Abstract
Bisphenol A (BPA) is a chemical used in the production of plastics (polycarbonate) that can pose risks to human health. This research aimed to evaluate contents of BPA in soft drink samples (samples packaged in different containers and different volumes) by MSPEGC/MS (Magnetic Solid-Phase Extraction-Gas Chromatography/Mass Spectrometry) technique. Moreover, health risks of human were assessed for Iranian consumers by MCS method. Our results revealed, the average level of BPA in all samples was 1.83 ± 1.16 ppb (ranging from 0.45 to 5.10 ppb). BPA levels differed significantly among the samples depending on the type of packaging and pH level (P < 0.05). Also, the highest and lowest average levels of BPA in different soft drinks of different flavours/tastes were in cola soft drink samples (2.53 ppb) and energy drink samples (0.85 ppb), respectively. Furthermore, the highest and lowest average levels of BPA in soft drinks with different volumes were observed in 1500 cc soft drink samples (2.87 ppb) and 500 mL soft drink samples (0.9 ppb), respectively. The results of the MCS (Monte Carlo Simulation) indicated the 95th percentile for the THQ (Target Hazard Quotient) of BPA in soft drink samples was 9.61E + 1 for adults and 1.37E + 2 for children (6 years) (THQ > 1). Overall, these results imply that exposure to BPA could represent a potential health risk for individuals who frequently consume soft drinks packaged in PET (Polyethylene Terephthalate) or cans, especially for children.
Similar content being viewed by others
Introduction
BPA or Bisphenol A is a synthetic organic compound, which has become the focus of public health debate and regulatory scrutiny due to its widespread use and potential adverse impacts on human health. This substance (bisphenol A) was first synthesized in the early 1900s. BPA is primarily applied in the manufacture of epoxy resins and plastics (polycarbonate) that are used in a variety of consumer products, comprising containers (foodstuff and beverage), thermal paper, and dental materials. The chemical structure of BPA, a diphenylmethane derivative, is strongly linked to its estrogenic activity1,2. Endocrine disruption has been the main focus of much of the toxicological research surrounding BPA. Unlike classical hormones, endocrine disruptors can mimic or oppose the effects of natural hormones, disrupting the body’s complex hormonal signaling pathways3,4. Endocrine disruption can have many consequences during the human lifespan, affecting various physiological processes including growth, reproduction, and metabolism. The effects of endocrine disruption are often subtle and can appear years, even decades after exposure, challenging epidemiological studies and highlighting the need for robust analytical methods to quantify BAP levels in food/drink5,6. The consumption of soft drinks has emerged as a highly visible and controversial public health and policy issue. Researchers often consider soft drinks to be a primary cause of obesity and associate them with health issues, especially in children7.The versatility and durability of BPA-containing materials have led to their widespread use in many applications in modern life, thus raising significant concerns about the safety of various products, particularly in relation to food and beverages8,9. The EU Regulation No. 2024/3190 banned, the use of BPA from 20 January 202510.
In the case of BPA in foodstuffs, the Commission of EU (European Union) has set a migration limit of 600 µg/kg11. The Reference Dose (RfD) of BPA was established at 50 µg/kg bw/day by the US.EPA (Environmental Protection Agency)12. In 2023, the European Food Safety Authority (EFSA) conducted a new safety review of bisphenol A (BPA), resulting in a substantially lower tolerable daily intake (TDI) of 0.2 ng/kg body weight per day. This updated limit is roughly 20,000 times stricter than the former provisional TDI of four micrograms per kilogram and aligns with EFSA’s revised analysis of the health risks associated with BPA exposure13,14. Additionally, the TDI of BPA recommended by the Canada Health Organization is 25 µg kg−1 bw day[− 1 15.
Several studies have documented the existence of BPA in a variety of food and beverage matrices, including canned foods, bottled water, and even infant formula16,17,18,19,20,21. However, BPA levels in soft drinks, which are a significant part of many people’s diets, remain an area of ongoing research. Soft drinks are a significant category of beverages that frequently come into contact with materials containing BPA. Many soft drinks are packaged in plastic bottles or aluminum cans lined with epoxy resins that may contain BPA. Research has revealed BPA compounds can migrate from these packaging materials into beverages, especially under conditions such as heat or prolonged storage, leading to potential exposure for consumers17,22,23,24,25. Given the popularity of soft drinks among various populations, understanding the levels of BPA in these beverages is important to assess potential health risks9,26,27.
