic acid equivalents (GAE)/ per g of dry weight (mg GAE/g DW).
Total flavonoid compounds were assayed based at the technique mentioned with the aid of Chang et al.,22, utilizing aluminum chloride. Initially, 20 µl methanolic extract, 3.4 ml methanol (30%), 80 µl distilled water, 6 µl sodium nitrite (0.5 M), 6 µl aluminum chloride h (0.3 M) and 80 µl sodium hydroxide (1.0 M) was taken in a test tube and mixed well. The absorbance of the solution was determined against the reagent blank at 510 nm wavelength. The data were expressed as mg of rutin equivalents (RE) per g of dry weight (mg RE/g DW).
Ferric reducing activity power (FRAP) assay
The approach outlined by Benzie and Strain23 turned into hiring. The fresh FRAP reagent was prepared by mixing 300 mmol/l acetate buffer (pH 3.6), 10 mmol/l TPTZ in 40 mmol/l HCl, and 20 mmol/l FeCl3 at 10:1:1 (v/v/v). The reaction mixture was kept for 30 min at 37 °C. The absorption was then measured at 593 nm. A standard curve was generated using ferrous sulfate solution in concentrations ranging from 0.25 to 8 mmol dm − 3 (0.5–10 mg/ml). The result was expressed as µmol Fe+ 2 per g of DW.
Total anthocyanin content (TAC)
For this purpose, 0.04 ml of methanolic extract of the flower samples was poured into two separate tubes, 3.6 ml of potassium chloride buffer (0.025 M) at pH = 1 was added to one, and 3.6 ml of sodium acetate buffer (0.4 M) at pH = 4.5 was added to the second, and the absorbance of each tube was read at wavelengths of 510 and 700 nm by spectrophotometer24,25. The amount of TAC was calculated with the following formula based on milligrams of anthocyanin equivalent to Cyanidin-3-glucosid per gram:
A = (A510 – A700) pH1- (A510 – A700) pH4.5.
TAC = (A× MW× DF × 100)/ MA.
A = Absorption, MW = 499.2, DF = 100, MA = 26900.
Quantification of phenolic compounds by HPLC-DAD
Phenolic compounds were analyzed using a Knauer HPLC system from Germany, featuring dual Wellchrom-K1001 pumps and a K2800 PDA detector. The separation was carried out on an RP-C18 column with dimensions of 4.6 mm internal diameter and 250 mm length, manufactured by Eurosphr. The mobile phase consisted of methanol and HPLC-grade water. Peak detection was performed within a wavelength range of 200–600 nm. The sample injection volume was set at 20 µl, with the system maintained at a constant temperature of 25 °C. The pure phenol compound standards were sourced from Sigma Aldrich. Retention times and the analysis of the spiked oil extract, alongside a standard solution, were utilized to determine the presence of phenolic acids. Calibration curves were generated by injecting varying concentrations of standard compounds (5, 10, 20, 40, 60, 80, 100, 150, and 200 ppm) to quantify the phenolic acids. The results were expressed in milligrams per gram of dry weight (mg/g DW).
Proximate composition analysis
The analysis of the samples for ash, protein, fat, and fiber was conducted following the AOAC guidelines26. The ash content was quantified by subjecting the cultivars to high temperatures in a muffle furnace. Fat extraction was performed using a Soxhlet (SOX406, HANON Systems, South Korea) and its amount was determined. The micro-Kjeldahl method was employed to estimate protein levels, with the amount of protein calculated by multiplying the nitrogen content by a factor of 6.25. The fiber was derived following the digestion of samples with boiling solutions of diluted acid and alkali. The carbohydrate content was ascertained by deducting the combined weights of ash, protein, fiber, and fat from the overall dry matter.
Energy dispersive X-ray analysis (EDX)
Elemental analysis of Alcea flower samples was conducted using the EDX technique, specifically utilizing the INCA100 system from Oxford Instruments, U.K. This analysis focused on the detection of various elements, including carbon (C), oxygen (O), nitrogen (N), potassium (K), sulfur (S), phosphorus (P), calcium (Ca), sodium (Na), chlorine (Cl), magnesium (Mg), and silicon (Si).
Data analysis
All experiments conducted in this research were carried out in five replicates. The results obtained are presented as means ± standard deviation (SD). To assess significant disagreement among the cultivars regarding the measured traits, a one-way analysis of variance (ANOVA) was performed using SAS 9.4. A post-hoc analysis was conducted employing Duncan’s test with a significance level set at p < 0.05. The biplot was generated using Origin software version 2022, while cluster analysis was performed utilizing the Euclidean distance coefficient and Ward’s method. Additionally, R software was utilized to create the correlation plot.
