Abstract
Fish can be infected with metacercariae (the final larval stage) of different species of potentially zoonotic digenetic trematodes (flukes). The fish-borne zoonotic trematodes thus compromise food safety and present a major threat for human health. Reducing the risk of human infections requires careful assessment and accurate taxonomic identification of these parasites. Here, we analysed metacercariae in muscle tissue of tench (Tinca tinca), sampled between March and September 2022 from three fish farms and three natural waterbodies in Germany. Whenever possible, we combined morphological and molecular data from the very same individual metacercariae using mitochondrial cytochrome oxidase c subunit 1 (cox1) and nuclear internal transcribed spacer 1 (ITS1) for species identification. Three morphotypes of metacercariae were found in the muscle of tench, corresponding to the trematode species Pseudamphistomum truncatum Rudolphi, 1819 (Opisthorchiidae), Hysteromorpha triloba Rudolphi, 1819 (Diplostomidae), and Paracoenogonimus ovatus Katsurada, 1914 (Cyathocotylidae). The high prevalence of metacercariae of P. truncatum and P. ovatus, both with zoonotic potential, poses a risk for human infections if undercooked or raw tench is consumed.
Similar content being viewed by others
Introduction
Fish can serve as the second intermediate host of the metacercariae of many digenetic trematode species. Fish-borne trematodes that parasitize fish as their second intermediate host comprise about 12 systematic families1. Among them, the species with zoonotic potential belong to seven families: Opisthorchiidae, Clinostomidae, Heterophyidae, Echinochasmidae, Isoparorchiidae, Troglotrematidae and Plagiorchiidae2. Food-borne trematodiases are considered neglected parasitic diseases3 even though 750 million people are at risk4. The risk of human infections is related to social and cultural traits associated with certain dietary habits as the predilection for raw or undercooked fish5. The opisthorchiid liver flukes Opisthorchis felineus Rivolta, 1884, Opisthorchis viverrini Poirier, 1886 and Clonorchis sinensis Cobbold, 1875 are considered the most concerning fish-borne zoonotic trematodes6. Opisthorchiasis is a public health problem particularly in Asia, but also in Russia and the Ukraine7, whereas recent reports from other European countries are rare8.
Reliable identification tools are needed to assess the distribution of trematode infections of fish, particularly due to their zoonotic potential and consequent relevance to food safety, economy, and public health9,10. The identification to the species-level of most digeneans is based almost exclusively on morphological features of adult animals11. Only few morphological descriptions of immature stages are available and the poor development of certain organs in larval stages complicates their identification10. Moreover, intraspecific morphological variation of trematodes and accumulating evidence for widespread occurrence of cryptic species in this group increases the chance for errors12,13. Therefore, pure morphological identification of metacercariae should be taken with caution and ideally complemented with molecular techniques or the examination of adult specimens directly related to the metacercariae14. However, so far, molecular approaches are not exempt from challenges including the availability and taxonomic assignment of sequences in databases, and the difficult delimitation of the genetic variation among taxa and across taxonomic levels, depending on the genetic markers and the taxa15. Consequently, combination of morphological and molecular data, along with additional sources of information, such as biogeography, life history traits and ecology under one conceptual framework, thus termed integrative taxonomy16, has been recognized as a promising approach for the delimitation of trematode species13,17,18.
In Berlin and Brandenburg (Germany), metacercariae of O. felineus have been found in common roach (Rutilus rutilus Linnaeus, 1758), rudd (Scardinius erythrophthalmus Linnaeus, 1758), common bleak (Alburnus alburnus Linnaeus, 1758), common bream (Abramis brama Linnaeus, 1758), white bream (Blicca bjoerkna Linnaeus, 1758) and blue bream (Ballerus ballerus Linnaeus, 1758); whereas the adult trematodes have been reported in red foxes (Vulpes vulpes Linnaeus, 1758), muskrats (Ondatra zibethicus Linnaeus, 1766), and cats (Felis catus Linnaeus, 1758)19,20,21,22,23,24.
Remarkably, these studies do not include tench, although it is an important secondary species in pond culture25 and, while not among the top targeted species, highly valued by anglers26. There are only few systematic studies on the parasitofauna of tench in Germany, with only two trematode species, Tylodelphys clavata and Asymphlodora tincae, and 18 species of other metazoan parasites previously reported [27 and references therein]. None of these studies have so far looked specifically for potentially zoonotic metacercariae in the muscle of the tench. Due to two outbreaks of human infections with O. felineus reported after the consumption of marinated tench (Tinca tinca Linnaeus, 1758) from a lake in Italy27,28, and the knowledge gap about the parasitofauna of tench in Germany29, the aim of this study was to determine the diversity of trematode species in the muscle of tench and the occurrence of fish-borne zoonotic trematodes, using integrative morphological and molecular analyses of the same individual metacercaria in tench collected from fish farms and natural waterbodies of Germany.