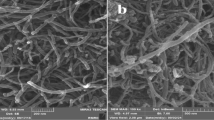
Analytical assessment of BPA composition in food matrices such as soft drinks (due to the simple matrix but various additives) presents significant challenges. The low concentrations typically encountered necessitate sensitive and selective analytical techniques17,18,28. GC-MS has emerged as a widely accepted and powerful method for the evaluation of BPA compound in various environmental and biological samples17,29,30. Its high sensitivity, coupled with its ability to provide both qualitative and quantitative data, makes it a suitable choice for this investigation. However, effective sample preparation is critical to remove interfering compounds and to concentrate the analyte, thereby improving the reliability and accuracy of the GC-MS analysis. SPE (solid-phase extraction) and LLE (liquid-liquid extraction) are generally applied procedures for this purpose, each possessing its advantages and disadvantages in terms of efficiency, cost, and ease of implementation. The selection of the optimal technique of sample preparation often depends on the specific matrix and the anticipated concentration of BPA. Magnetic multi-walled carbon nanotubes (MWCNTs) functionalized with iron oxide (Fe3O4) as MSPE (magnetic solid-phase extraction) method are employed as an effective adsorbent for the extraction and detection of BPA in beverages. The magnetic properties facilitate easy separation of the adsorbent from the solution after the adsorption process, enhancing the efficiency of BPA extraction. This method leverages the high area of surface and adsorption capacity of MWCNTs, allowing for sensitive and selective determination of BPA, which is crucial for assessing the safety of food and drink products9,31.
Assessment of human health risk is a systematic procedure applied to assess the potential human health risks related with exposure to hazardous substances, like BPA, in consumer products. This process typically comprises 4 main steps: hazard identification, dose-response assessment, exposure assessment, and risk characterization32,33.
In the context of BPA in soft drinks, hazard identification focuses on understanding the adverse health effects linked to BPA exposure, particularly its role as an endocrine disruptor. Assessment of dose-response examines the relationship between the level of exposure and the severity of health impacts, while assessment of exposure estimates the actual levels of BPA that consumers may encounter through soft drink consumption. Finally, risk characterization integrates these elements to provide an overall evaluation of the potential health risks to consumers, helping to inform regulatory decisions and public health recommendations. By conducting a thorough risk assessment, we can better understand the implications of BPA presence in soft drinks and guide efforts to protect consumer health5,34.
Since soft drinks are one of the components of the Iranian consumer basket, and no comprehensive study has been conducted in this field so far, the present study seems necessary. With this explanation, the aim of this study was to determine the level of BPA compound in different soft drink samples (orange drink, cola drink, energy drink and lemon soft drink) using the MSPE-GC/MS method and to assess the potential human health risks associated with the detected levels. By comparing our findings with the safety limits set by international regulatory agencies, we hope to provide valuable insights into the safety of soft drinks and their implications for consumer health.
Materials and methods
Chemicals
The MWCNTs were acquired from Nanoshel Co. (Panchkula, India), with specifications of 30–60 nm diameter and 5–30 µmlength. Bisphenol A was ordered from Sigma-Aldrich (West Chester, PA; USA). From Merck Co., (Darmstadt, Germany), sucrose, magnesium sulfate, sodium chloride (NaCl), acetonitrile, potassium carbonate, isoamyl alcohol, n-hexane, Fehling’s solution, methylene blue, sulfuric acid, tetrachloroethylene, acetic anhydride and other used chemicals were ordered. All chemicals were of analytical grade.
Preparation of magnetic adsorbent
The magnetic adsorbent (MWCNTs-Fe3O4) was prepared as described in previous studies18,35,36. The magnetic nanoparticle fabrication process consisted of two steps. (1) Functionalization of MWCNTs: First, the nanotubes were treated with HNO3/H2SO4 (1:3) and sonicated for 10 h to create carboxyl and hydroxyl groups on their surfaces. The magnetic nanoparticle fabrication process consisted of two steps. (1) Functionalization of MWCNTs: First, the MWCNTs were treated with HNO3/H2SO4 (1:3) and ultrasonicated for 10 h to create carboxyl and hydroxyl groups on their surfaces. The assembly of magnetic nanoparticles on acid-treated MWCNTs: An amount of 0.467 g of FeCl3.6H2O and 0.04 g of acid-treated MWCNTs were suspended in 25 mL of ethylene glycol in a glass vial. 1.2 g of sodium acetate was added and the solution was mixed (for 5 min) at room temperature by a magnetic stirrer. Afterwards, the glass vial was placed in an airtight steel container and heated in an oven for 14 h at 200 C. After cooling to room temperature, the synthetic product was washed with 25 mL ethanol and MWCNT-MNPs were recovered by applying a magnetic field via a magnet placed on the outer wall of the glass vial. This cleanup procedure was repeated 5 times. The magnetic MWCNTs composite obtained was stored in ethanol (25 mL) until needed.
Collection of samples
A total of 48 soft drink samples (in duplicate) (including 4 tastes (cola, lemon, orange and energy drinks), 2 packaging type (can and PET) and 6 different volumes (150, 250, 300, 330, 500 and 1,500 mL)) were purchased from supermarkets in Tehran City (5 districts by simple random sampling). Also, soft drink brands were selected from the most widely consumed brands by simple random sampling. They were transferred to the laboratory and retained at conditions mentioned on their labels until investigation.
