Abstract
The burning of excessive fossil fuel produces a high amount of noxious compounds, posing significant threats to environmental and human health. However, to elucidate these problems in this study biodiesel was produced from the locally available dropdown neem (Melia azadirachta) seeds derived oil by applying the widely practiced transesterification process via solution blending with high yield (around 86%). Using advanced instruments (such as GC, FTIR-ATR, CV analysis, etc.), the produced biodiesel was characterized and it’s overall qualities were investigated by optimizing it under different conditions, such as catalyst, temperature, time and stirring speed. The observed results showed biodiesels have possessed high calorific values (around 9562.22 ± 5.87 kcal/kg), remarkable physicochemical properties, active binding sites along with various saturated/unsaturated fatty acids. Therefore, due to these exceptional properties, the newly produced biodiesels would be beneficially used in various sectors as a suitable replacement of hazardous fossil fuels regarding the sustainable environmental protection.
Similar content being viewed by others
Introduction
To meet the luxurious life style as well as the massive demand of the growing population nowadays we are enormously reliant on various fossil based hazardous materials1,2,3. Whereas fuel oils like petrol, kerosene, diesel, octane, CNG, LPG, etc. are one of the most widely used ones. While burning or consuming these fossil fuels could have been easily pollutes out total environment hence they have lately been recognized as a breakneck tread on for all the air, water, and soil as well as human health safety/security4,5,6. The unrelenting burning of fossil fuels has caused a crisis of environmental deterioration and public health crises. According to7, air pollution alone causes 8.7 million premature deaths per year, and CO2 emissions will reach 36.8 billion tons in 20238.
So this particular problem should be solved urgently by replacing the hazardous fossil fuels by biobased ecofriendly energy sources/fuels for the sustainable environmental protection. While biodiesel, primarily derived from naturally occurring plant materials, can serve as an alternative to fossil fuels, it stands out as a renewable energy source, especially as the demand for fossil fuels decreases. Plus, it has the potential to enhance a nation’s socioeconomic value9. Among all energy system about 80% primary energy comes from fossil fuel where from oil 32.8%, coal 27.2%, natural gas 20.9%10. Fossil fuels are extremely used in case of transportation, industrial development and agriculture11. Burning of the fossil fuels produces various toxic gases (COx, SOx, NOx), and organic compounds which are all released into the atmosphere and cause air pollution12,13.Inadequate concentration of carbon dioxide and a number of other greenhouse gases, raising fears that the planet may warm by 1–5 °C over the course of the next century14. On the other hand, fossil fuel-related pollution are responsible for around 65% of excess mortality worldwide15. Inhaling of polluted air might potentially lead to serious health problem. Recently several research have found that polluted air shows pulmonary health effect, asthma, lung cancer, respiratory infection, chronic obstructive pulmonary disease (COPD) etc16,17,18. Beside all these uneven distribution of fossil fuels in the energy production system not only poses health and environmental risks, but it also poses a major threat to maintaining energy security19. From 2005 to 2030 oil demand will grow about 1.3% per annum20. Oil consumption increased from 84 million barrels per day in 2005 to 99 million barrels per day by 2015 and 116 million barrels per day by will be in 203021. Since fossil fuels are a form of nonrenewable energy, there are issues for the next generation to think about and their complete depletion should be avoided22. As biofuel is produced from organic materials by chemical reactions, which are abundant in nature, this type of raw material is easy to collect. Hence, according to International Energy Agency, biofuel provide a low-carbon alternative to current technologies, such as light duty automobiles in the near term and heavy-duty trucks, ships, and aircraft with few other viable and affordable options in the long run, biofuels are particularly significant in the decarbonization of transportation23. In comparison to conventional fuels, biofuels typically burn cleaner, and when biodiesel and ethanol blends are used in car engines, particulate matter (PM), carbon monoxide (CO), and unburned volatile organic compounds (VOC) emissions are reduced24. However, for better understanding the global energy demand/consumption including all possible source of energy has been shown in Fig. 1, while the data were taken according to the resent year from a strong database such as8.
Global energy consumption in 2023. Data taken from (IEA, 2024)8.
After over 350 oil-bearing crops were examined, some of them were suitable to be taken into consideration as possible diesel engine alternative fuels11. Melia azadirachta is a type of neem which is found abundant is south Asian countries like Bangladesh, Pakistan, India. It can grow on soils of almost all kinds and in adverse climatic conditions except long lasting cold and freezing. Neem trees begin to produce edible seeds in three to five years, reach maximum output in ten years, and continue to do so for 150 to 200 years after they are planted25. When this tree’s fruit is ripens, birds either consume it or it is wasted. Although the seeds from these trees cannot be utilized as cattle feed, their abundant local availability and ability to thrive under normal climatic conditions make them a promising non-edible feedstock for sustainable biodiesel production. Because this particular neem seeds have shown a high theoretical oil quantity which could be varied from the range inter between 20 and 45%. Production of biodiesel from this plant seed could be a much more beneficial source of renewable energy as a sustainable alternative of hazardous fossil based fuels/fuel oils26,27,28. In the past few decades, researchers from all over the world have been exasperating to develop a new route of production of biofuels from the nonedible plant seeds/seed oils to solve the environmental pollution issues. Hence, the production of biodiesel from the available nonedible seeds of various natural plants would be a promising ones regarding the sustainable development of the total environment including all the air, water, and soil. However, for better understanding a list of comparison addressing the production of biodiesel from different natural sources indicating the name of seed oils/sources, method of production of biodiesel, name of the used catalyst, and yield percent of the final products i.e., biodiesel with relevant references has been shown in Table 1.