Results and discussion
Statistical descriptive parameters of the studied traits
The results showed significant variation among different Alcea species in terms of functional, phytochemical, proximate, and mineral traits, which allows for selection for these traits among different Alcea species (Table 3). The highest coefficient of variation (CV) was obtained for rutin (103.45%), chlorogenic acid (85.98%), antioxidant activity (75.60%), flower dry weight (65.08%), number of lateral branches (53.91%), calcium (51.86%) and TFC (51.02%) and the lowest was for Kaempferol (4.97%), moisture (6.84%), ash (6.32%) and carbohydrates (11.75%) (Table 3). The high CV indicates a wider range of that trait in terms of quantity. The higher the CV in a trait, the greater the range of selection and the greater the selection power of the breeder, which leads to an increased response to selection18.
Agro-morphological features
Significant variation was observed among the agromorphological traits of different Alcea species (p < 0.01). Based on the results of the comparison of means, the plant height of different Alcea species varied from 85.40 to 190.40 cm. The highest plant height was observed in A. rechingeri (190.40 cm) and A. koelzii (179.80 cm) and the lowest plant height was observed in A. xanthochlora (85.40 cm) (Fig. 1a). The highest (13.8) and lowest (2.6) number of lateral branches were obtained in A. koelzii and A. lavateriflora species, respectively (Fig. 1c). A. rechingeri had the highest (174.0) number of flowers per plant and A. xanthochlora had the lowest (45.6) number of flowers per plant (Fig. 1b). The flower diameter varied between the different species from 7.80 to 19.60 cm, which was obtained in A. kurdica and A. koelzii, respectively (Fig. 1d). The dry weight of the flower per plant, an important economic trait in the genus Alcea, varied between the different species from 200.18 to 1220.48 g/plant. The species A. rechingeri (1220.48 g/plant), A. koelzii (935.60 g/plant) and A. tehranica (477.42 g/plant) had the highest dry weight of the flower per plant, and the lowest was related to A. xanthochlora (200.18 g/plant) (Fig. 1e(. The species analyzed in this research are all classified under the genus Alcea, demonstrating considerable phenotypic diversity. A separate investigation of a hollyhock population comprising 162 specimens identified significant variability across 11 morphological characteristics and established a notable positive correlation among certain quantitative traits27. Additionally, other research has highlighted variations in leaf and flower attributes among hollyhock plants, further contributing to the examination of their morphological features28.
Histogram of plant height (a), number of flowers (b), number of lateral branches (c), flower diameter (d), and flower dry weight (e) among Alcea species. The mean comparisons were performed using the Duncan test at p ≤ 0.05 significant level. Means followed by the same letter(s) are not significantly different.
Mucilage content
As shown in Fig. 2, the mucilage content varied significantly (p < 0.01) among different Alcea species, ranging from 12.37 to 32.91%. The species A. lavateriflora (32.91%), A. tehranica (23.11%), and A. rechingeri (16.40%) had the highest mucilage content. On the other hand, the lowest mucilage content was found in the species A. xanthochlora (16.40%) and A. koelzii (12.37%) (Fig. 2). Azadeh et al.8 reported mucilage content for four Alcea species (A. koelzii, A. arbelensis, A. aucheri var. lobata and A. aucheri var. aucheri) ranging from 8.6 to 32%. Mucilage, a class of natural substances, is widely used in the food industry due to its beneficial physical properties. It also has applications in the pharmaceutical sector due to its adhesive, thickening, stabilizing, and wetting properties29. The unique properties of mucilage, including its ability to stabilize, emulsify, and suspend, contribute to its popularity in food, pharmaceutical, and cosmetic applications. Furthermore, mucilage is often preferred over semi-synthetic and synthetic alternatives because it is non-toxic, affordable, readily available, and gentle on the skin30. Notably, Alcea flowers are known for their high mucilage content. This mucilage is used as a pharmaceutical supplement as a binder in tablet formulations, as a gelling agent in nasal gels containing diazepam, and as a disintegrant in fast-dissolving amlodipine besylate tablets31. In this study, the species A. lavateriflora and A. tehranica were superior in terms of mucilage content and these species can be used for breeding and cultivation programs.