Measurement of BPA
Sample Preparation
First, 0.1 g of MWCNT-Fe3O4 composite was mixed with 10 mL of the degassed soft drink samples, 0.5 g of salt (NaCl) and 100 µL of phenol (internal standard) and mixed for 5 min with a magnetic stirrer and left to stand for 10 min. Then the supernatant was removed and an external magnet was located on the vial’s outer wall to gather the MWCNT-Fe3O4 composite. Next, with 2 mL of dichloromethane, the composite was washed and then the magnet was placed outside again to separate the composite from the dichloromethane containing the analyte, and finally the solution containing the analyte was transferred to the GC vial with syringe filters and kept in a refrigerator. To the sample extracted from the previous step, 50 µL of MSTFA (N-Methyl-N-trimethylsilyltrifluoroacetamide) derivatizing was added and incubated at 50 C for 1 h. Finally, one microliter was injected into the instrument18,28.
CG-MS operating conditions
In this study, a GC (Agilent 7890, Palo Alto, CA, USA) with a MS detector (Agilent 5975) was used, equipped with a capillary column (HP5-MS; internal diameter: 0.25 mm; length of column: 30 m; thickness of film: 0.25 μm). The GC conditions such as temperatures, times, carrier gas, etc. were in accordance with the previous study17. The GC-MS operating conditions were: the carrier gas: helium (99.999%); flow rate: 1 mL min−1; the injection mode: splitless; the injection volume: 2 µL. The oven temperature instructions were as follows: the temperature of the injector: 280 °C, the temperature initial: 100 °C with 1 min hold, ramp to 225 °C for 5 min at 20 °C min−1, and then ramp to 325 °C at 35 °C min−1 and held for. The monitoring m/z ions for IS were 94, 187 and 188 and for BPA were 357 and 285 for confirmation ions (m/z) and 285 for qualifier ion (m/z).
Method performance
From BPA standard solution, the curve of calibration was constructed ranging from 10 to 100 (ng L−1) in methanol. The limit of quantification(LOQ) and Limit of Detection (LOD) were calculated applying the calibration curve slope (S) and response standard deviation (σ)37,38,39 according to the following formula:
By spiking 3 replicas of BPA real sample, the recovery rate was evaluated. The Relative Standard Deviation (RSD) was evaluated through investigation of 6 BPA compound.
Measurement of sugar and pH
The sugar content in the samples was measured according to a previous study17. First, 6–10 mL of hydrochloric acid with 25 g of sample were added to a volumetric flask (100 mL) and then located in a water bath at 70 °C for 10 min. Next, the volumetric flask was cooled, a few drops of phenolphthalein were added and it was neutralized with 40% sodium hydroxide and 0.1 N sodium hydroxide to create a stable light pink color. Then, Fehling solution A (5 mL) and Fehling solution B A (5 mL) were poured into the Erlenmeyer flask and mixed. Then, a few glass beads, 3 to 4 drops of methylene blue, and 20 mL distilled water were added to the Erlenmeyer flask. The resulting solution was heated on a hot plate to boil for 2 min. Then, the neutralized solution was poured into the burette and, while boiling the Fehling’s solution, the neutralized solution was slowly added to the Erlenmeyer until it turned a reddish-brown Cu2O color. The volume of consumption was recorded. It was calculated according to the following formula:
where, F is the Fehling factor; V is the volume of solution consumed in mL, and N is total sugars (sugar after hydrolysis) in grams per % gram.
In our study, the soft drink sample (10 mL) was transferred to a beaker and the solution’s pH was analyzed with a pH meter (electronic, Hach, USA)17.
Hazard identification and exposure assessment
The human health risk assessment model is a comprehensive approach designed to evaluate the likelihood and severity of negative health effects from exposure to carcinogenic and non-carcinogenic environmental threats within human-environment interactions over a defined timeframe.
Estimated daily intake
To evaluate the health risks posed by BPA, it is important to determine the how much, how often, and how long of human exposure to this contaminant by tracking the exposure path into the human.
Since the primary entry pathway is oral, the health risk assessment model calculates the estimated daily intake (EDI) of Bisphenol A using the equation provided by the USEPA17.
A summary of the parameters used for the risk assessment can be found in Supplemental Table S1.
Dose-response assessment refers to a quantitative evaluation that illustrates the extent or frequency of risks linked to a specific amount of a contaminant. Based on a comprehensive food study of household food consumption patterns and nutritional status in Iran, the ingestion rate of soft drinks was set at 0.012 kg/day for children and 0.03 kg/day for adults40.This process includes determining toxicity benchmarks, such as the tolerable daily intake (TDI) for non-carcinogenic risks. According to the European Food Safety Authority (EFSA), the TDI for bisphenol A (BPA) is set at 0.2 ng kg−1 body weight per day13.
The non-carcinogenic aspects of Bisphenol A, indicated by the target hazard quotient (THQ), are calculated as the ratio of the estimated daily intake of the contaminant from ingestion to its respective tolerable daily intake (TDI) and oral reference doses (RfD)12, as expressed by the following equation17:
Thus, when THQ ≤ 1, it displays no risk, whereas THQ > 1 suggests a significantly elevated non-cancer risk. Monte Carlo simulation (MCS) is a valuable tool for conducting probabilistic health risk evaluations in situations with uncertain and variable conditions, which are important in real-world scenarios where contaminant levels can fluctuate. In contrast to deterministic techniques, MCS provides a statistical distribution of possible outcomes, enabling a more comprehensive and realistic representation of risk exposure41,42,43,44. To thoroughly evaluate the health risks associated with Bisphenol A in soft drink samples, a Monte Carlo simulation was conducted using the Crystal Ball software tool (Crystal Ball v 11.1.2.3.0 software, Oracle@ Crystal Ball, Oracle Corporation, USA) for uncertainty analysis. It is crucial to determine the number of simulation steps at the beginning of the simulation. Monte Carlo Simulation (MCS) quantified uncertainty distributions by running 10,000 iterations with randomized input values—including measured BPA concentrations, ingestion rates, body weights, and exposure durations. This process generated probability distributions for model outputs, with final results reported at confidence levels ranging from 1 to 99%.