Though, a limited number of works has been done on the production of biodiesel from different sources. But biodiesel production from the specific species of Melia azadirachta neem, no one performed the research previously. Again transesterification process carried out for production of biodiesel. High calorific value of biodiesel made the work unique as well. Therefore, production of ecofriendly biodiesel from this species of neem seed oils could be very much new, innovative, and beneficial as well as more useful for much heating duties in case of engine and so on. Hence, a harsh understanding about the effective extraction of the organic neem oils from the dropdown useless neem seeds (consider as an agro-waste materials) to produce a new class of high efficiency biobased ecofriendly fuel oils namely biodiesel with high yield percent by employing a prominent cost-effective method namely transesterification to enhance their overall physical along with chemical properties is very much crucial indeed. Thus, the main aim of this current work is to (i) Extract neem oil by soxhlet apparatus, (ii) Biodiesel production from the neem oil by transesterification, (iii) Optimization of biodiesel by varying reaction conditions, (iv) Physio-chemical properties of biodiesel observation, and (v) Fatty acid analysis of produce biodiesel.
Experimental
Materials
Unripe fresh neem seeds specious of Melia azadirachta were collected from Rajshahi, Bangladesh. N-hexane, methanol (CH3OH), KOH, acetic acid (CH3COOH), potassium iodide (KI), Sodium thiosulfate (Na2S2O3⋅ 5H2O), chloroform all were purchased from BDH (England), Starch solution was prepared in the lab. Ultra-pure water and DI water were collected from the Oils, Fats, and Waxes Research Division, BCSIR Rajshahi Laboratory, Rajshahi-6206.
Method for extraction of neem oil from neem seeds
First, clean water was used to wash the entire neem seed. Next, it was dried in a microwave oven set to 105 °C for 24 h of retention. In a grinder, whole dried neem seeds were pulverized. After the material was sieved, it was confirmed that every particle was less than 5 mm. The analytical balance was then used to weigh 50 g of neem powder. Filter paper was used to hold this sample. A stapler pin was then used to seal it. The soxhlet chamber was filled with the sample. A 300 mL round-bottom flask containing the solvent n-hexane was filled. After heating n-hexane to 70 °C, the extraction process took almost two hours32. The extracted oil was simply heated to 70 °C in an oven to separate oil from the solvent. However, for better clarity the whole process of the extraction of neem seed oil by following Soxhlet solvent extraction during the experimental session of this current study has been shown in Fig. 2.
Noteworthy that the yield percent (%) of the extracted neem seed oil can be measured by using the following equation (Eq. 1):
Methods of physicochemical parameters analysis of the extracted neem oil
Acid value determination
1.4028 g of KOH was thoroughly mixed with 100 mL of distilled water into a conical flask so that it does dissolve in water. Additional amount of water added to make 250 mL solution. 50 mL from this solution was taken into burette. 10 mL of absolute ethanol shifted into conical flask and some drop of phenolphthalein indicator was added into it. Then ethanol was neutralized to do blank titration by adding 0.1 N KOH solution drop wise from burette until light pink color was just attained. At this time burette reading was 0.1 mL. After this 2 g of extracted neem seed oil was taken into another conical flask. Then neutralized abs. ethanol was mixed with this amount of oil by ultrasonic bath where temperature was 60 °C and heating time was 20 min. 2/3 drops phenolphthalein indicator was added with oil sample.0.1 N KOH solution was added drop wise from burette into the oil sample until light pink color attained. At that time burette reading was 1.4. Acid value can be measured by the following equation (Eq. 2):
Where, S = Volume of KOH (mL) required in titration; B = Volume of KOH (mL) required in blank titration; N = Normality of KOH; M = Molecular weight of KOH; W = Weight of sample.
Free fatty acid determination
Free fatty acid content (FFA) can be determined by the following equation (Eq. 3):
Biodiesel production and analysis
The significant route of production of biodiesel from the extracted neem seed oil by employing the prominent method namely transesterification and solvent washing has been described below. For better clarity the flow diagram along with the required chemical reaction have also been presented.
Transesterification of neem seed oil
In the context of biodiesel, transesterification is a chemical reaction that is commonly used to produce mono esters (biodiesel) and glycerol by reacting triglycerides and alcohol (Sekhar et al. 2009). Transesterification can be represented by (Eq. 4).
The base catalyzed transesterification was carried out using 10 mL of neem oil in a magnetic stirrer. The alcohol and catalyst used was methanol and potassium hydroxide (KOH) respectively.
Potassium hydroxide (KOH) was selected as the catalyst for transesterification due to its high catalytic activity, cost-effectiveness, and ability to tolerate moderate levels of free fatty acids. Its efficiency in promoting the conversion of triglycerides to methyl esters has been well-documented in literature. However, one known drawback of alkali catalysts like KOH is the possibility of soap formation when free fatty acids are present, which can hinder separation and reduce biodiesel yield. Although soap formation was not quantitatively measured in this study, its potential influence on the reaction efficiency is acknowledged. Similarly, while methanol was used in excess to drive the reaction forward, no methanol recovery was performed, and the recovery rate was not quantified. These aspects are considered limitations of the current study and are identified as targets for future optimization and sustainability assessments. Due to the more reactive and less expensive methanol (CH3OH) is more preferred than other alcohols. Compared to another catalyst, potassium hydroxide (KOH) is more preferred due to being more catalytically active, tolerant of higher levels of FFA, need of lower concentration. Neem oil was mixed with methanol in the presence of a catalyst KOH. The catalyst was helped to accelerate the reaction and is often referred to as an alkali catalyst. The triglycerides in neem oil was reacted with methanol in the presence of KOH. This reaction was responsible in the formation of biodiesel (fatty acid methyl esters) and glycerol. After the transesterification process the mixture of biodiesel was shifted to a separating funnel. After allowing to settle for 24 h biodiesel was at upper layer and glycerol was at lower layer. Then the biodiesel layer was separated from glycerol for better purity. However, for better clarity and to stand out the originality, the whole process of the production of biodiesel by following the prominent method namely trans esterification during the experimental session of this current study has been shown in Fig. 3.
Washing of biodiesel
The biodiesel was washed to remove impurities and water soluble contaminants. Biodiesel and water was mixed together in a conical flask. After phase separation, the biodiesel layer was washed three times with warm distilled water (20% v/v of biodiesel). Each wash involved 2 min of stirring, followed by 15 min of settling to remove residual catalyst, glycerol, and water-soluble impurities. The washed biodiesel was dried at 105 °C for 30 min to eliminate moisture, adhering to American Society for Testing and Materials (ASTM) D6751 standards. However, for better understanding the scope, contribution, novelty, selectivity, and significances of the applied method as well as to stand out the originality of this current study, the overall experimental process addressing the extraction of neem oil, production of biodiesel, and processing of the newly produced crude biodiesel by following the beneficial purification methods during the real-time experimental session has been shown in Fig. 4, by providing an easily readable flow diagram.
