Abstract
Relative fat mass (RFM) serves as an emerging metric for estimating body fat composition. This study evaluates the association between RFM and chronic kidney disease (CKD) using data from the National Health and Nutrition Examination Survey (NHANES) covering the years 2005–2018. Descriptive statistics were applied to characterize the study population, and logistic regression models assessed the relationships between body mass index (BMI), waist circumference (WC), RFM, and CKD. To further elucidate the RFM–CKD association, subgroup analyses, restricted cubic spline (RCS) modeling, sensitivity analyses, and mediation analyses were conducted. A positive trend was observed, with higher RFM values correlating with increased CKD risk. Compared to the lowest RFM quartile, individuals in the highest quartile exhibited a significantly elevated CKD risk (OR = 1.82; 95% CI 1.46–2.27; p < 0.001). Mediation analysis identified neutrophil-to-lymphocyte ratio, hemoglobin, glycated hemoglobin, and uric acid as significant mediators in this association, with hemoglobin exerting a potentially protective influence. RFM is associated with CKD, suggesting that attention to RFM may be helpful for CKD research.
Similar content being viewed by others
Introduction
Chronic kidney disease (CKD) represents a critical global public health concern. The 2017 Global Burden of Disease (GBD) study estimated that approximately 698 million individuals were affected by CKD worldwide, corresponding to a global prevalence of 9.1%. Notably, the prevalence among females exceeded that of males by approximately 30%1,2. Between 1990 and 2017, global all-age CKD prevalence increased by 29.3%, primarily due to population aging and growth, while the age-standardized prevalence remained nearly constant, with a marginal change of 1.2%. Over the same period, CKD-related mortality rose by 41.5%, although the age-standardized mortality rate fluctuated insignificantly by 2.8%, without statistical significance. CKD advanced from the seventeenth to the twelfth leading cause of death globally between 1990 and 20171. In the United States, recent data from the Centers for Disease Control and Prevention (CDC) indicate that approximately 14% of adults are affected by CKD3. Projections considering shifts in demographic profiles, obesity, and diabetes trends suggest that CKD prevalence could escalate to 29–68% by 20304.
Obesity, characterized by excessive adipose tissue accumulation, arises from a combination of genetic, environmental, and behavioral influences. According to the World Health Organization (WHO), obesity is defined by a BMI exceeding 30 kg/m2, while a BMI between 25 and 30 kg/m2 denotes overweight status5. However, BMI presents limitations in accurately evaluating obesity, as individuals with similar BMI values may differ substantially in fat distribution and body composition6. Furthermore, BMI inadequately captures abdominal adiposity. While waist circumference (WC) offers improved insight into abdominal obesity and is associated with visceral fat, it does not distinguish between subcutaneous and visceral adipose compartments7,8. To address these limitations, Woolcott et al. developed the relative fat mass (RFM) index, which incorporates height and WC to provide a more accurate estimate of total body fat percentage in adults9. RFM has demonstrated superior precision in reflecting body fat compared to BMI9 and is strongly associated with chronic conditions such as hypertension, type 2 diabetes, and heart failure10,11,12.
Given these findings, RFM appears to hold considerable potential as a predictor of chronic disease risk. However, the relationship between RFM and CKD remains insufficiently explored. It is hypothesized that RFM may serve as a robust anthropometric marker for identifying individuals at risk for CKD. This study employed data from the NHANES to examine the association between RFM and CKD and compare the predictive strength of BMI, WC, and RFM in relation to CKD. In summary, this study advances understanding of the role RFM may play in elucidating the link between obesity and CKD risk.