TAC
Anthocyanins are natural plant pigments that cause a variety of colors in plants. Anthocyanins are produced in plants under environmental stress conditions such as cold temperature, dryness and UV light, which have anti-inflammatory, antioxidant, antimicrobial, anti-allergic, antiviral, anti-cancer, anti-proliferative and anti-mutagenic effects and play an important role in the prevention of various diseases32. Based on the results, the TAC in different species varied from 11.47 to 30.39 mg C3G/g DW. A. tehranica and A. kudica had the highest TAC with values of 30.39 and 21.14 mg C3G/g DW, respectively. The lowest TAC was observed in A. koelzii (Fig. 3). Cherries (3-143.3 mg/100 g), berries (0.80-794.1 mg/100 g), pomegranates (9.8-155.3 mg/100 g), plums (5.6–36.6 mg/100 g) and red apple (36.1 mg/100 g) are known as anthocyanin-rich plants33, with the TAC of Alcea flowers being significantly higher. According to previous studies, harvest time, growing season, geographical location, and genotype all significantly affect the type of compounds and the TAC34. Given the positive and significant correlation between total antioxidant capacity and anthocyanin content, TAC is considered a strong indicator of the quality of plant materials and their potential benefits for human health35.
Phenolic acids
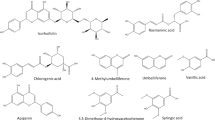
In order to study the phenolic acid content of different Alcea species, the extracts of the samples were analyzed by HPLC. Four main phenolic compounds (apigenin, kaempferol, chlorogenic acid, rutin) were identified (Fig. 4). The apigenin content varied from 0.81 to 1.91 mg/g dry weight among different Alcea species, with the highest content observed in A. tehranica and the lowest in A. xanthochlora. The highest (1.06 mg/g DW) and lowest (0.91 mg/g DW) kaempferol content were found in A. koelzii and A. lavateriflora, respectively. The highest rutin content was found in A. lavateriflora, A. rechingeri and A. koelzii, respectively, with 1.01, 0.52 and 0.35 mg/g DW. The lowest levels of rutin were also observed in A. tehranica (0.02 mg/g DW) and A. xanthochlora (0.03 mg/g DW). The species A. tehranica and A. lavateriflora had the highest (0.44 mg/g DW) and the lowest (0.01 mg/g DW) levels of chlorogenic acid, respectively. Syringic acid, p-hydroxybenzoic, ferulic acid, p-hydroxyphenylacetic, p-coumaric, and caffeic acids have been reported from the extract of A. rosea var. nigra flowers36. In another study, phenolic compounds such as caffeic acid, p-hydroxybenzoic acid, alicylic acid, and quercetin were reported from A. pallida and A. apterocarpa species37. Benbassat et al.38 reported flavonoid compounds such as kaempferol and quercetin in Althaea officinalis L. root extracts. The degree of variation in phenolic compounds of the different Alcea species studied may be due to the type of genetics, extraction and analysis method, and geographical and climatic factors39.
TPC, TFC, and antioxidant activity
A statistically significant disparity (p < 0.01) was identified among various species of Alcea concerning the aggregate content of phenolic compounds and flavonoids. The TPC within Alcea exhibited a range from 17.49 to 48.42 mg GAE/g DW. The A. tehranica (48.42 mg GAE/g DW) and A. lavateriflora (44.75 mg GAE/g DW) species demonstrated the most elevated levels of TPC, whereas the A. koelzii (21.52 mg GAE/g DW) and A. kurdica (18.27 mg GAE/g DW) and species the lowest concentration (Fig. 5a). On the other hand, The A. tehranica species exhibited the highest average of 34.32 mg RE/g DW in total flavonoid content, while the A. kurdica and A. xanthochlora populations had the lowest (11.22 and 10.75 mg RE g DW, respectively) (Fig. 5b).
In a study, the TPC of four Alcea species from Iran ranged from 27.39 to 82.59 mg GAE/g DW, those of which were related to the species A. aucheri and A. koelzii11. The TFC in the species A. koelzii, A. arbelensis, A. aucheri var. lobata and A. aucheri var. aucheri has been reported to be 8.06 to 16.15 mg QE/g DW8. Based on the results obtained, a significant variation was observed in the phenolic and flavonoid compounds of different Alcea species, which can be influenced by genetics, ecological factors, climatic factors, developmental stage, geographical coordinates, and soil conditions40. The antioxidant activity of Alcea species was also assayed via the ferric-reducing antioxidant power (FRAP) method. The highest (20.54 µmol Fe Π/ml) and the minimum (1.20 µmol Fe Π/ml) antioxidant capacity were observed in A. tehranica and A. koelzii species, respectively (Fig. 5c). Species with higher phenolic and flavonoid compounds showed higher antioxidant activity, which is consistent with other results on Salvia species41, Dracocephalum moldavica42 and Scutellaria altissima43.