Biodiesel analysis
Different types of significant physical and chemical properties including density, viscosity, specific gravity, refractive index, peroxide value, para-anisidine, calorific value, etc., were investigated by ASTM standard protocol. However, for better understanding a short description of these particular methods including the experimental conditions have been provided below:
Density measurement
The density was determined by measuring the mass and volume of each sample. The mass of test sample was measured to an accuracy of 0.0001 g by using an analytical balance. And the volume was measured by measuring cylinder. Temperature of the sample can affect density33. That’s why each biodiesel sample was taken at 20 °C. As much as accurately biodiesel sample was filled in a measuring cylinder at the level of 5 mL. Then the weight of sample was measured by an analytical balance. Then density was measured by the following equation (Eq. 5):
Specific gravity
Specific gravity gives information about any substance is how much lighter or denser comparing with the density of water. It provides clear insight about fluid behavior of biodiesel. Specific gravity (S.G) was measured by using the following equation (Eq. 6).
Refractive index
Refractive index method determines light bending or refraction when light enters that medium or exits. Refractive index assesses the valuable insight about the purity and overall quality of biodiesel. Refractive index was determined by using digital refractometer (Model number: DR 102, China) with high accuracy by maintaining the protocol of ASTMD1218.
Peroxide value
2 g of biodiesel sample was taken in a 250 mL conical flask. 25 mL chloroform to acetic acid (2:3) mixture was added with the sample. 1 mL of saturated KI solution was added with sample. Then the conical flask was shacked for 1 min and kept it for 5 min in a dark place. Side wall of flask was washed by distilled water. 0.5 mL of 1% starch solution was added with the sample very carefully. Then titration was done using 0.05 N Na2S2O3⋅ 5H2O solution. The peroxide value was determined by the following equation (Eq. 7)34:
Where: S = Volume of Na2S2O3⋅ 5H2O (mL) required in titration; B = Volume of Na2S2O3⋅ 5H2O (mL) required in blank titration; N = Normality of Na2S2O3⋅ 5H2O; W = Weight of biodiesel sample in gram.
Para-anisidine
The para-anisidine value (PAV) is a measure of the oxidation products in fats and oils, including biodiesel. It assesses the degree of oxidative degradation and is an important parameter to evaluate the stability and quality of biodiesel. The determination procedure was done by conducting a special types of CDR Foodlab Analyzer device by maintaining the protocol of ASTM D4492.
Calorific value
Calorific value, sometimes called heating value or energy value, is the amount of energy generated during the whole burn of a fuel. It is an important factor to consider while evaluating the effectiveness and acceptability of various fuels for different purposes35. The calorific value of the biodiesel was obtained using bomb calorimeter (which have a model number: JULIUS PETERS, BERLIN NW21, GERMANY) with the pressure vessel 30 bar with around 99% pure oxygen gas as a carrier. Here crucible hold the sample which is basically equipped in the bomb. For adequate combustion oxygen is pressurized inside bomb. Bomb placed into calorimeter which is basically a container. Also there remain water which absorb heat when ignition occurs. Accurate determination of the heat of combustion can be achieved by measuring the subsequent temperature change, usually using a thermometer36. Theoretical equation (Eq. 8) for bomb calorimeter can be expressed as follows-
Where, ‘m’ is mass of water, ‘c’ is the specific heat capacity of the water and ‘ΔT’ is measured temperature change.
Viscosity analysis
Viscosity can be described as resistance to flow. Viscosity is an important physical properties of biodiesel. Higher viscosity can show adverse effect in case of performance of engine and can causes higher energy demand37. Viscosity decreases with as long as temperature increases. Viscosity of biodiesel were measured using a special types of viscometer with a model number (Fungilab, Smart Series, Serial number: TSML: 261536, Code number: V210003, Barcelona, Spain). However, for better accuracy at least 3 replicas were recorded for every single individual samples, while experiments were carried out in room temperature by following the actual procedure.
Gas chromatography analysis
One of the most recognized and sophisticated analytical techniques for separating and evaluating components in a mixture is gas chromatography. Gas chromatography enables it to be feasible to with accuracy and delicately analyze heterogeneous mixtures. In this study GC (which have a model number: Shimadzu GC-2010 plus, country of origin: Japan) has been used for analyzing the subjected sample of biodiesel. The sample was injected into a heated column, and specific components were carried out by the inert gas acting as a mobile phase38. Hydrogen and nitrogen are used as inert gases. 20 mL/min of nitrogen and 40 mL/min of hydrogen were consistently maintained. There was 400 mL/min of air flow. As components move into columns, they become separated according to characteristics such as affinity, volatility, and polarity39. Based on different retention time separated compound detected.
FTIR analysis
FTIR-ATR is one of the most known method that has been widely used for identification of specific functional groups within a sample and to know molecular bonds and arrangements40,41,42. It provides insightful information for chemical analysis. Based on intramolecular vibration which identify purity of compound43,44. For analyzing the sample FTIR-ATR (which have a model number: PerkinElmer, L1600300 spectrum TWO, Serial Nmber:115061, made in Llantrisant, UK, country of origin: England) has been used. Updated software version was 10.6.2. Sample was placed on diamond prism. Recorded spectra was in the range of 400–4000 cm-1 while particular resolution was 5 cm-145,46. However, for better accuracy at least 3 replicas were recorded for every single individual samples, meanwhile the investigations were carried out in room temperature and before each trials the sample holder was cleaned properly by using isopropyl alcohol solution47,48.
Results and discussions
Analysis of extracted neem oil
Yield of oil have influence on production of biodiesel. But yield of oil can vary depending on type of species and maturity of the seeds49. Moderately mature species of Melia azadirachta whole neem seeds oil was found to be around 20%. Though higher oil content favorable for biodiesel production.