Methods
Study design and population
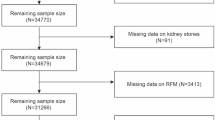
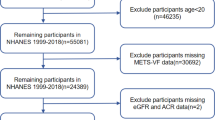
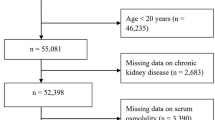
The NHANES is a nationally representative, cross-sectional survey administered by the National Center for Health Statistics (NCHS), a division of the CDC in the United States. Utilizing a complex, multistage probability sampling design, NHANES collects detailed health and nutritional data from a diverse U.S. population to assess public health status at the national level13. The survey protocol is approved by the NCHS Research Ethics Review Board to ensure compliance with ethical standards, and written informed consent is obtained from all participants (NCHS Research Ethics Review Board Approval). For this analysis, NHANES data spanning from 2005 to 2018 were used, encompassing an initial cohort of 70,190 participants. Exclusion criteria were applied to enhance data integrity: individuals under 18 years of age (n = 28,047), those with missing CKD status (n = 2318), incomplete RFM data (n = 4104), and absent values for key covariates (n = 8336) were excluded. The participant selection process is illustrated in Fig. 1.
Exposure variable
The exposure variable was RFM, calculated using WC, height, and gender. Anthropometric measurements were performed by trained health technicians at Mobile Examination Centers (MECs). WC was measured at the upper lateral border of the right iliac crest using a non-stretchable measuring tape, with participants standing upright at the end of normal expiration. Height was recorded using a portable stadiometer. The RFM was calculated using the formula: RFM = 64−(20 × height / WC) + (12 × gender), where gender was coded as 1 for females and 0 for males9.
Outcome variable
The outcome variable was CKD, defined in accordance with the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines14. CKD was diagnosed based on the presence of either an estimated glomerular filtration rate (eGFR) below 60 mL/min/1.73 m2 or a urine albumin-to-creatinine ratio (ACR) of 30 mg/g or greater. eGFR was calculated using the 2021 Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, adjusted for age and sex15. Thus, CKD was defined as eGFR < 60 mL/min/1.73 m2 and/or ACR ≥ 30 mg/g.
Covariates
Key covariates included demographic variables, health behaviors, hypertension, diabetes mellitus (DM), hemoglobin (HGB), neutrophil-to-lymphocyte ratio (NLR), cardiovascular disease (CVD), physical activity level, and uric acid (UA). Demographic data comprised age, WC, educational attainment, BMI, marital status, poverty income ratio (PIR), and race. Health behaviors encompassed smoking and alcohol use. Age was categorized into three groups: 18–45, 46–60, and ≥ 60 years. PIR was classified as < 1.00, 1.00 < 2.00, and ≥ 2.00. Racial groups included Mexican American, non-Hispanic White, non-Hispanic Black, and Other (Other Hispanic and other non-Hispanic race including non-Hispanic multiracial). Marital status was divided into married, never married, divorced/widowed, and other. Education levels were grouped as less than 11th grade, high school graduate, some college, and college graduate or above. Smoking status was defined as current (regular smoking, ≥ 100 cigarettes), former (previously smoked ≥100 cigarettes but not currently smoking), and never (< 100 cigarettes over a lifetime). Alcohol use was classified as ever (≥ 12 drinks in a lifetime) or never (< 12 drinks in a lifetime). Physical activity level was stratified into inactive, moderate, vigorous, and combined moderate-vigorous, based on self-reported participation in high-intensity activities (e.g., running, basketball, fitness training) and moderate-intensity activities (e.g., brisk walking, swimming, cycling at a steady pace). Hypertension was defined by self-reported history, current use of antihypertensive medications, physician diagnosis, or measured systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg16. DM diagnosis was based on self-reported history, glycated hemoglobin (HbA1c) ≥ 6.5%, use of hypoglycemic agents or insulin, random or 2-h OGTT blood glucose ≥ 11.1 mmol/L, or fasting blood glucose ≥ 7.0 mmol/L17. CVD status was determined by the presence of coronary heart disease, congestive heart failure, myocardial infarction, stroke, or angina. NLR, a marker of systemic inflammation, was calculated as the ratio of absolute neutrophil count to absolute lymphocyte count, derived from complete blood count data using a Beckman Coulter automated hematology analyzer in the MEC, and expressed as × 103 cells/µL 18. The specific covariates included NLR, HGB, glycated hemoglobin, and UA, which were incorporated into the mediation analysis.