Proximate composition
Table 4 shows the approximate composition of flower including carbohydrates, protein, fat, and fiber of different Alcea species. Significant disagreements were observed between different Alcea species in terms of the approximate composition content of flowers. The carbohydrate varied from 42.44 g/100 g DW in A. xanthochlora to 58.62 g/100 g DW in A. koelzii. The flower protein content of different varieties varied from 6.06 to 11.30 g/100 g DW, with the lowest and maximum being determined in A. tehranica and A. kurdica species, respectively. The protein content of Agave salmiana44, Arbutus xalapensis45, and Borage46 flowers was 11.58, 11.3 and 22.69 g/100 g DW, respectively. A. xanthochlora showed the highest (2.53 mg/100 g DW) fat content and A. lavateriflora showed the lowest (1.30 mg/100 g DW) fat content. Among the approximate composition, the lowest amount was related to fat. In general, flowers have a lower fat content than other plant organs. According to the results of other studies, the fat content in Calendula47 and Borage46 has been reported to be 5.33 and 4.93 g/100 g DW, respectively. The maximum (28.64 mg/100 g DW) and lowest (13.34 mg/100 g DW) fiber content was determined in A. xanthochlora and A. koelzii species, respectively. Jakubczyk et al.48 reported fiber content (mg/100 g DW) of the flower of some plants including Calendurla officinalis (62.33), and Centaurea cyanus (53.06). Fiber, which plays a substantial role in the prevention of cardiovascular diseases, gastrointestinal diseases, diabetes and some cancers, is about 3–6 times higher in this study than the amount found in the US Food Database (4.5 g/100 g)49. The maximum ash was achieved in A. lavateriflora (8.3 mg/100 g DW), followed by A. xanthochlora (7.83 mg/100 g DW), and A. kurdica (7.34 mg/100 g DW).
EDX elemental analysis
There was a significant difference among different Alcea species in terms of flower mucilage mineral content (p < 0.05). The EDX analysis of flower mucilage of different species is shown in Fig. 6. The oxygen and carbon content varied between samples, ranging from 29.1 to 44.2% and 15.8–28.9%, respectively. A. lavateriflora and A. xanthochlora had the highest (8.1%) and lowest (2.7%) nitrogen levels, respectively. The highest amount of potassium was found in the A. tehranica (22.8%) followed by A. xanthochlora (19.4%), A. lavateriflora (14.8%) and A. rechingeri (14.6%). The lowest amount (7.4%) of potassium was found in the A. koelzii. The amount of phosphorus varied from 1.7% in the A. rechingeri to 3.9% in the A. tehranica. The species A. lavateriflora, A. xanthochlora and A. tehranica had the maximum calcium amount with 16.9, 13.5 and 7.5% respectively and the lowest calcium content (4.7%) was observed in the A. kurdica. The highest magnesium was determined in the A. tehranica (5.1%) and A. kurdica (4.12%). The lowermost magnesium amount (1.4%) was in the A. koelzii. The highest levels of sodium (4.2%) and silicon (0.9%) were observed in A. lavateriflora and A. rechingeri, respectively. Elemental analysis via EDX of Althaea officinalis seed mucilage revealed the following elemental composition: nitrogen at 5.34%, oxygen at 18.32%, carbon at 26.59%, magnesium at 2.04%, chlorine at 9.4%, potassium at 26.39%, sulfur at 4.22%, and calcium at 7.69%50. In another study on Alcea species, the highest levels of carbon (49.2%), oxygen (42.8%), nitrogen (8.1%), potassium (53.1%), phosphorus (3.4%), and calcium (53.1%) were found in the A. tehranica, A. remotiflora, A. kurdica, A. rechingeri, A. schiraziana and A. rechingeri species, respectively7. Additionally, Procacci et al.51 reported that mucilage extracted from Opuntia ficus-indica cladodes contained oxygen at 48.46%, carbon at 39.4%, calcium at 4.85%, potassium at 0.88%, sulfur at 0.27%, magnesium at 3.84%, and phosphorus at 0.22%. Variations in these results compared to other studies may be influenced by factors such as plant organs analyzed, genetic differences, and environmental conditions during cultivation7.