Statistical analysis
In our study, the data were analyzed using SPSS version 22 (for Windows, version 22.0; SPSS Inc., Chicago, Illinois, USA). The Kolmogorov–Smirnov test was conducted to assess the distribution of the research parameters. The results were evaluated using Spearman Correlation, with statistical significance set at p < 0.05. Additionally, the Kruskal–Wallis test was employed to determine significance between groups.
Results and discussion
Analytical method validation
In our investigation, R2 (determination coefficient), relative standard deviation (RSD), LOQ (quantification limit), LOD (detection limit) and recovery rate were 0.994, 12.3%, 0.0035 ng mL−1, 0.001 ng mL−1 and 92.5%, respectively. The results obtained in this study were similar to other confirmed study methods. In similar study, Jiao et al. used MWCNT-MNP and GC-MS/MS for the measurement of BPA and they reported the recovery was in the range of 90.3–103.7% with RSD lower than 10%. The determination coefficient (R2 = 0.9988) was achieved. Also, they reported the LOD was 0.001 µg L[− 1 18. In other study, Zang et al. prepared graphene grafted magnetic microspheres and applied as the adsorbent in MSPE method for the measurement of BPA. They reported that the LOD (S/N = 3) was 10.0 ng L−1, the recovery was in the range from 93.5 to 99.5% (inter-day) and from 93.9 to 104.3% (intra-day), with the RSD varying from 3.1 to 5.7% (inter-day) and from 2.1 to 5.8% (intra-day)28. Furthermore, Hazrati-Raziabad et al. analyzed BPA concentration with method of derivatization by GC/MS and reported the determination coefficient (R2), recovery, LOQ, LOD, and RSD were 0.991, 99.8%, 0.35 ng/g, 0.1 ng g−1, and 8.9%, respectively17.
Comparison of pH between soft drinks with different flavours/tastes
Table 1 shows the pH levels in soft drinks with different flavours/tastes. The average pH in all samples was 3.06 ± 0.26. In addition, the highest and lowest average pH levels among the soft drinks with different flavours/tastes were lemon soft drink (3.26) and cola soft drink (2.77), respectively. According to the results, the order of average pH was lemon soft drink > energy drink > orange soft drink > cola soft drink. It is worth noting that there was a significant difference between the samples, as determined by the Kruskal-Wallis test (Table 1).
The pH levels in different soft drinks such as energy drink, orange, lemon, and cola soft drinks depend on several factors. One of the main reasons is the type of acids present in these drinks; For example, lemon and orange soft drinks usually contain citric acid, which can lower the pH and make them more acidic. On the other hand, cola soft drinks may contain phosphoric acid, which also affects the acidity level. In addition, the sweeteners, flavors, and carbon dioxide gas used in the production process of these drinks can also affect the final pH. In general, the formulation and composition of the raw materials of each type of drink causes their pH to vary8,9,45.
Comparison of sugar content between soft drinks with different flavours/tastes
Table 2 shows the sugar content in soft drinks with different flavours/tastes. The average sugar content in all samples was 29%. Also, the highest and lowest average sugar content in soft drinks with different flavours/tastes were orange soft drink (30.59%) and lemon soft drink (25.74%), respectively. According to the results, the order of average sugar content in different soft drinks was orange soft drink > cola soft drink > energy drink > lemon soft drink. As determined by the Kruskal-Wallis test (Table 1), there was no significant difference between the samples.
The sugar content in different soft drinks such as energy soft drink, orange soft drink, lemon soft drink and cola soft drink depends on several factors, comprising the type of flavoring, the purpose of production and the target market. Some energy drinks and orange soft drink typically contain higher amounts of sugar to provide a stronger energy and flavor, while lemon and cola drinks may require less sugar due to the use of natural acids and flavors. Also, in soft drinks, the amount of sugar depends on the formulation and brand; some brands use more sugar to balance the taste and sweetness. In addition, consumer demand and health trends can also affect the amount of sugar in these drinks, with some manufacturers moving towards reducing sugar and using artificial or natural sweeteners8,34,46.
Bisphenol A levels in soft drink samples
Table 3 indicates the BPA levels in all soft drink samples. The average BPA level in all samples was 1.83 ± 1.16 ppb (ranging from 0.45 to 5.10 ppb). According to the results obtained, the level of BPA contamination in the soft drink samples was less than the existing standards (such as EU standard level that is 600 ppb in food products)11.