Statistical analysis
Statistical analyses were conducted using R software (version 4.4.1). To improve population representativeness, data weighting procedures were applied. Statistical significance was defined as a two-sided P-value < 0.05. RFM was stratified into quartiles (RFMQ): 7.75 ≤ Q1 < 29.19, 29.19 ≤ Q2 < 34.98, 34.98 ≤ Q3 < 42.78, 42.78 ≤ Q4 ≤ 57.29, with Q1 designated as the reference group for comparison purposes. Descriptive statistics for continuous variables were reported as means with standard deviations or medians with interquartile ranges, while categorical variables were expressed as proportions. Linear regression models were employed to assess the associations of BMI, WC, and RFM with CKD prevalence. Four sequential regression models were constructed: the unadjusted baseline model; Model 1, adjusted for age, sex, and race; Model 2, further adjusted for marital status, PIR, and educational level; and Model 3, additionally adjusted for smoking status, alcohol use, hypertension, CVD, and physical activity level. To further investigate the association between RFM and CKD, restricted cubic spline (RCS) analysis was performed. Subgroup analyses were stratified by sex, age, smoking status, race, hypertension, and CVD status. Mediation analysis was conducted to examine whether selected variables mediated the association between RFM and CKD. Considering the simplicity and fitting performance of the model, covariates from Model 2 were retained, and HbA1c, HGB, UA, and NLR were selected as mediators. Causal mediation analysis within the potential outcomes framework was performed to estimate indirect effects of RFM on CKD through these mediators, with decomposition into average causal mediation effect (ACME), average direct effect (ADE), and total effect. A nonparametric bootstrap approach with 1,000 resamples was applied to derive confidence intervals (CIs) and assess statistical significance. The percentile method was used to construct 95% CIs, thereby avoiding assumptions regarding the normality of effect distributions. Sensitivity analyses were performed using the “mice” package to address missing data and enhance the robustness of the findings.
Results
Characteristics of the participants
A total of 27,385 individuals were included in the analysis, with a mean age of 46 years. Participant characteristics, stratified by RFM quartiles, are summarized in Table 1. The gender distribution was balanced, with 49.05% male and 50.95% female. Hypertension and diabetes were reported in 32.92% and 9.78% of participants, respectively, with significantly higher prevalence observed in the Q4 group relative to lower quartiles. Non-Hispanic White individuals represented the largest ethnic subgroup (69.54%). Young adults (18–45 years) constituted the largest age group (47.98%), whereas older adults formed the smallest subgroup. The average BMI was 27.90 kg/m2, and the mean WC was 97.90 cm. The overall prevalence of CKD was approximately 14.09%, with a rate nearly threefold higher in Q4 compared to Q1.
Relationship between RFM and CKD
Associations between BMI, WC, RFM, and CKD are detailed in Table 2. In unadjusted models, each standard deviation (SD) increase in BMI and WC was associated with a 2% and 3% elevated risk of CKD, respectively. Additionally, each 1 SD increase in RFM corresponded to a 4% increase in CKD risk. To further examine these relationships, RFM was categorized into quartiles. Following multivariable adjustment for gender, age, race, PIR, marital status, educational attainment, smoking, alcohol use, hypertension, CVD, and physical activity, adjusted odds ratios (ORs) for each RFM quartile relative to Q1 were computed (Table 3). The highest quartile (Q4) demonstrated a significantly elevated CKD risk compared to the lowest (Q1), with an OR of 1.82 (95% CI 1.46–2.27; P < 0.01). Moreover, a significant positive trend was observed across RFM quartiles in relation to CKD prevalence (P for trend < 0.001). Dose–response analysis revealed a statistically significant non-linear association between RFM and CKD risk (P-non-linear < 0.0001), as illustrated in Fig. 2.
Subgroup analysis
Subgroup analyses were conducted to explore the relationship between RFM and CKD across different populations, stratified by age, gender, race, hypertension, and CVD. The findings from these analyses are presented in Fig. 3. Age and gender significantly influenced the correlation between RFM and CKD (all P for interaction < 0.05), while no significant interactions were found for race, hypertension, or CVD. Among individuals aged over 46 years, a significant association between RFM and CKD was observed. Within racial subgroups, stronger associations were found in non-Hispanic Black, Mexican American, and non-Hispanic White populations.