Free fatty acid (FFA) and acid value (AV)
FFA and AV are important factor of oil. Lower FFA and AV means higher shelf life of oil50. Large amount of FFA and AV causes need for esterify the excess amount of free fatty acid before transesterification51. FFA and AV was found to be 1.82 mg KOH/g and 3.64 mg KOH/g.
Factor affecting production of biodiesel
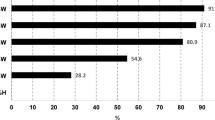
Due to low FFA and AV direct transesterification was done to produce the biodiesel. Molar ratio of alcohol to oil, reaction time, amount of catalysts, stirring speed, and temperature influence the transesterification process52. However, for better understanding the selectivity, contribution, significant and effects of the influential factors like effect of molar ratio of oil and methanol, effect of catalyst quantity, effect of reaction time, effect of stirring speed, and effect of applied temperature on the yield percent of the newly produced biodiesels, the observed findings have been shown in Fig. 5a and e.
Effect of molar ratio of oil to methanol
The molar ratio of oil to methanol had a major influence on the synthesis of biodiesel from neem seed oil. This ratio was a key indicator because it controlled the availability of reactants throughout the transesterification process. Neem seed oil includes triglycerides, and the molar ratio determined how much methanol molecules were mixed with these triglycerides to produce biodiesel and glycerol. At molar ratio of 1:02 reaction didn’t start might be because of lack of methanol solution. While molar ratio was at 1:03 showing increment of biodiesel yield. When the molar ratio of oil to methanol was 1:06, highest yield of biodiesel had been achieved which was about 86% when other influential parameters were fixed at their optimum level (in Fig. 5a). This happened due to the reversible reaction of transesterification, as equilibrium shifted to the product side for the excess amount of methanol and increased oil conversion53. After the limit varying molar ratio at 1:08 to 1:10 decreased the yield percent. It could be suggested that the oil’s active site is preventing methanol from reacting fully.
Effect of catalyst weight (%)
Catalyst (KOH) facilitated conversion of triglyceride in the oil to biodiesel and glycerol. Optimal weight% of KOH was important for efficient conversion rate and achieving high yield. In this Fig. 5b, with increasing catalyst percentage biodiesel yield also increases and reached at maximum level 86% when percentage is 1.5%. At that percentage reaction reached its equilibrium. After this limit increasing catalyst concentration yield percentage started to decrease because as the catalyst concentration is already beyond the reaction equilibrium. The quicker reaction to equilibrium and increased oil conversion are caused by the presence of KOH in the mixture54.
Effect of time
Change of reaction time was also a critical aspect of transesterification process influenced by the intricate kinetics of the transesterification process. The time parameter directly affected the extent of conversion of triglycerides into biodiesel and glycerol. Adequate reaction time was essential for complete conversion, which ensured optimal yield and purity of the biodiesel product. Effect of reaction time studied ranging from 60 to 150 mints. Beginning of the process as the time was increasing yield of biodiesel also started to increase. Figure 5c revealed that 120mints was enough to covert neem oil to biodiesel at optimum level. And longer time decreases production of biodiesel at alkaline condition because of there might be probability of hydrolysis of biodiesel55.
Effect of variation of stirrer speed
The stirring speed directly affected the mass transfer and mixing efficiency during the transesterification reaction between the neem oil and methanol in the presence of a catalyst. Magnetic stirrer was used for the production of biodiesel. And the production was carried out by varying stirring speeds: 250, 400, 500, and 750 revolution per minute (rpm). The optimal stirring speed, more than 500 rpm ensured the effective dispersion of reactants, promote greater contact between the neem seed oil and methanol molecules. This enhanced interaction facilitated the conversion of triglycerides into biodiesel and glycerol. However, excessive stirring (more than 500 rpm) was led to energy inefficiency and unnecessary turbulence, while inadequate stirring (less than 500 rpm) was resulted in incomplete mixing and it reduced reaction rates (in Fig. 5d).
Effect of temperature
Temperature also affected the overall reaction kinetics and fluid properties during the transesterification process. Higher temperatures generally accelerated the reaction rate, led to faster conversion of triglycerides into biodiesel and glycerol. However, excessively high temperatures like more than 65 °C were promoted side reactions and caused thermal degradation of the biodiesel product. That’s why the yield of biodiesel was lower at those conditions. On the other hand, lower temperatures like less than 60 °C was responsible for the result in sluggish reaction rates and incomplete conversion. When the temperature was 60 °C and 65 °C, we have got the higher yield of biodiesel (in Fig. 5e). But 60 °C was the optimum temperature because of lower consumption of energy.
FTIR analysis of biodiesel and neem seed oil
FTIR-ATR analysis gives important information about presence of functional group with the particular peak. Here at the Fig. 6 FTIR-ATR analysis of neem seed oil and biodiesel are shown and in Table 2 responsible functional groups as well as their monochromatic light absorption peak have been detected carefully. 3500–3000 cm− 1 absorption was observed for NSO and biodiesel due to presence of N–H of amine56,57. NSO have shown absorption peak in the range of 3000–2850 cm− 1 while biodiesel have shown absorption peak 2950 –2760 because of presence of C–H vibration58. Absorption peak in the range of around 2000 to 1800 cm− 1 might be responsible for presence of C=O stretching of ester59,60. Correspondingly absorption peaks 1600 to 1400 cm− 1 were for C=O. But the new absorption peak in the range of 1250 to 1000 cm− 1 only has been detected for the produce biodiesel while not found in case of NSO. This new peak indicates the presence of C-O stretching vibration of ester41,42. Again 900 to 800 cm− 1 notable peak has been observed only for the newly produced biodiesel. C–H bending of 1,3-disubstituted could be the functional group that causes the significance changes in the absorption peak. Moreover peaks around in the range 785 to 400 cm− 1 specify the presence of N–O, C–H in aromatic ring, S-S stretching42,61,62,63, both in NSO and in biodiesel. Noteworthy that some absorption peaks are absent in the NSO but in biodiesel presence of those peak indicates the successful fabrication. This is due manifestation of transesterification which converted the C=O stretching of ester to single bonded C–O stretching of ester. These findings made the study completely successful for ensuring effective production of biodiesel (Table 2).