Results
Fish samples and parasitological parameters
The mass of tench differed significantly between the localities sampled during this study (Table 1; Kruskal-Wallis test: H (5) = 61.187, p < 0.001).
Three different types of metacercariae were found in the musculature of tench (Fig. 1). Considering all the sampling sites, metacercariae were detected in the muscles of 77 tench (79.4%). From the infected fish, 42 tench (54.5%) harbored only one type of metacercaria. Co-infections with two types of metacercaria were detected in 32 tench (41.6%), and three tench (3.9%) were infected with all three species of metacercaria detected during the study. Based on the morphology of cysts and excysted metacercariae, these were assigned to the families Opisthorchiidae, Diplostomidae and Cyathocotylidae.
Opisthorchiid metacercariae were found in fish from all natural waterbodies, but only in one fish farm, with prevalences ranging from 20 to 100%, and overall, more prevalent in natural waters (Table 1). Diplostomid metacercariae were likewise detected in tench from all sampling sites, excluding River Spree, with prevalences ranging from 9 to 48%. Cyathocotylid metacercariae were only absent in tench from fish farm 3, whereby the prevalences showed no pattern in terms of occurrence in fish farms or natural waters and ranged between 36% and 100%.
The mean infection intensity of opisthorchiid metacercariae ranged from 1.0 in fish farm 1 to 5.9 in River Spree (Table 1) and although highly variable, no significant differences between localities were detected (Kruskal-Wallis H-test: H (3) = 7.767, p = 0.051).
Morphological description of opisthorchiid metacercariae
The cysts were round and composed of two distinct layers. The outer layer was light yellow-brownish in color, and the inner layer was hyaline (Fig. 1a, b). Within the cyst, the metacercaria was usually folded and its characteristic dark excretory bladder was easily observed. The body was flat, elongated, slightly oval and tapering anteriorly (Fig. 1c). The integument was covered with small spines. The subterminal oral sucker was slightly smaller than the ventral sucker. The pharynx was followed by a short oesophagus and the intestinal caeca that reached up to the posterior end of the excretory bladder. Below the bifurcation of the intestines the cephalic glands were visible. The ventral sucker was located toward the upper end of the posterior half of the body. The excretory bladder placed below the ventral sucker, occupied almost one third of the total body length and was filled with small, rounded granules. Morphometric data of metacercariae are given in Table 2.
Molecular characterization of opisthorchiid metacercariae
Thirty newly generated partial sequences of the mitochondrial cox1 gene were aligned with sequences of the cox1 gene used for phylogenetic study of the family Opisthorchiidae by Sokolov et al.30. The analysis involved 84 sequences (Supplementary table S1), and the alignment was 1010 bp long.
Sequences of Pseudamphistomum truncatum (KP869078 and KP869080 to KP869085) were the most closely related retrieved by BLAST searches in NCBI (GenBank, consulted on 15/01/2024), when sorted by the percentage of identity with similarity values between 100% and 98.3%, although the query coverage was only 30%. Due to this low alignment coverage with P. truncatum sequences, BLAST search results were sorted by e-value and the best matches corresponded to Metorchis xanthosomus (NC079699) and M. bilis (NC079698), with similarity values between 86 and 87%, and 84–85%, respectively.
The phylogenetic analysis based on cox1 sequences retrieved a well-supported clade (Bootstrap support 99%) including all the samples from this study and isolates of P. truncatum deposited in GenBank (Fig. 2a). The pairwise distances of the cox1 sequences obtained in this study varied between 0% and 2.4%, and between 0% and 3.9% for all P. truncatum sequences. The TCS haplotype network analysis of the cox1 dataset including the haplotypes of Sherrard-Smith31, identified nine haplotypes (Fig. 3a). Four haplotypes were found only once in German samples, three of them in River Spree and one in Lake Müggelsee. More than one haplotype was detected in three tench from River Spree, the locality with the highest number of haplotypes. Specimens from Germany, United Kingdom, Sweden and Denmark shared the most frequently detected haplotype in terms of samples. The cox1 dataset had 10 segregating sites, 5 of which were parsimony informative. The nucleotide diversity (Pi) was 0.00143 and Tajima’s D was − 2.63323 (p < 0.05).