Mediation analysis
Additionally, mediation analysis identified HGB, HbA1c, NLR, and UA as mediators in the RFM-CKD relationship. All four mediators significantly contributed to the association, with NLR accounting for 4.45%, HGB for 2.11%, HbA1c for 35.20%, and UA for 5.85% of the effect (P < 0.001). Notably, HGB exerted an inhibitory effect on the relationship between RFM and CKD, as shown in Fig. 4.
Sensitivity analysis
To address potential biases from missing data, multiple imputation with 5 replications was performed. After imputation, the OR for the highest RFM quartile compared to the lowest was 1.85 (95% CI 1.48–2.31; P < 0.001). The trend test remained significant for both groups (P for trend < 0.0001), as detailed in Table 4.
Discussion
This cross-sectional study, utilizing NHANES data from 2005 to 2018, identified a statistically significant positive association between higher RFM values and an increased risk of CKD.
Obesity has long been linked to a range of chronic conditions, including CVD, diabetes, and hypertension 10,11,12. Individuals with obesity are at an elevated risk of developing CKD compared to those with normal weight or those who are overweight. This relationship is thought to stem from the detrimental effects of obesity on renal function, which can lead to inflammation and insulin resistance. Overweight and obesity have recently been recognized as independent risk factors for CKD and end-stage renal disease (ESRD)19. A comprehensive cohort study by Hsu et al. in Northern California further highlighted overweight and obesity as significant, modifiable risk factors for ESRD20. Lipotoxicity and ectopic lipid deposition in the kidneys (fatty kidney) have emerged as key mechanisms in obesity-related CKD, alongside other contributing factors such as insulin resistance, disrupted adipocytokine balance, hypertension, and glomerular hypertension21,22.
Although BMI is the most commonly used metric for diagnosing obesity, it has notable limitations. BMI does not distinguish between different components of body composition, such as fat, muscle, and bone, nor does it account for fat distribution (e.g., abdominal vs. peripheral fat)23,24. Fluid retention can also skew BMI measurements in clinical settings25. While magnetic resonance imaging (MRI) can accurately measure abdominal obesity and explore its relationship with complications, its complexity and impracticality make it unsuitable for routine clinical use26. In light of BMI’s limitations, alternative indicators such as waist-to-hip ratio (WHR) and WC have been employed to investigate the link between abdominal obesity and CKD. Previous research has shown an independent association between WC and CKD incidence, regardless of BMI27,28,29. RFM represents a newer obesity indicator 9, demonstrating stronger correlations with body fat percentage as estimated by dual-energy X-ray absorptiometry (DXA) than BMI, across large cohorts from the United States, Chile, and Korea9,30,31. Compared to traditional measures like BMI and WHR, RFM is more accurate in estimating total body fat percentage32. To date, however, no studies have explored the association between RFM and CKD.
Weight loss in obese individuals with CKD leads to reduced urinary protein levels and slowed decline in GFR33. In linear regression, a strong association between the obesity indicators (BMI, WC, and RFM) and CKD was observed (P<0.001). Without adjustment for covariates, a 1 SD increase in BMI, WC, and RFM was associated with a respective 3%, 2%, and 4% increased risk of CKD. Notably, RFM quartiles showed a significantly stronger association with CKD than BMI and WC quartiles (Supplementary Table 1). When the lowest RFM quartile was used as the reference, the highest quartile was linked to a markedly increased risk of CKD (OR = 1.82; 95% CI: 1.46–2.27; P < 0.001). Moreover, as RFM was stratified into quartiles, the positive association between RFM and CKD prevalence not only persisted but also became more pronounced with increasing RFM (P for trend < 0.001). The correlation between RFM and CKD was significantly influenced by age (P for interaction < 0.05). Subgroup analyses revealed a significant association between RFM and CKD incidence in individuals older than 46 years, likely due to the compounded effects of aging and obesity on renal function. Aging results in increased glomerular permeability, reduced glomerular volume, glomerular sclerosis, and a decrease in nephron number, which may amplify the adverse effects of obesity on renal function in older individuals34. Furthermore, RCS analysis revealed a significant non-linear positive correlation between RFM and CKD (P-non-linear < 0.001).