Fatty acid profiling of newly produced biodiesel by GC analysis
When separating and identifying chemicals in a sample, gas chromatography (GC) analysis is a potent analytical method GC analysis widely used in case of determining Fatty Acid Methyl Ester (FAME). The obtained findings addressing the saturated, and unsaturated (both mono and poly unsaturated) fatty acid methyl ester of the newly produced biodiesel sample according to the GC analysis have been shown in Fig. 7 as well as Tables 3, 4 and 5. The existence of the fatty acid.
methyl esters into the spectra of the subjected biodiesel are clearly observed from the above figure (Fig. 6). There were a quite number of saturated, and unsaturated including both the monounsaturated and polyunsaturated fatty acid methyl esters observed. However, for better understanding all these fatty acid methyl ester have been listed and presented in Tables 3, 4 and 5):
From the table main fatty acids are linoleic acid 69.696%, oleic acid 17.519%, stearic acid 2.761% and palmitic acid 7.495%.Linoleic and oleic acid is in high proportion. 97.471% of the fatty acids in neem seed oil are these specific fatty acids. In fact, validate the oil’s unsaturated behavior.
Physicochemical properties of the newly produced biodiesel
The overall physicochemical properties regarding the considered neem seed oil derived biodiesel including the density, sp. gravity, viscosity, calorific value, flash point, acid value, saponification value, iodine value, para-anisidine value, peroxide value, refractive index value, etc., have been listed and presented in Table 6 indicating their particular units and experimental conditions.
The physicochemical properties of the neem-derived biodiesel were evaluated against global standards EN 14214 (EU), ASTM D6751 (USA), and GB/T 20828 (China) to assess its viability as a sustainable alternative fuel. The density (874.12 kg/m³) falls within the permissible ranges of all three standards (EU: 860–900, US: 870–900, China: 860–900 kg/m³), demonstrating compatibility with conventional diesel engines. However, the kinematic viscosity (6.05 cSt) exceeds the EU limit (3.5–5.0 cSt) but complies with the broader ASTM (1.9–6.0 cSt) and Chinese specifications, likely due to the high unsaturated fatty acid content (e.g., 69.7% linoleic acid). The acid value 1.819 mg NaOH/g surpasses EU/US thresholds ≤ 0.50 mg NaOH/g but aligns with China’s less stringent limit ≤ 0.80 mg NaOH/g, indicating residual free fatty acids that could be mitigated through acid pre-treatment. Notably, the flash point 142 °C far exceeds all standards (EU: ≥101 °C, US: ≥93 °C, China: ≥130 °C), ensuring safe storage and handling. The calorific value 956222 Kcal/kg outperforms China’s baseline (≥ 9000 kcal/kg) and approaches fossil diesel’s energy content (~ 10,000 kcal/kg), underscoring its efficiency. While the peroxide value(3.98 meq/kg complies with EU limits (≤ 20 meq/kg), it exceeds China’s stricter threshold (≤ 10 meq/kg), suggesting mild oxidative instability that could be addressed with antioxidants. Non-standardized parameters (e.g., refractive index, para-anisidine value) are reported for holistic characterization. Overall, the biodiesel meets critical benchmarks for density, flash point, and energy content globally but requires refinement in acid value and viscosity for stricter EU/US compliance, achievable through feedstock pretreatment or blending with diesel.
Conclusion
Neem seed is a type of feedstock from which, with minimal environmental pollution, biodiesel has been produced. As well as neem seed is renewable in nature. From the useless dropped neem oil was extracted by using soxhlet apparatus where 20% oil was found. In this study, the transesterification method was explored for the efficient production of biodiesel magnificently. simultaneously optimum condition for the produce biodiesel was also observed by varying reaction parameter such as molar ratio of oil to alcohol, catalyst concentration, temperature, time, stirring speed and subsequent resulting conditions found that molar ratio of oil to alcohol 1:6, catalyst concentration 1.5%, temperature 60 °C, time 120 minutes, stirring speed 500 rpm. A notable amount of biodiesel has been produced, which was around 86%. Besides a considerable yield of oil it possessed outstanding physicochemical properties. Sophisticated instruments and techniques are used for those analyses. Noteworthy results reveal that high calorific value 9562.22 ± 5.87 kcal/kg. This work is even more superior because of its remarkable implementation. Likewise, from the experimental data, it indicates that eco-friendly biodiesel from useless neem seed could have a major impact due to its outstanding properties and would be used as a suitable alternative to conventional fossil fuel to mitigate environmental pollution as well as to build a greener environment and a cleaner society. However, it is still needed to scale up this work by further research, whereas piloting as well as direct applications on real-time engines would be much more important and beneficial. Additionally, the purity and overall properties of the newly produced biodiesel can be improved by conducting more trials and errors regarding future research.
Data availability
Data should be available on request to the corresponding author (Md. Mahmudur Rahman via the Email address: shamrat.acce@gmail.com).
References
Benti, N. E. et al. Biodiesel production in Ethiopia: current status and future prospects. Sci. Afr. 19, e01531. https://doi.org/10.1016/j.sciaf.2022.e01531 (2023).
Rahman, M. M. & Rahman, M. S. Green synthesis of Chitosan from shrimp shell and fabrication of Chitosan- modified clay nanofilter composite for the purification of drinking water. https://doi.org/10.13140/RG.2.2.27864.67844 (2022).
Sheikh, M. S., Rahman, M. M., Rahman, M. S., Yildirim, K. & Maniruzzaman, M. Fabrication of nano composite membrane filter from graphene Oxide(GO) and banana rachis cellulose nano Crystal(CNC) for industrial effluent treatment. J. Ind. Eng. Chem. 128, 196–208. https://doi.org/10.1016/j.jiec.2023.07.048 (2023).
El Messaoudi, N. et al. Recent developments in the synthesis of tetraethylenepentamine-based nanocomposites to eliminate heavy metal pollutants from wastewater through adsorption. Bioresource Technol. Rep. 28, 101982. https://doi.org/10.1016/j.biteb.2024.101982 (2024).