Correlation, cluster analysis and PCA
The outcomes of the analysis concerning the interrelations among traits, as determined by the Pearson correlation coefficient, is illustrated in the accompanying Fig. 7. Positive communication are depicted in blue, while negative correlations are indicated in red. Notably, a positive and statistically significant relationship was observed between apigenin and carbohydrates with fiber. Conversely, total anthocyanin demonstrated a negative and statistically significant relationship with flower diameter. Additionally, a significant positive relationship was identified among chlorogenic acid, P, Si, and Mg. The flower dry weight had a positive and significant correlation with number of flower, and plant height. Furthermore, TPC, TFC, and mucilage content all showed a positive and significant correlation with antioxidant activity. Numerous research has documented a significant positive association between antioxidant activity and phenolic compounds, aligning with the findings of this investigation41,43.
In this research, principal component analysis (PCA) was conducted focusing on agro-morphological, bioactive compounds and minerals across various Alcea species. Table 5 illustrates the relationships among the species and the studied traits. The analysis revealed that the first eight PC accounted for 95.67% of the variance among the species. Specifically, PC1, PC2, PC3, and PC4 contributed 39.85%, 17.02%, 14.63, and 4.32% of the total variance, respectively. The first component was predominantly influenced by traits such as antioxidant activity, TAC, potassium, magnesium, carbon, TPC, oxygen, kaempferol, TFC, flower diameter, silicon, number of flowers, flower dry weight and mucilage content. The second component was primarily affected by nitrogen, fat, apigenin, sodium, carbohydrates, plant height, and ash. The third component highlighted traits such as number of lateral branches, moisture, proteins, chlorogenic acid, and calcium with the highest coefficients. Fiber and rutin also had the greatest coefficients on component four (Table 5). This analysis serves to elucidate the key differentiating factors among the species under study.
The results of the cluster analysis conducted on various cultivars based on agro-morphological and bioactive compounds are presented in Fig. 8. The cluster analysis revealed that the species could be classified into three primary groups. The A. koelzii and A. rechingeri species, which were superior in terms of the number of lateral branches, number of flowers, flower diameter, flower dry weight, and plant height, were placed in the first group (I). The species A. tehranica, A. lavateriflora and A. kurdica were placed in the second group (II), which were superior to other species in terms of TAC, mucilage, TFC, TPC, calcium, silicon, antioxidant activity, potassium and phosphorus traits. The A. xanthochlora species, which was superior in terms of fiber and chlorogenic acid traits, was placed in the third group (III). A biplot generated from PC1 and PC2 illustrated the diversity among the cultivars and corroborated their classification into three distinct groups (Fig. 9). This biplot reinforced that the findings related to functional traits and bioactive compounds were largely consistent with those derived from the cluster analysis.
Conclusion
The study highlights the significant potential of certain Iranian Alcea species as sources of valuable bioactive compounds. Among the species examined, A. tehranica and A. kurdica exhibited the highest levels of TAC. The species A. lavateriflora, A. tehranica, and A. rechingeri had the highest mucilage content. Apigenin and rutin were identified as the uttermost phenolic acids present in these species. Additionally, the flowers of the Alcea species demonstrated a commendable level of fats, proteins, and carbohydrates. Furthermore, the TPC, TFC, and phenolic compounds in the flowers significantly contributed to their antioxidant potential. A. lavateriflora, A. tehranica, and A. rechingeri are recommended for further agricultural production and research, particularly for their potential uses in the pharmaceutical and food industries, as well as their suitability as breeding candidates.
Data availability
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
References
Shi, Y. et al. Designing perennial landscapes: plant form and species richness influence the gaze perception associated with aesthetic preference. Land 11 (10), 1860. https://doi.org/10.3390/land11101860 (2022).
Ikten, H. et al. Assessment of the genetic diversity and population structure of Vaccaria hispanica (Mill.) Rauschert germplasms of Türkiye based on SRAP and SSR markers. Turk. J. Agric. For. 47 (6), 983–998. https://doi.org/10.55730/1300-011X.3143 (2023).
Dinesha, S. et al. Underutilized edible fruit species of the Indo-Gangetic plains: A systematic review for food security and land degradation neutrality. Turk. J. Agric. For. 48 (3), 443469. https://doi.org/10.55730/1300-011X.3193 (2024).