Dendrogram based on maximum-likelihood analysis of (a) partial cox1 sequences and (b) ITS1 sequences of the opisthorchiid isolates obtained in this study (bold) and sequences obtained from GenBank. Only bootstrap values > 80% are shown. R, redia; C, cercaria; M, metacercaria; A, adult; CHN, China; CZE, Czech Republic; DEU, Germany; DNK, Denmark; ECU, Ecuador; ESP, Spain; GBR, United Kingdom; IND, India; KOR, Korea; LAO, Laos; FRA, France; PAK, Pakistan; SWE, Sweden; RUS, Russia; USA, United States of America; VNM, Vietnam.
Forty-two partial ITS1 sequences were generated. The closest matches to our isolates were two sequences deposited in GenBank under the names Pseudamphistomum truncatum (MZ266334) and Metorchis orientalis (MW001042).
The dataset for phylogenetic analysis based on ITS1 included our sequences and additional opisthorchiid isolates available from GenBank (Supplementary table S1). The final alignment comprised 684 bp. A cluster with moderate statistical support (bootstrap 85%) grouped all our isolates, P. truncatum (MZ266334- MZ266336) and ‘M. orientalis’ (MW001042) (Fig. 2b). The pairwise distances of the ITS sequences of our opisthorchiids and the closest matches in the GenBank ranged between 0.00% and 1.69%.
The TCS-haplotype network analysis of ITS1 (Fig. 3b) distinguished four haplotypes among P. truncatum, the most dominant haplotype was shared by samples from Germany, Denmark and Finland; three haplotypes were found only in Germany, two of them detected in single individuals. The dataset contained eight segregating sites, one parsimony informative. The nucleotide diversity (Pi) was low 0.00098, and Tajima’s D was − 2.0075 (p < 0.05).
Morphological description of diplostomid metacercariae
Most diplostomid metacercariae were found free, not encapsulated in the muscle. The few specimens found encysted had a granular, round, single-layered, and extremely fragile cyst (Fig. 1d). The body of the metacercaria had a pyriform shape and was divided in two distinct parts (Fig. 1e). The tegument was covered by small papillae. The forebody was round and ventrally concave pseudosuckers were located at both sides of the subterminal oral sucker. The trilobated holdfast organ was situated ventrally in the middle of the forebody. The ventral sucker located near the anterior margin of the holdfast organ was slightly bigger than the oral sucker (U = 8.5, p = 0.01). In some cases, the ventral sucker was partially or totally covered by the holdfast organ and not visible. The hindbody consisted of a cylindrical to subtriangular prolongation, widest at the junction with the forebody. The intestinal caeca reached the posterior part of the hindbody. Morphometric data of metacercariae are provided in Table 2.
Molecular characterization of diplostomid metacercariae
Two sequences of the complete ITS1–5.8 S–ITS2 region were highly similar (> 99%) to 14 isolates of Hysteromorpha triloba and Hysteromorpha sp. from GenBank (MG649479, MG649481, MG649482, MG649486, MG649487, MG649490, MG649491, MH521250, MN179274-MN179276, MW135094, MW135095, MW135097) and four isolates assigned to Hysteromorpha corti (JF769486, HM064925-HM064927), although identified as H. triloba in their respective references32,33. These sequences were co-analyzed with other diplostomid sequences available in GenBank, comprising a total of 94 sequences (Supplementary table S1). The alignment was 1063 bp long. In the ML tree, both isolates clustered with sequences of H. triloba, H. corti and Hysteromorpha sp. obtained from GenBank (Fig. 4). in a clade with strong bootstrap support (100%). The pairwise distances among Hysteromorpha in the dataset varied from 0 to 0.3%.
Dendrogram based on maximum-likelihood analysis of ITS sequences of the diplostomid isolates of this study (bold) and sequences obtained from GenBank. Only bootstrap values > 80% are shown. C, cercaria; M, metacercaria; A, adult; BRA, Brazil; CAN, Canada; CHN, China; DEU, Germany; DNK, Denmark; ECU, Ecuador; GRC, Greece; HUN, Hungary; IND, India; KEN, Kenya; MEX, Mexico; TZA, Tanzania; USA, United States of America.
Morphological description of cyathocotylid metacercariae
The cysts of cyathocotylid metacercariae were round, the most internal layer was thinner than the others, and almost completely occupied by the metacercaria (Fig. 1f). The body of the metacercaria was sub-oval in shape, narrow anteriorly (Fig. 1g). The pharynx was slightly smaller than the subterminal oral sucker, the esophagus rather short, and the intestinal branches extended to the posterior end of the body (Fig. 1g). The body had a shallow ventral concavity in the posterior half of the body, where the round holdfast organ was located. The ventral sucker, situated anteriorly to the holdfast organ, a little behind the center of the body (Fig. 1g), was almost half the size of the oral sucker (U = 193.5, p < 0.001). The ventral sucker was not visible in all individuals. The channels of the secondary excretory system covered almost entirely the body and masked most of the organs. Small round granules were visible within the tubules. Morphometric data of metacercariae are given in Table 2.