Rahman, M. M. et al. Adsorptive abatement of Pb2 + and crystal Violet using chitosan-modified coal nanocomposites: A down flow column study. Groundw. Sustainable Dev. 23, 101028. https://doi.org/10.1016/j.gsd.2023.101028 (2023d).
Rahman, M. M. et al. Simultaneous removal of Ni2 + and congo red from wastewater by crystalline nanocellulose - Modified coal bionanocomposites: continuous adsorption study with mathematical modeling. Groundw. Sustainable Dev. 26, 101244. https://doi.org/10.1016/j.gsd.2024.101244 (2024a).
Lelieveld, J. et al. Air pollution deaths attributable to fossil fuels: observational and modelling study. Bmj 383. https://doi.org/10.1136/Bmj-2023-077784 (2023).
IEA. World Energy Outlook 2024, IEA, Paris https://www.iea.org/reports/world-energy-outlook-2024, Licence: CC BY 4.0 (report); CC BY NC SA 4.0 (Annex A). (2024).
Priya, Deora, P. S., Verma, Y., Muhal, R. A., Goswami, C. & Singh, T. Biofuels: an alternative to conventional fuel and energy source. Mater. Today Proc. 48, 1178–1184. https://doi.org/10.1016/j.matpr.2021.08.227 (2022).
Höök, M. & Tang, X. Depletion of fossil fuels and anthropogenic climate change—A review. Energy Policy. 52, 797–809. https://doi.org/10.1016/j.enpol.2012.10.046 (2013).
Hosseini, S. E. & Wahid, M. A. Necessity of biodiesel utilization as a source of renewable energy in Malaysia. Renew. Sustain. Energy Rev. 16 (2012), 5732–5740. https://doi.org/10.1016/j.rser.2012.05.025 (2012).
Babir, F., Veziroǧlu, T. N. & Plass, H. J. Environmental damage due to fossil fuels use. Int. J. Hydrog. Energy. 15, 739–749. https://doi.org/10.1016/0360-3199(90)90005-J (1990).
Rahman, M. M. et al. Cellulose nanocrystal (CNC) from Okra plant (Abelmoschus esculentus L.) stalks as a reinforcement in Bionanocomposite fabrication: extraction, processing, and characterization study. Carbohydr. Polym. Technol. Appl. 8, 100581. https://doi.org/10.1016/j.carpta.2024.100581 (2024b).
Wuebbles, D. J. & Jain, A. K. Concerns about climate change and the role of fossil fuel use. Fuel Process. Technol. 71, 99–119. https://doi.org/10.1016/S0378-3820(01)00139-4 (2001).
Lelieveld, J. et al. Effects of fossil fuel and total anthropogenic emission removal on public health and climate. Proceedings of the National Academy of Sciences. 116, 7192–7197. (2019). https://doi.org/10.1073/pnas.1819989116
Guarnieri, M. & Balmes, J. R. Outdoor air pollution and asthma. Lancet 383, 1581–1592. https://doi.org/10.1016/S0140-6736(14)60617-6 (2014).
Schikowski, T. et al. Ambient air pollution: a cause of COPD. Eur. Respiratory Soc. 43, 250–263. https://doi.org/10.1183/09031936.00100112 (2014).
Kurt, O., Zhang, J. & Pinkerton, K. E. Pulmonary health effects of air pollution. Curr. Opin. Pulm. Med. 22, 138–143. https://doi.org/10.1097/MCP.0000000000000248 (2016).
Narula, K. Global energy system and sustainable energy security. In: The Maritime Dimension of Sustainable Energy Security 68, 23–49. https://doi.org/10.1007/978-981-13-1589-3_2 (2019).
Shafiee, S. & Topal, E. An econometrics view of worldwide fossil fuel consumption and the role of US. Energy Policy. 36, 775–786. https://doi.org/10.1016/j.enpol.2007.11.002 (2008).
Leblond, D. Iea: fossil energy to dominate market through 2030. Oil Gas J. 104, 28–29 (2006).
Martins, F., Felgueiras, C., Smitkova, M. & Caetano, N. Analysis of fossil fuel energy consumption and environmental impacts in European countries. Energies 12 (6), 964. https://doi.org/10.3390/en12060964 (2019).
IEA. Tracking Clean Energy Progress 2023, IEA, Paris. https://www.iea.org/reports/tracking-clean-energy-progress-2023, Licence: CC BY 4.0. (2023).
Khan, M. A. H. et al. Investigation of biofuel as a potential renewable energy source. Atmosphere 12, 1289. https://doi.org/10.3390/atmos12101289 (2021).
Karmakar, A., Karmakar, S. & Mukherjee, S. Biodiesel production from Neem towards feedstock diversification: Indian perspective. Renew. Sustain. Energy Rev. 16, 1050–1060. https://doi.org/10.1016/j.rser.2011.10.001 (2012).
Rabby, M. M. R. et al. Production of CNC from agro-waste biomass (maize shells) as a potential reinforcement in bio-nanocomposites: extraction, modification, and characterization study. Carbohydr. Polym. Technol. Appl. 9, 100671. https://doi.org/10.1016/j.carpta.2025.100671 (2025).
Dewan, S. et al. Isolation and characterization of CNC from waste maize cob available in Bangladesh as a potential candidate for the fabrication of multifunctional bio-nanocomposites: A new approach. S. Afr. J. Chem. Eng. 51, 287–301. https://doi.org/10.1016/j.sajce.2024.12.007 (2025).
Hassan, M. M., Rahman, M. M., Ghos, B. C., Hossain, M. I. & Zuhanee, M. K. A. Extraction, and characterization of CNC from waste sugarcane leaf sheath as a reinforcement of multifunctional bio-nanocomposite material: A waste to wealth approach. Carbon Trends. 17, 100400. https://doi.org/10.1016/j.cartre.2024.100400 (2024).
Yuan, H., Yang, B., Zhang, H. & Zhou, X. Synthesis of biodiesel using Castor oil under microwave radiation. Int. J. Chem. Reactor Eng. 9 https://doi.org/10.1515/1542-6580.2562 (2011).