Chaachouay, N. & Zidane, L. Plant-Derived natural products: A source for drug discovery and development. Drugs Drug Candidates. 3 (1), 184–207. https://doi.org/10.3390/ddc3010011 (2024).
Nawaz, M. A. et al. Global metabolome profiles of Lonicera caerulea L. and Lonicera caerulea ssp. kamtschatica (Sevast.) Gladkova. Turk. J. Agric. For., 48 (5), 745–759. (2024). https://doi.org/10.55730/1300-011X.3216
Tiwari, D. et al. Predicting phytochemical diversity of medicinal and aromatic plants (MAPs) across eco-climatic zones and elevation in Uttarakhand using generalized additive model. Sci. Rep. 13, 10888 (2023).
Sharifi, B. et al. Unveiling the mucilage complexity in a multispecies exploration of the genus Alcea from Iran. Ind. Crops Prod. 220, 119275. https://doi.org/10.1016/j.indcrop.2024.119275 (2024).
Azadeh, Z., Saeidi, K., Lorigooini, Z., Kiani, M. & Maggi, F. Organ–oriented phytochemical profiling and radical scavenging activity of Alcea spp. (Malvaceae) from Iran. SN Appl. Sci. 2, 927 (2020).
Al-Snafi, A. E. The pharmaceutical importance of Althaea officinalis and Althaea rosea: A review. Int. J. Pharm. Res. 5 (3), 1378–1385 (2013).
Fahamiya, N., Shiffa, M., Aslam, M. & Muzn, F. Unani perspective of Khatmi (Althaea officinalis). J. Pharmacogn Phytochem. 5 (6), 357–360 (2016).
Kumar, S. S., Sudhakar, S., Kapil, S. & Snigdha, T. Ethnopharmacological review on Althaea officinalis. World J. Pharm. Pharm. Sci. 5 (7), 425–432 (2016).
Mousavi, S. F., Razavi, S. M. A. & Koocheki, A. Marshmallow (Althaea officinalis) Flower Gum397–423 (Rheology and Functions, 2019).
Reinelt, N. & Melzig, M. F. Der echte Eibisch Althaea officinalis L. Zeitschrift. Fur Phytotherapie. 38 (2), 91–96 (2017).
AnjuIdris, M. A brief review on a Unani drug: Khatmi (Althaea officinalis). Asian J. Pharm. Sci. 4 (4), 394–398 (2018).
Yashaswini, S., Hegde, R. & Venugopal, C. Health and nutrition from ornamentals. Int. J. Res. Ayurveda Pharm. 2, 375e382 (2011).
Ghareaghajlou, N., Hallaj-nezhadi, S. & Ghasempour, Z. Red cabbage anthocyanins: stability, extraction, biological activities and applications in food systems. Food Chem. 365, 130482. https://doi.org/10.1016/j.foodchem.2021.130482 (2021).
Pourhosseini, S. H. et al. Diversity of phytochemical components and biological activities in Zataria multiflora boiss. (Lamiaceae) populations. S Afr. J. Bot. 135, 148–157. https://doi.org/10.1016/j.sajb.2020.08.024 (2020).
Hatami, H., Karimi, M., Aghaee, A., Bovand, F. & Ghorbanpour, M. Morphological diversity, phenolic acids, and antioxidant properties in eryngo (Eryngium caucasicum Trautv): Selection of superior populations for agri-food industry. Food Sci. Nutr. 10, 3905–3919 (2022).
Esmaeili, H., Karami, A., Hadian, J., Nejad Ebrahimi, S. & Lars-Gernot, O. Genetic structure and variation in Iranian licorice (Glycyrrhiza glabra L.) populations based on morphological, phytochemical and simple sequence repeats markers. Ind. Crops Prod. 145, 112–140 (2020).
Husain, M., Wadud, A., Hamiduddin Sofi, G., Perveen, S. & Hafeez, K. A. Physicochemical standardization of mucilage obtained from Althaea officinalis Linn– root. Pharmacogn Mag. 15 (62), 155–161 (2019).
Slinkard, K. & Singleton, V. L. Total phenol analysis: automation and comparison with manual methods. Am. J. Enol. Vitic. 28, 49–55. https://doi.org/10.5344/ajev.1977.28.1.49 (1977).