The BPA levels in different types of soft drinks depend on several factors. First, the type of packaging of soft drinks plays an important role; many soft drinks are packaged in plastic bottles or aluminum cans, which may or may not comprise BPA. Also, the process of manufacturing and storing soft drinks is also influential; some manufacturers use alternatives to BPA to ensure the health of consumers. Furthermore, the type of soft drink formulation and additives used can also affect the concentrations of BPA. In general, differences in foodstuff quality and safety standards in different countries can also lead to variations in the amount of BPA in soft drinks8,26,34,46.
The results of the investigating of bisphenol A levels in previous studies are shown in Table 4, and the differences may be owed to differences in raw materials, preparation methods, packaging types, and measurement procedures for this compound in different beverages8,34,46,47.
Comparison of BPA levels in soft drinks with different flavours/tastes
Table 3 shows the BPA levels in soft drinks with different flavours/tastes. The highest and lowest average levels of BPA were in cola soft drink samples (2.53 ppb) and energy drink samples (0.85 ppb), respectively. Also, the average levels of BPA in orange and lemon soft drinks were 1.27 and 1.98 ppb, respectively. The order of average levels of BPA in soft drinks was cola soft drinks > lemon soft drinks > orange soft drinks > energy drinks. It is worth noting that the p-value is 0.055, which is close to the border of significance of 0.05. A p-value of 0.05 or greater is frequently referred to as “borderline significant” or “not significant.” This suggests that the result is likely due to chance, and the null hypothesis cannot be rejected.
The BPA levels in soft drink samples depend on several factors, which can be related to differences in packaging, formulation, and manufacturing processes. Cola drinks are usually packaged in aluminum cans or plastic bottles, which may contain BPA, which can lead to increased contamination in these types of drinks. Also, the formulation and additives in different drinks can affect the amount of BPA; for example, lemon and orange drinks may use ingredients (such as natural acids (citric acid), artificial flavors and colors) that help reduce exposure to BPA. Finally, energy drinks usually contain various ingredients (such as antioxidants ingredients (ascorbic acid (vitamin C) and tocopherol (vitamin E)), natural acids (citric acid and malic acid), herbal extracts (extract of green tea or extract of grape seed) and artificial flavors or colors) that may reduce the amount of BPA. Therefore, the results indicate that cola drinks have the highest contamination (not significantly), with lemon, orange, and energy drinks gradually decreasing in contamination8,45,48,47.
Comparison of BPA concentrations in soft drink samples with different packaging (can and PET)
Table 5 shows the BPA concentrations in different soft drink packaging types (cans and PET). According to this table, the average BPA level in PET packaging (2.31 ppb) was higher than in can packaging (1.21 ppb). It is worth noting that since the p-value is 0.025, the difference is significant.
The amount of bisphenol A (BPA) in different types of beverage packaging, such as cans and PET, varies for several reasons. BPA is a chemical compound commonly applied in the production of PET plastics and can leach into the beverage contents under certain packaging conditions. PET packaging may cause more contamination than aluminum cans, which usually have internal protective layers, due to its higher permeability and reactivity of plastic materials with the liquid content. Storage conditions, temperature, and time of storing can also impact the amount of BPA in beverages. For this reason, the results show that PET packaging generally has more contamination than can packaging1,8,16.
Comparison of BPA concentrations in samples of soft drink with different volumes
Table 6 shows the BPA levels in samples of soft drink with different volumes. The highest and lowest average levels of BPA were observed in 1500 cc soft drink samples (2.87 ppb) and 500 cc soft drink samples (0.9 ppb), respectively. The order of the average levels of BPA in samples of soft drink with different volumes was 1500 cc > 300 cc > 250 cc > 330 cc > 150 cc > 500 cc, respectively. It is worth noting that since the p-value is 0.48, the difference is not significant.
The BPA levels in samples of soft drinks with different volumes may vary for several reasons, including the type of packaging, storage time, and conditions of storing. Nonetheless, the outcomes demonstration there is no difference (significant) in BPA levels amongst different volumes, meaning that changes in volume do not have a significant effect on BPA concentrations. These findings suggest that while the volume of the soft drink may be influential, other factors must also be considered to identify the exact reasons for the differences in BPA levels8,45,47,48.
Exposure assessment
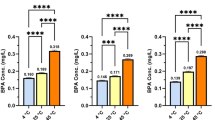
The findings of the EDI risk assessment concerning the presence of BPA from the soft drinks are presented in Table 7. The EDI index (95th percentile) of BPA in soft drink samples for children and adults were 2.61E-5, and 1.78E-5 mg kg−1 bw day−1, respectively (Table 7). Additionally, the order of EDI indices in samples was cola drink > orange drink > energy drink > lemon drink. The EDI risk assessment results for both children and adults exceeded the TDI index of BPA (2 E-7 mg/kg bw/day) established by the EFSA13,51, while these values in the soft drink samples were lower than the maximum acceptable risk level (reference dose, 0.05 mg Kg−1 bw day−1) for BPA permitted by USEPA hazard assessment12. The findings of the THQ index risk assessment concerning the presence of BPA from the soft drinks are presented in Fig. 1. The results of the Monte Carlo method revealed the THQ index in the form of a frequency histogram for a continuous distribution. The THQ index (95th percentile) of BPA in soft drink samples for children and adults were 1.37E + 2, and 9.61E + 1, respectively. Also, the order of EDI values in samples was cola drink > orange drink > energy drink > lemon drink. According to the EFSA opinion, the presence of BPA in soft drink samples obtained from Iran posed a significant non-carcinogenic health risk. However, the same samples were found to pose no significant health risk based on USEPA criteria. These predicted non-carcinogenic harms in the study area underscore a concerning situation for the public, suggesting a heightened likelihood of serious health risks for individuals in the coming years unless an immediate remediation strategy is implemented.