Bahadur, S., Goyal, P., Sudhakar, K. & Prakash Bijarniya, J. A comparative study of ultrasonic and conventional methods of biodiesel production from Mahua oil. Biofuels 6, 107–113. https://doi.org/10.1080/17597269.2015.1057790 (2015).
Sirajudin, N., Jusoff, K., Yani, S., Ifa, L. A. & Roesyadi, A. Biofuel production from catalytic cracking of palm oil. Catalyst 5 (9). https://doi.org/10.5829/idosi.wasj.2013.26.nrrdsi.26012 (2013).
Rahman, M. M. et al. Fabrication of CNC-AC bionanosorbents from the residual mass of Magnolia champaca l. Bark after methanol extraction for wastewater treatment: continuous column adsorption study. Environ. Nanatechnol. Monit. Manage. 22, 101015. https://doi.org/10.1016/j.enmm.2024.101015 (2024d).
Felipe, L. & Verduzco, R. Density and viscosity of biodiesel as a function of temperature: empirical models. Renew. Sustain. Energy Rev. 19, 652–665. https://doi.org/10.1016/j.rser.2012.11.022/article/pii/S1364032112006338) (2013).
Rahman, M. M., Islam, M. R., Islam, M. R. & Naznin, S. Extraction and characterization of lipid from Pangus fish (P. Pangasius) available in Bangladesh by solvent extraction method. Am. J. Zool. 1 (2), 28–34. https://doi.org/10.11648/j.ajz.20180102.11 (2018).
Ozcanli, M., Gungor, C. & Aydin, K. Biodiesel fuel specifications: a review. Energy Sources Part A Recov. Util. Environ. Effects 35, 635. https://doi.org/10.1080/15567036.2010.503229 (2013).
Nabizadeh, R. et al. Biodiesel production from supernatant waste cooking oil by a simple one- step technique: calorific value optimization using response surface methodology (RSM) based on D-optimal design. J. Mater. Cycles Waste Manag. 25, 3567–3583. https://doi.org/10.1007/s10163-023-01779-5 (2023).
Baroutian, S., Aroua, M. K., Raman, A. A. & Sulaiman, N. M. Viscosities and densities of binary and ternary blends of palm oil + palm biodiesel + diesel fuel at different temperatures. J. Chem. Eng. Data. 55 (1), 504–507. https://doi.org/10.1021/je900299x (2010).
Yeasmin, M. S., Chowdhury, T. A., Rana, G. M. M., Rahman, M. M. & Ferdousi, L. Characteristics of Sesame (Sesamum Indi-cum L.) seed meal grown in the Northern region of Bangladesh. Biomed. J. Sci. Tech. Res. 38 (1), 29944–29949. https://doi.org/10.26717/BJSTR.2021.38.006083 (2021).
Yeasmin, M. S. et al. Physico-Chemical properties and GCMS analyses of Indigenous rice Bran and mustard seed oils and their blends. Biomed. J. Sci. Tech. Res. 34 (5), 27167–27172. https://doi.org/10.26717/BJSTR.2021.34.005620 (2021).
Rahman, M. M., Maniruzzaman, M., Islam, M. R. & Rahman, M. S. Synthesis of Nano- cellulose from Okra fibre and FTIR as well as morphological studies on it. Am. J. Polym. Sci. Technol. 4, 42–52. https://doi.org/10.11648/j.ajpst.20180402.11 (2018a).
Rahman, M. M. et al. Adsorptive removal of toxic heavy metal and dyes from wastewater by rice husk (lignocellulosic biomass) derived activated biochar: A fixed-bed column adsorption study. Carbohydr. Polym. Technol. Appl. 9, 100698. https://doi.org/10.1016/j.carpta.2025.100698 (2025a).
Rahman, M. M. et al. A state-of-the-art review focusing on the fabrication technique of activated chitosan-bitumin coal based multifunctional bionanocomposites for industrial wastewater treatment: production, characterization, and fixed bed column adsorption study. J. Environ. Chem. Eng. 13 (2), 115908. https://doi.org/10.1016/j.jece.2025.115908 (2025b).
Rahman, M. M., Maniruzzaman, M. & Yeasmin, M. S. A State-of-the-Art review focusing on the significant techniques for naturally available fibers as reinforcement in sustainable Bio-composites: extraction, processing, purification, modification, as well as characterization study. Result Eng. 20, 101511. https://doi.org/10.1016/j.rineng.2023.101511 (2023b).
Rahman, M. M., Maniruzzaman, M. & Zaman, M. N. Fabrication and characterization of environmentally friendly biopolymeric nanocomposite films from cellulose nanocrystal of banana M. Oranta (Sagar kala) tree rachis fibers and Poly lactic acid: A new route. S. Afr. J. Chem. Eng. 100619 https://doi.org/10.1016/j.sajce.2024.10.002 (2024).
Rahman, M. M., Islam, M. M. & Maniruzzaman, M. Preparation and characterization of biocomposite from modified α-cellulose of Agave cantala leaf fiber by graft copolymerization with 2-hydroxy Ethyl methacrylate. Carbohydr. Polym. Technol. Appl. https://doi.org/10.1016/j.carpta.2023.100354 (2023c). .6,100354.
Rahman, M. M. et al. Production of cellulose nanocrystals from the waste banana (M. Oranta) tree rachis fiber as a reinforcement to fabricate useful Bionanocomposite. Carbohydr. Polym. Technol. Appl. 8, 100607. https://doi.org/10.1016/j.carpta.2024.100607 (2024e).
Rahman, M. M. & Maniruzzaman, M. Preparation of shrimp shell Chitosan-Clay-Nanofilter for the purification of drinking water. Int. J. Food Eng. Technol. 2, 17–26. https://doi.org/10.11648/j.ijfet.20180202.12 (2018).
Rahman, M. M. & Maniruzzaman, M. Extraction of nanocellulose from banana rachis (Agro-waste) and Preparation of nanocellulose-Clay nanofilter for the industrial wastewater purification. J. Bioremediat. Biodegradation. 12, 485. https://doi.org/10.4172/2155-6199.1000485 (2021).