Chang, C. C., Yang, M. H., Wen, H. M. & Chern, J. C. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug Analys. 10 (3), 3. https://doi.org/10.38212/2224-6614.2748 (2002).
Benzie, I. F. F. & Strain, J. J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal. Biochem. 239 (1), 70–76. https://doi.org/10.1006/abio.1996.0292 (1996).
Sellappan, S., Akoh, C. C. & Krewer, G. Phenolic compounds and antioxidant capacity of Georgia-grown blueberries and blackberries. J. Agric. Food Chem. 50 (8), 2432–2438 (2002).
Sellappan, S. & Akoh, C. C. Flavonoids and antioxidant capacity of Georgia-grown Vidalia onions. J. Agric. Food Chem. 50 (19), 5338–5342 (2002).
AOAC. Official Methods of Analysis 15th edn, Vol. 1 (Association of Official Analytical Chemists, 1990).
Wang, Y. et al. Genetic diversity and population structure analysis of Hollyhock (Alcea rosea Cavan) using high-throughput sequencing. Horticulturae 9 (6), 662 (2023).
Ghasemi, M. & Atakishiyeva, Y. Investigation of the antibacterial effect of native Peganum harmala, Mentha pulegium and Alcea rosea hydro-alcoholic extracts on antibiotic resistant Streptococcus pneumoniae and Klebsiella pneumonia isolated from baku, Azerbaijan. Infect. Epidemiol. Micr. 2 (2), 12–14 (2016).
Waghmare, R., Moses, J. A. & Anandharamakrishnan, C. Mucilages: sources, extraction methods, and characteristics for their use as encapsulation agents. Crit. Rev. Food Sci. Nutr. 62 (15), 4186–4207. https://doi.org/10.1080/10408398.2021.187373 (2022).
Chandravanshi, K. et al. Isolation of mucilage from herbal plants and its evaluation as a pharmaceutical excipients. J. Pharmacogn Phytochem. 14 (3), 171–178. https://doi.org/10.52711/0975-4385.2022.00031 (2022).
Nayak, A. K., Hasnain, M. S., Nayak, A. K. & Hasnain, M. S. Fenugreek seed mucilage based multiple units for oral drug delivery. Plant polysaccharides-based multiple-unit systems for oral drug delivery. 93–112. (2019). https://doi.org/10.1007/978-981-10-6784-6_8
Liu, J. et al. Anthocyanins: promising natural products with diverse Pharmacological activities. Molecules 26 (13), 3807. https://doi.org/10.3390/molecules26133807 (2021).
Lakshmikanthan, M. et al. A comprehensive review on anthocyanin-rich foods: insights into extraction, medicinal potential, and sustainable applications. J. Agric. Food Res. 17, 101245. https://doi.org/10.1016/j.jafr.2024.101245 (2024).
Chai, Z. et al. A comparative analysis on the anthocyanin composition of 74 blueberry cultivars from China J. Food Compos. Anal. 102, 104051. https://doi.org/10.1016/j.jfca.2021.104051 (2021).
Kim, J. G., Kim, H. L., Kim, S. J. & Park, K. S. Fruit quality, anthocyanin and total phenolic contents, and antioxidant activities of 45 blueberry cultivars grown in Suwon. Korea J. Zhejiang Univ. Sci. B. 14, 793799. https://doi.org/10.1631/jzus.B1300012 (2013).
Dudek, M., Matławska, I. & Szkudlarek, M. A. Phenolic acids in the flowers of Althaea rosea var. Nigra. Acta Pol. Pharm. 63 (3), 207–211 (2006).
Ertas, A. et al. Fatty acid, essential oil and phenolic compositions of Alcea pallida and Alcea apterocarpa with antioxidant, aanticholinesterase and antimicrobial activities. Chiang Mai J. Sci. 43 (1), 89–99 (2016).
Benbassat, N. et al. Influence of the extraction solvent on antioxidant activity of Althaea officinalis L. root extracts. Cent. Eur. J. Biol. 9 (2), 182–188. https://doi.org/10.2478/s11535013-0245-2 (2014).
Krasteva, D., Ivanov, Y., Chengolova, Z. & Godjevargova, T. Antimicrobial potential, antioxidant activity, and phenolic content of grape seed extracts from four grape varieties. Microorganisms 11, 395 (2023).
Moshari-Nasirkandi, A. et al. Screening of 20 species from Lamiaceae family based on phytochemical analysis, antioxidant activity and HPLC profiling. Sci. Rep. 13, 16987. https://doi.org/10.1038/s41598-023-44337-7 (2023).