Obesity is characterized by chronic low-grade systemic inflammation, which has been shown to play a critical role in the pathogenesis of chronic renal failure. CKD is a chronic inflammatory condition where inflammation serves as a key initiator of progressive tubulointerstitial fibrosis, ultimately leading to ESRD35,36,37. NLR is a well-established marker of systemic inflammation 38 and an independent risk factor for the progression of renal disease in patients with stages 1–4 CKD39. In light of these findings, it is hypothesized that NLR may mediate the relationship between RFM and CKD. The present study indicates that NLR significantly mediates the association between RFM and CKD, accounting for 4.45% of the effect. In addition, interactions between HGB and serum UA have been observed in the context of incident CKD, highlighting the synergistic effects of anemia and hyperuricemia in the onset of CKD 40. Chronic low-grade systemic inflammation in obese individuals can contribute to anemia41, while UA is recognized as an independent risk factor for CKD42. Based on these findings, it is postulated that HGB may also mediate the relationship between RFM and CKD. This study demonstrates that HGB significantly mediates the association between RFM and CKD, acting as a protective factor. The mediation proportion for HGB was 2.11%, while the mediation proportion for UA was 5.85%. This effect may be attributed to the ability of anemia and hyperuricemia to induce oxidative stress and inflammation in the extracellular matrix and endothelial cells, resulting in glomerular hypertension, sclerosis, increased renal vascular resistance, inadequate renal blood perfusion, and decreased renal eGFR34,43,44. Moreover, the decline in renal function exacerbates anemia and impairs the clearance of blood UA45,46,47. Furthermore, prolonged obesity leads to insulin resistance, which increases the risk of developing diabetes—a recognized contributor to CKD. Mediation analysis also revealed that glycated hemoglobin plays a significant mediating role between RFM and CKD, with an estimated mediation proportion of approximately 35.20%.
This study has several notable strengths. First, it is the first to explore the association between RFM and the risk of CKD using a large and representative sample of the US population, while effectively adjusting for potential confounders. Second, compared to DXA, RFM measurement is more accessible, does not involve radiation exposure, and offers a practical alternative for clinical use. Third, even after employing multiple imputation to address missing data, a significant association between RFM and CKD remains evident.
However, several limitations must be acknowledged. First, due to the cross-sectional design, this study cannot establish a causal relationship between RFM and the development of CKD. Second, the use of a single time-point (< 3 months) measurement of serum creatinine and urine albumin from the NHANES may introduce bias in the diagnosis of CKD. Additionally, certain variables, such as medical history and healthcare utilization, are based on self-reported data, which may be subject to recall and response biases. Finally, the RFM variable, calculated from height and WC rather than direct body fat measurements, may introduce measurement inaccuracies, potentially affecting the study’s validity.
Conclusion
In conclusion, the findings of this study reveal a significant positive association between RFM and CKD. Furthermore, NLR, HGB, HbA1c, and UA were identified as substantial mediators in the relationship between RFM and CKD, with HGB may server as a protective factor.
Data availability
The analysis in this study utilized publicly available datasets, which can be accessed at https://www.cdc.gov/nchs/nhanes/.
References
Bikbov, B. et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global burden of disease study 2017. Lancet 395(10225), 709–733 (2020).
Aminu, K. et al. Assessment of global kidney health care status. JAMA 317(18), 1864–1881 (2017).
Te, L. et al. Chronic kidney disease and cognitive performance: NHANES 2011–2014. BMC Geriatr 24(1), 351 (2024).
McCullough, K. et al. Projecting ESRD Incidence and prevalence in the United States through 2030. J Am Soc Nephrol 30(1), 127–135 (2019).
Yau, K. et al. Obesity and the kidney: Mechanistic links and therapeutic advances. Nat Rev Endocrinol 20(6), 321–335 (2024).