In a related study conducted in Australia, Marchiandi et al. reported that the estimated daily intake of BPA from beverages packaged in cans, plastic, and glass for adults and children ranged from 89 to 423 ng/kg−1/bw day−1, respectively, which is 2000 times higher than the safety limit. The revised tolerable daily intake (TDI) by EFSA13 for BPA indicates that drinking non-alcoholic beverages from BPA containers may present health risks, especially for young children who are more susceptible to chemical exposure. Young children typically eat larger amounts of food relative to their body weight than adults do, mainly because of their lower body mass. This can result in a higher estimated daily intake of certain substances. Due to their higher intake levels and smaller body size, children are more vulnerable to the adverse effects of potentially toxic substances. Moreover, children who drink sugary beverages face an elevated risk because of the combined impact of sugar and BPA, potentially resulting in cardiometabolic issues like insulin resistance and high blood pressure, especially in overweight or obese children with elevated BPA levels50.
A proposed ban on BPA in food contact materials (FCMs) raises concerns about substituting it with alternatives that may pose similar or unknown risks. To prevent regrettable substitutions, it is essential to gather toxicological data on these substitutes and implement proper monitoring. Currently, knowledge about the usage and exposure levels of these alternatives is limited. Thus, increased toxicological research and monitoring of these substances in FCMs are necessary to avoid poor replacement decisions for BPA.
Conclusion
The purpose of the present research was to assess BPA levels in soft drink samples distributed in Tehran, Iran by MSPE-GC/MS method. According to the results obtained, the concentration of BPA contamination in all soft drink samples was lower than the existing standards (such as EU standard level that is 600 ppb in food products). Significant differences were observed among the samples in terms of packaging type, and pH level (P < 0.05). Also, the order of average pH in samples was lemon drink > energy drink > orange drink > cola drink, and the order of average sugar content in different soft drink samples was orange drink > cola drink > energy drink > lemon drink. Furthermore, the order of average levels of BPA in soft drink samples with different flavors were cola soft drinks > lemon soft drinks > orange soft drinks > energy drinks, and the order of the average levels of BPA in soft drink samples with different volumes was 1500 cc > 300 cc > 250 cc > 330 cc > 150 cc > 500 cc. Finally, the order of average BPA levels in different soft drink packaging types was PET packaging > can packaging. The uncertainty analysis of health risk findings indicates that consuming soft drinks poses a significant health threat (HQ > 1). Limitations of this study include the lack of monitoring by relevant agencies as well as financial constraints, suggesting that it is important to assess the long-term effects of BPA exposure from daily diets, which regulatory agencies should address to truly protect public health. Addressing these issues will likely lead to better management of BPA and possibly other similar bisphenols. Other food products vulnerable to BPA contamination, such as dairy products and various beverages (including mineral water and fruit juice), should also be assessed.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Cao, X. L., Popovic, S. & Dabeka, R. W. Trends of bisphenol A occurrence in canned food products from 2008–2020. Food Addit. Contam. Part. A. 40, 781–786 (2023).
Ghahremani, M. H. et al. Bisphenol A in dairy products, amount, potential risks, and the various analytical methods, a systematic review. Food Chem: X, 21,101142 (2024).
Madani, A. & Rashedinia, M. An overview of the migration status of bisphenol A from different food containers and packages. Nutr Food Sci 54, (12) (2024).
Rudakov, Y. O., Selemenev, V., Khorokhordin, A. & Volkov, A. Chromatographic methods for determining free bisphenol A in technical and food products. J. Anal. Chem. 79, 1387–1393 (2024).
Wang, X. et al. Human health risk assessment of bisphenol A (BPA) through meat products. Environ. Res. 213, 113734 (2022).
Çiftçi, S., Yalçın, S. S. & Samur, G. Comparison of daily bisphenol A intake based on dietary and urinary levels in breastfeeding women. Reprod. Toxicol. 106, 9–17 (2021).
Yüksel, B., Ustaoğlu, F., Yazman, M. M., Şeker, M. E. & Öncü, T. Exposure to potentially toxic elements through ingestion of canned non-alcoholic drinks sold in istanbul, türkiye: A health risk assessment study. J. Food Compost Anal. 121, 105361. https://doi.org/10.1016/j.jfca.2023.105361 (2023).
Cao, X. L., Corriveau, J. & Popovic, S. Levels of bisphenol A in canned soft drink products in Canadian markets. J. Agric. Food Chem. 57, 1307–1311 (2009).