Dubey, K. K. D. et al. Biodiesel production from Hiptage benghalensis seed oil. Ind. Crops Prod. 144, 112027. https://doi.org/10.1016/j.indcrop.2019.112027 (2020).
Ouilly, J. T. et al. Chemical composition, physicochemical characteristics, and nutritional value of Lannea kerstingii seeds and seed oil. J. Anal. Methods Chem. 2017, 1–6. https://doi.org/10.1155/2017/2840718 (2017).
Canakci, M. & Gerpen., J. V. Biodiesel production from oils and fats with high free fatty acids. Am. Soc. Agric. Biol. Eng. 44, 1429–1436. https://doi.org/10.13031/2013.7010 (2001).
Sahoo, P. K. & Das, L. M. Process optimization for biodiesel production from jatropha, Karanja and Polanga oils. Fuel 88, 1588–1594. https://doi.org/10.1016/j.fuel.2009.02.016 (2009).
Guan, G., Kusakabe, K., Moriyama, K. & Sakurai, N. Continuous production of biodiesel using a microtube reactor. Chem. Eng. Trans. 14, 237–244 (2008).
Rashid, W. N. W. A., Uemura, Y., Kusakabe, K., Osman, N. B. & Abdullah, B. Synthesis of biodiesel from palm oil in capillary millichannel reactor: effect of temperature,methanol to oil molar ratio, and KOH concentration on FAME yield. Procedia Chem. 165–171. https://doi.org/10.1016/j.proche.2014.05.020 (2014).
Okwundu, O. S., El-Shazly, A. H. & Elkady, M. Comparative effect of reaction time on biodiesel production from low free fatty acid beef tallow: a definition of product yield. SN Appl. Sci. 1, 140. https://doi.org/10.1007/s42452-018-0145-1 (2019).
Rahman, M. M. et al. Simultaneous abatement of Ni2 + and Cu2 + effectually from industrial wastewater by a low cost natural clay-chitosan nanocomposite filter: synthesis, characterization and fixed bed column adsorption study. Environ. Nanatechnol. Monit. Manage. 20, 100797. https://doi.org/10.1016/j.enmm.2023.100797 (2023a).
Rahman, M. M. & Maniruzzaman, M. Environmentally friendly strength bio-composite Preparation by grafting of HEMA onto shrimp Chitosan without destroying original microstructure to enrich their physicochemical, thermomechanical, and morphological properties. S. Afr. J. Chem. Eng. 47, 300–311. https://doi.org/10.1016/j.sajce.2023.12.005 (2024).
Rahman, M. M. et al. Fabrication of Chitosan coated bentonite clay multifunctional nanosorbents from waste biomass for the effective elimination of hazardous pollutants from waterbodies: A fixed bed biosorption, mechanism, and mathematical model study. Int. J. Biol. Macromol. 282 (6), 137439. https://doi.org/10.1016/j.ijbiomac.2024.137439 (2024f).
Rahman, M. M. & Maniruzzaman, M. A new route of production of the mesoporous Chitosan with well-organized honeycomb surface microstructure from shrimp waste without destroying the original structure of native shells: extraction, modification and characterization study. Result Eng. 19, 101362. https://doi.org/10.1016/j.rineng.2023.101362 (2023).
Uddin, M. J. et al. Morphostructural studies of pure and mixed metal oxide nanoparticles of Cu with Ni and Zn. Heliyon 10, e30544. https://doi.org/10.1016/j.heliyon.2024.e30544 (2024).
Rahman, O., Rahman, M. M. & Maniruzzaman, M. Removal of dye and heavy metals from industrial wastewater by activated charcoal-banana rachis cellulose nanocrystal composite filter. Int. J. Environ. Anal. Chem. 1–19. https://doi.org/10.1080/03067319.2022.2039647 (2022).
Rahman, M. M., Maniruzzaman, M. & Saha, R. K. A green route of antibacterial films production from shrimp (Penaeus monodon) shell waste biomass derived chitosan: physicochemical, thermomechanical, morphological and antimicrobial activity analysis. S. Afr. J. Chem. Eng. https://doi.org/10.1016/j.sajce.2024.11.005 (2024 g).
Rahman, M. M. et al. Production of functionalized clay-CNC based biopolymeric nanocomposite from agro-waste biomass for bulky industrial wastewater treatment via continuous column adsorption study with mathematical modeling: A critical review. J. Clean. Prod. 518, 145883. https://doi.org/10.1016/j.jclepro.2025.145883 (2025c).
Acknowledgements
All authors of this paper devoutly acknowledge the chairman of Bangladesh Council of Scientific and Industrial Research (BCSIR), as well as the Ministry of Science and Technology (MOST), People’s Republic of Bangladesh for their financial support and for consenting us to conduct their highly sophisticated instruments for experimentation. Furthermore, we want to acknowledge our lab attendant Md. Mofijul Islam and Mst. Ronjina Khatun for their cordial support during the experimental session and drafting.
Funding
The authors extend their appreciation to the Ministry of Science and Technology (MOST), the People’s Republic of Bangladesh, and the Bangladesh Council of Scientific and Industrial Research (BCSIR), for their joint funding to conduct this current work through Research and Development Project under grant number of (G.O.39.02. 0000.011.14.180.2024/1116 and G.O. 39.02.0000.011.14.169.2023/877).
Author information
Authors and Affiliations
Contributions
M.K.A.Z.: Sample collection & preparation, Conceptualization, Software. M.M.R.: Conceptualization, Fund acquisition, Methodology, Software, Validation, Formal analysis, Data Curation, Writing - Review & Editing. M.S.Y.: Sample collection & preparation. M.M.H.: Writing - Review & Editing. M.I.H.: Data Curation. B.C.G.: Data analysis. G.M.M.R.: Formal analysis. M.S.H.: Visualization. N.A.: Data Curation. M.N.R.: Formal analysis, Data Curation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zuhanee, M.K.A., Rahman, M.M., Hassan, M.M. et al. Production, characterization and yield optimization of biodiesel from waste neem Melia azadirachta seeds available in Bangladesh. Sci Rep 15, 33913 (2025). https://doi.org/10.1038/s41598-025-09251-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-09251-0