Marie-Eve, P. et al. Overview of epidemiology and contribution of obesity and body fat distribution to cardiovascular disease: An update. Progr Cardiovasc Diseases 61(2), 103–113 (2018).
Ford, E. S., Maynard, L. M. & Li, C. Trends in mean waist circumference and abdominal obesity among US adults, 1999–2012. JAMA 312(11), 1151–1153 (2014).
Buyun, L., et al., Trends in obesity and adiposity measures by race or ethnicity among adults in the United States 2011–18: Population based study. 2021. 372(0).
Woolcott, O. O. & Bergman, R. N. Relative fat mass (RFM) as a new estimator of whole-body fat percentage: A cross-sectional study in American adult individuals. Sci Rep 8(1), 10980 (2018).
Peng, Y. et al. Predictive value of relative fat mass algorithm for incident hypertension: A 6-year prospective study in Chinese population. BMJ Open 10(10), e038420 (2020).
Navin, S. et al. Associations of relative fat mass, a new index of adiposity, with type-2 diabetes in the general population. Eur J Internal Med 109, 73–78 (2023).
Navin, S. et al. Relative fat mass, a new index of adiposity, is strongly associated with incident heart failure: data from PREVEND. Sci Rep 12(1), 147 (2022).
Peter, R. et al. Prevalence of chronic kidney disease in type 1 diabetes among adults in the US. Diabetes Care 47(8), 1395–1399 (2024).
Rovin, B. H. et al. KDIGO 2021 Clinical practice guideline for the management of glomerular diseases. J Kidney Int. 100(4), S1–S276 (2021).
Cynthia, D. et al. A unifying approach for GFR estimation: Recommendations of the NKF-ASN task force on reassessing the inclusion of race in diagnosing kidney disease. J Am Soc Nephrol 32(12), 2994–3015 (2021).
Thomas, U., et al., 2020 International society of hypertension global hypertension practice guidelines. 2020. 75(6).
Farmer, A., Fox, R. Diagnosis, classification, and treatment of diabetes. 2011. 342(0).
Bogdan-Sorin, T. et al. Assessment of inflammatory hematological ratios (NLR, PLR, MLR, LMR and monocyte/HDL-cholesterol ratio) in acute myocardial infarction and particularities in young patients. Int J Mol Sci 24(18), 14378 (2023).
Radovan, H. et al. Chronic kidney disease and obesity. Nephron 147(11), 660–664 (2023).
Chi-yuan, H. et al. Body mass index and risk for end-stage renal disease. Ann Internal Med 144(1), 21–28 (2006).
Przemyslaw, R. et al. Renal disease in obesity: The need for greater attention. J Renal Nutr 16(3), 216–223 (2006).
Aiko, P. J. et al. Fatty kidney: Emerging role of ectopic lipid in obesity-related renal disease. Lancet Diabetes Endocrinol 2(5), 417–426 (2014).
Eknoyan, G. Adolphe Quetelet (1796–1874): The average man and indices of obesity. Nephrol Dial Transplant 23(1), 47–51 (2007).
Humphreys, S. The unethical use of BMI in contemporary general practice. Br J Gener Pract 60(578), 696–697 (2010).
Carrero, J. J. Misclassification of obesity in CKD: Appearances are deceptive. Clin J Am Soc Nephrol 9(12), 2025–2027 (2014).
Sophia, M. et al. Anthropometric estimators of abdominal fat volume in adults with overweight and obesity. Int J Obes 47(4), 306–312 (2023).
Martin, T. et al. Abdominal obesity is associated with microalbuminuria and an elevated cardiovascular risk profile in patients with hypertension. Vasc Health Risk Manag 5(4), 577–585 (2009).
Sara-Joan, P.-S. et al. A central body fat distribution is related to renal function impairment, even in lean subjects. Am J Kidney Dis 41(4), 733–741 (2003).
Holly, K. et al. Waist circumference, body mass index, and ESRD in the REGARDS (reasons for geographic and racial differences in stroke) study. Am J Kidney Dis 67(1), 62–69 (2015).