Gallart-Ayala, H., Moyano, E. & Galceran, M. Analysis of bisphenols in soft drinks by on-line solid phase extraction fast liquid chromatography–tandem mass spectrometry. Anal. Chim. Act. 683, 227–233 (2011).
(EU), C. R. /3190 of 19 December 2024 on the use of bisphenol A (BPA) and other bisphenols and bisphenol derivatives with harmonised classification for specific hazardous properties in certain materials and articles intended to come into contact with food,. (2024). (2024).
EC. Commission directive 2004/19/EC of 1 March 2004 amending directive 2002/72/EC relating to plastic materials and articles intended to come into contact with foodstuffs. OJEC 71, 8–21 (2004).
EPA. (U.S. Environmental Protection Agency) Bisphenol A Action Plan. (2010). Available at: https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/bisphenol-bpa-summary
EFSA Panel on Food Contact Materials. Re-evaluation of the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA J. 21, e06857 (2023).
EFSA. Opinion of the Scientific Panel on Food Additives. Flavourings, processing aids and materials in contact with food (AFC) on a request from the commission related to butylbenzylphthalate (BBzP) for use in food contact materials. EFSA J. 241, 1–14 (2005).
Shariatifar, N. et al. Levels of polycyclic aromatic hydrocarbons in milk and milk powder samples and their likely risk assessment in Iranian population. J. Food Compost Anal. 85, 103331 (2020).
Goodson, A., Summerfield, W. & Cooper, I. Survey of bisphenol A and bisphenol F in canned foods. Food Addit. Contam. 19, 796–802 (2002).
Hazrati-Raziabad, R. et al. Bisphenol A concentration in canned fruits and vegetables and their risk assessment using Monte Carlo simulation in Iran. Sci. Rep. 14, 31305 (2024).
Jiao, Y. et al. Determination of bisphenol A, bisphenol F and their diglycidyl ethers in environmental water by solid phase extraction using magnetic multiwalled carbon nanotubes followed by GC-MS/MS. Anal. Methods. 4, 291–298 (2012).
Thomson, B. & Grounds, P. Bisphenol A in canned foods in new zealand: an exposure assessment. Food Addit. Contam. 22, 65–72 (2005).
Li, Y., Zhang, S., Song, C. & You, J. Determination of bisphenol A and alkylphenols in soft drinks by high-performance liquid chromatography with fluorescence detection. Food Anal. Methods. 6, 1284–1290 (2013).
Georgescu, B. & Georgescu, C. E. Bisphenol A levels in commercial milk, infant formula and dairy products. Anim Biol. Anim. Husb 5, 171–174. (2013).
Kang, J. H., Kito, K. & Kondo, F. Factors influencing the migration of bisphenol A from cans. J. Food Prot. 66, 1444–1447 (2003).
Mercea, P. Physicochemical processes involved in migration of bisphenol A from polycarbonate. J. Appl. Polym. Sci. 112, 579–593 (2009).
Munguia-Lopez, E., Gerardo-Lugo, S., Peralta, E., Bolumen, S. & Soto-Valdez, H. Migration of bisphenol A (BPA) from can coatings into a fatty-food simulant and tuna fish. Food Addit. Contam. 22, 892–898 (2005).
Wang, X. et al. A probabilistic approach to model bisphenol A (BPA) migration from packaging to meat products. Sci. Total Environ. 854, 158815 (2023).
Toptancı, İ. Risk assessment of bisphenol related compounds in canned convenience foods, Olives, Olive oil, and canned soft drinks in Turkey. Environ. Sci. Pollut Res. 30, 54177–54192 (2023).
Agarwal, A. et al. Food contamination from packaging material with special focus on the Bisphenol-A. Crit. Rev. Biotechnol. 45, 69–79 (2025).
Zang, X., Chang, Q., Hou, M., Wang, C. & Wang, Z. Graphene grafted magnetic microspheres for solid phase extraction of bisphenol A and triclosan from water samples followed by gas chromatography-mass spectrometric analysis. Anal. Methods. 7, 8793–8800 (2015).
Becerra, V. & Odermatt, J. Detection and quantification of traces of bisphenol A and bisphenol S in paper samples using analytical pyrolysis-GC/MS. Analyst 137, 2250–2259 (2012).
Mead, R. N. & Seaton, P. J. GC–MS quantitation and identification of bisphenol-A isolated from water. J. Chem. Educ. 88, 1130–1132 (2011).
Kumar, P. et al. Bisphenol A contamination in processed food samples: an overview. Int. J. Environ. Sci. Technol. 20, 13975–13994 (2023).
Farsani, G. M. et al. Determination of trace elements content of fruits from tehran’s market using ICP-OES method: a risk assessment study. Environ. Monit. Assess. 196, 784 (2024).
Karimi, F. et al. Probabilistic health risk assessment and concentration of trace elements in meat, egg, and milk of Iran. Int. J. Environ. Anal. Chem. 103, 6940–6951 (2023).
Farooq, M. U., Jalees, M. I., Hussain, G., Anis, M. & Islam, U. Health risk assessment of endocrine disruptor bisphenol A leaching from plastic bottles of milk and soft drinks. Environ. Sci. Pollut Res. 28, 57090–57098 (2021).