Moshari-Nasirkandi, A. et al. Chemometrics-based analysis of the phytochemical profile and antioxidant activity of Salvia species from Iran. Sci. Rep. 14, 17317. https://doi.org/10.1038/s41598-024-68421-8 (2024).
Weremczuk-Jeżyna, I., Grzegorczyk-Karolak, I., Frydrych, B., Królicka, A. & Wysokińska, H. Hairy roots of Dracocephalum moldavica: Rosmarinic acid content and antioxidant potential. Acta Physiol. Plant. 35, 2095–2103. https://doi.org/10.1007/s11738-013-1244-7 (2013).
Grzegorczyk-Karolak, I., Kuźma, L. & Wysokińska, H. Study on the chemical composition and antioxidant activity of extracts from shoot culture and regenerated plants of Scutellaria altissima L. Acta Physiol. Plant. 37, 1–9. https://doi.org/10.1007/s11738-014-1736-0 (2015).
Pinedo-Espinoza, J. M. et al. Nutritional composition, bioactive compounds and antioxidant activity of wild edible flowers consumed in semiarid regions of Mexico. Plant. Foods Hum. Hum. Nutr. 75, 413–419 (2020).
Sotelo, A., López-García, S. & Basurto-Peña, F. Content of nutrient and antinutrient in edible flowers of wild plants in Mexico. Plant. Foods Hum. Hum. Nutr. 62, 133–138 (2007).
Fernandes, L., Ramalhosa, E., Pereira, J. A., Saraiva, J. A. & Casal, S. Borage, camellia, Centaurea and pansies: nutritional, fatty acids, Ffree sugars, vitamin E, carotenoids and organic acids characterization. Food Res. Int. 132, 109070 (2020).
Pires, T. C. S. P., Dias, M. I., Barros, L. & Ferreira, I. C. F. R. Nutritional and chemical characterization of edible petals and corresponding infusions: valorization as new food ingredients. Food Chem. 220, 337–343 (2017).
Jakubczyk, K., Koprowska, K., Gottschling, A. & Janda-milczarek, K. Edible flowers as a source of dietary fibre (total, insoluble and soluble) as a potential athlete’s dietary supplement. Nutrients 14, 2470 (2022).
Food Data Central (FDC). (2021). Available at: https://fdc.nal.usda.gov/fdc-app.html#/fooddetails/168165/nutrients (Accessed 1 Dec 2021).
Moazzezi, S., Elhamirad, A. H., Nateghi, L., Haddad Khodaparast, M. H. & Zarei, F. Studies on physicochemical and structural properties of marshmallow (Althaea officinalis) seed mucilage. J. Food Biosci. Technol. 12 (1), 29–38. https://dorl.net/dor/20.1001.1.22287086.2022.12.1.4.3 (2022).
Procacci, S. et al. Opuntia ficus-indica pruning waste recycling: recovery and characterization of mucilage from cladodes. Nat. Resour. 12, 91–107 (2021). https://www.scirp.org/journal/nr)
Acknowledgements
The authors are grateful to the Shahid Beheshti University Research Center for their support in providing research facilities.
Author information
Authors and Affiliations
Contributions
Yasaman Mashhadi Tafreshi: Methodology, Carried out field and lab work, Phenotyping, Data curation. Ghasem Eghlima: Sample collection, Supervision, Conceptualization, Methodology, Data curation, Investigation, Formal analysis, Writing-original draft. Samad Nejad Ebrahimi: Conceptualization, Methodology, Writing-review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tafreshi, Y.M., Eghlima, G. & Ebrahimi, S.N. Phenotypic yield-attributed traits and phytochemical composition of the flowers from Alcea species in Iran. Sci Rep 15, 22362 (2025). https://doi.org/10.1038/s41598-025-08706-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-08706-8
Keywords
This article is cited by
-
Variability in lutein and zeaxanthin content, fatty acid and phytosterols profiles, and genetic parameters of some Tagetes spp. cultivars
BMC Plant Biology (2026)
-
Morphological and biochemical diversity among wild-grown barberry (Berberis vulgaris L.) genotypes from Isfahan province, Iran
Scientific Reports (2025)
-
Evaluation of performance, essential oil composition, and genetic parameters of Grammosciadium platycarpum populations toward developing high-linalool industrial cultivars
Scientific Reports (2025)