Jeong Ki, P. et al. Usefulness of relative fat mass in estimating body adiposity in Korean adult population. Endocr J 66(8), 723–729 (2019).
Carolina, A. et al. Relative fat mass as an estimator of body fat percentage in Chilean adults. Eur J Clin Nutr 78(9), 782–787 (2024).
Di, W. et al. Association between two novel anthropometric measures and type 2 diabetes in a Chinese population. Diabetes, Obes Metab 26(8), 3238–3247 (2024).
Bolignano, D. & Zoccali, C. Carmine, effects of weight loss on renal function in obese CKD patients: A systematic review. Nephrol Dial Transplant 28(4), 82–98 (2013).
Jun Ling, L. et al. Association of age and BMI with kidney function and mortality: A cohort study. Lancet Diabetes Endocrinol 3(9), 704–714 (2015).
Imig, J. D. & Ryan, M. J. Immune and inflammatory role in renal disease. Compr Physiol 3(2), 957 (2013).
Virginia, W., et al., The economic burden of chronic kidney disease and end-stage renal disease. 2016. 36(4).
Youhua, L. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol 7(12), 684–696 (2011).
Zhiwei, H. et al. Prognostic value of neutrophil-to-lymphocyte ratio in sepsis: A meta-analysis. Am J Emerg Med 38(3), 641–647 (2019).
Ryota, Y. et al. High neutrophil/lymphocyte ratio is associated with poor renal outcomes in Japanese patients with chronic kidney disease. Renal Fail 41(1), 238–243 (2019).
You-Chi, C. et al. Synergetic association between anemia and hyperuricemia on new-onset chronic kidney disease in a large Taiwanese population follow-up study. Int J Environ Res Public Health 20(2), 1421 (2023).
Ramadan A, S. and Q.J.E.R.H. Haitham Mohammed Habib, The relationship between anemia and obesity. 2022. 15(10).
Mihai-Emil, G. et al. Uric acid and oxidative stress-relationship with cardiovascular, metabolic, and renal impairment. Int J Mol Sci 23(6), 3188 (2022).
Ahmed, B., Sultana, R. & Greene, M. W. Adipose tissue and insulin resistance in obese. Biomed Pharmacother 137, 111315 (2021).
Spoto, B., Pisano, A. & Zoccali, C. Insulin resistance in chronic kidney disease: A systematic review. Am J Physiol-Renal Physiol 311(6), F1087–F1108 (2016).
Nosratola D, Oxidative stress in uremia: Nature, mechanisms, and potential consequences. 2004. 24(5).
Jonathan, H. & Raymond, M. Oxidative stress in uremia. Curr Opin Nephrol Hypertens 12(6), 593–598 (2003).
Laura, G. et al. Role of oxidative stress in the renal abnormalities induced by experimental hyperuricemia. Am J Physiol-Renal Physiol 295(4), F1134–F1141 (2008).
Acknowledgements
The authors express their sincere gratitude to all participants and investigators involved in the NHANES study. Their invaluable contributions have significantly facilitated the progression of this research.
Funding
This study was funded by the National Natural Science Foundation of Shandong Province (ZR2024MH196).
Author information
Authors and Affiliations
Contributions
WZD conceived the study, analyzed the data, interpreted the results, and wrote the manuscript. LWX, ZXS, LLN, LY, LX, and ZSG contributed to the interpretation of the results and the revision of the manuscript. ZW performed the data analysis and contributed to the interpretation of the findings. LGY conceived the study idea and provided guidance on interpretation and manuscript writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Institutional review board statement
This study utilized publicly available NHANES data. The NHANES data collection received approval from the NCHS Research Ethics Review Board. Use of the public-use datasets does not require IRB review or an exempt determination.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, Z., Li, W., Lv, L. et al. Association between relative fat mass (RFM) and chronic kidney disease (CKD): data from NHANES 2005–2018. Sci Rep 15, 25673 (2025). https://doi.org/10.1038/s41598-025-09334-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-09334-y