Shariatifar, N. et al. Polycyclic aromatic hydrocarbons (PAHs) in potato and related products in tehran: a health risk assessment study. Int J. Environ. Anal. Chem, 104(12728), 1–14 (2022).
Sharifiarab, G. et al. Evaluation of polycyclic aromatic hydrocarbons (PAHs) in bottled water samples (non-carbonated, mineral, carbonated and carbonated flavored water) in Tehran with MSPE-GC/MS method: a health risk assessment. Appl. Biol. Chem. 65, 1–12 (2022).
Öncü, T., Yüksel, B., Binay, E. & Şen, N. Ann Pharm. Fr 72–83 (Elsevier) (2024).
Yüksel, B., Öncü, T. & Şen, N. Assessing caffeine levels in soft beverages available in istanbul, turkey: an LC-MS/MS application in food toxicology. Tox Anal. Clin. 35, 33–43 (2023).
Prueitt, R. L. & Goodman, J. E. Evidence evaluated by European food safety authority does not support Lowering the temporary tolerable daily intake for bisphenol A. Toxicol. Sci. 198, 185–190 (2024).
Kalantari, N. G. M. National Report of the Comprehensive Study on Household Food Consumption Patterns and Nutritional Status of I.R.Iran (Nutrition Research Group, National Nutrition and Food Technology Research Institute,Shaheed Beheshti University of Medical Sciences, Ministry of Health, 2015).
Roudbari, A. et al. Concentration and health risk assessment of polycyclic aromatic hydrocarbons in commercial tea and coffee samples marketed in Iran. Environ. Sci. Pollut Res. 28, 4827–4839 (2021).
Kargarghomsheh, P. et al. Evaluation of trace elements in coffee and mixed coffee samples using ICP-OES method. Biol Trace Elem. 202(5), 2338–2346.(2023).
Khalili, F. et al. The analysis and probabilistic health risk assessment of polycyclic aromatic hydrocarbons in cereal products. Environ Sci. Pollut Res 29(21), 31099–31109 (2022).
Yazman, M. M., Ustaoğlu, F. & Yüksel, B. Nutritional profiling of Oncorhynchus mykiss from çamlıgöze dam, türkiye: health risk assessment through Monte Carlo simulation and elemental source attribution using positive matrix factorization. Process. Saf. Environ. Prot. 198, 107172. https://doi.org/10.1016/j.psep.2025.107172 (2025).
Kumar, A. et al. Bisphenol A in canned soft drinks, plastic-bottled water, and household water tank from punjab, India. J. Hazard. Mater. Adv. 9, 100205 (2023).
Fasano, E. et al. Bisphenol A contamination in soft drinks as a risk for children’s health in Italy. Food Addit. Contam. Part. A. 32, 1207–1214 (2015).
Tzatzarakis, M. N. et al. Bisphenol A in soft drinks and canned foods and data evaluation. Food Addit. Contam. Part. B. 10, 85–90 (2017).
Yazdinezhad, S. R., Ballesteros-Gómez, A., Lunar, L. & Rubio, S. Single-step extraction and cleanup of bisphenol A in soft drinks by hemimicellar magnetic solid phase extraction prior to liquid chromatography/tandem mass spectrometry. Anal. Chim. Act. 778, 31–37 (2013).
Cao, X. L., Corriveau, J. & Popovic, S. Sources of low concentrations of bisphenol A in canned beverage products. J. Food Prot. 73, 1548–1551 (2010).
Marchiandi, J., Alghamdi, W., Dagnino, S., Green, M. P. & Clarke, B. O. Exposure to endocrine disrupting chemicals from beverage packaging materials and risk assessment for consumers. J. Hazard. Mater. 465, 133314 (2024).
Vom Saal, F. S. et al. The conflict between regulatory agencies over the 20,000-Fold Lowering of the tolerable daily intake (TDI) for bisphenol A (BPA) by the European food safety authority (EFSA). Environ. Health Perspect. 132, 045001 (2024).
Acknowledgements
This work was supported by the deputy of research of Zanjan University of Medical Sciences (Grant No: A-12-769-68) with ethical approval number (IR-ZUMS.BLC.1403.018).
Author information
Authors and Affiliations
Contributions
Author’s contributions: Nabi Shariatifar: Supervision, Writing- Reviewing. Samira Koti: Writing- Original draft, Design of study, Methodology. Mir-Jamal Hosseini: Writing- Reviewing and Editing Conceptualization. Adel Mirza Alizadeh, Fatemeh Izadpanah: Data curation, Visualization, Investigation, Software, Validation, Methodology.Funding: Financial budget is not used in this article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent to participate
Not applicable.
Consent to publish
was not possible.
Ethical Approval
The research doesn’t comprise any testing of human or animal.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Koti, S., Hosseini, MJ., Shariatifar, N. et al. Determination of bisphenol A in different types of soft drink samples by MSPE-GC/MS technique and contribution to risk assessment. Sci Rep 15, 22353 (2025). https://doi.org/10.1038/s41598-025-09306-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-09306-2