Molecular characterization of cyathocotylid metacercariae
Sequences of the complete ITS1–5.8S–ITS2 region of three specimens were obtained. The closest matches found in GenBank corresponded to isolates designated as Holostephanus sp. (MT668941 to MT668949 and MT668951) or therein assigned as Paracoenogonimus ovatus (PP093043 and PP093044) with similarity values ranging from 99.5 to 100%. The phylogenetic analysis involved 24 sequences (Supplementary table S1) and the alignment comprised 418 bp. A relatively well-supported clade (90%) included cyathocotylid metacercariae from tench, together with metacercaria assigned as ‘Holostephanus sp.’ and adults deposited as Paracoenogonimus ovatus in GenBank (Fig. 5).
Two of our specimens (PV428971 and PV428972) were almost identical to metacercaria of Holostephanus sp. and adults of P. ovatus (PP093043 and PP093044) with distances between 0% and 0.4%. The genetic divergence between these isolates and our specimen PV428973 varied between 1.4% and 1.9%, and two metacercaria identified as P. ovatus (PP093040 and PP093041) differed between 1.2 and 3.4% from our samples PV428971-973.
Dendrogram based on maximum-likelihood analysis of ITS sequences of the cyathocotylid isolates of this study (bold) and sequences obtained from GenBank. Only bootstrap values > 80% are shown. M, metacercaria; A, adult; DEU, Germany; EGY, Egypt; HUN, Hungary; ITA, Italy; RUS, Russia; USA, United States of America.
Discussion
Previous cases of human infections from Italy showed that the consumption of raw or marinated tench fillets can be the cause of opisthorchiasis27,28,34. Furthermore, in the endemic areas of central Italy, tench serves as a particularly suitable host for Opisthorchis felineus, with a proven prevalence of almost 90% in the Bolsena and Bracciano lakes35. Although in the past opisthorchiid trematodes have been recorded in north-eastern Germany with high prevalence in cats and red foxes as definitive hosts19,21,23 and various cyprinid fish species as second intermediate hosts19,20,22, tench, which are popular as food fish, have not yet been examined for muscle metacercariae in Germany. Based on both morphological and molecular identification, we have detected metacercariae of the Opisthorchiidae, Cyathocotylidae and Diplostomidae families in muscle tissue of tench.
An unequivocal identification of opisthorchiid metacercariae only based on morphometrics is challenging, as the metacercariae of the European liver flukes O. felineus, M, bilis, M. xanthosomus and P. truncatum are morphologically very similar, differing mainly in their size, while size ranges of these species may even overlap, with P. truncatum regarded as the largest species20. Based solely on the comparison of the morphometric data of the opisthorchiid metacercariae isolated from tench in this study with data from the above-mentioned references, a possible co-infection with P. truncatum and O. felineus could be assumed, and the presence of the smaller Metorchis spp. could not be excluded. Moreover, all of them have been reported from Germany20,22. However, in the interspecific phylogenetic assignment based on cox1, all sequences generated from our opisthorchiid metacercariae were placed in a well-supported clade with adult specimens of Pseudamphistomum truncatum31, indicating their conspecificity and excluding the other morphologically similar species. The values of distances based on cox1 sequences were slightly higher than the range of genetic distances within species of the family Opisthorchiidae36, but lower than its interspecific divergence37, consistent with variation in other trematode families38. In the phylogenetic tree based on ITS1, a cluster included our sequences and a metacercaria isolate of P. truncatum (MZ266334) from Russia, and a cercaria of M. orientalis (MW001042) collected from the lymnaeid snail Stagnicola palustris in freshwater ponds in Denmark39. A probable misidentification by Duan et al.39 was suggested by Sokolov et al.30 taking into account further phylogenetic analysis, geographical distribution and because lymnaeid snails are not known as intermediate hosts of opisthorchiid trematodes.
The genetic divergence of the ITS1 sequences was similar or slightly higher compared with the intraspecific one within Opisthorchiidae36,40,41 or other trematode families38, without reaching interspecific values40. Irrespective of the large morphological variation observed in our metacerariae from tench and beyond those previously reported for the species42,43,44,45, the relatively low genetic distances below interspecific limits and their clustering with P. truncatum in both phylogenetic trees, particularly with adult specimen of P. truncatum31 strongly support their conspecificity with P. truncatum.
Combined morphological and molecular analyses of the diplostomid metacercariae from tench led to an unambiguous identification as Hysteromorpha triloba sensu lato. For a long time, H. triloba was considered cosmopolitan, until it was recently subdivided into the Palaearctic H. triloba and the Nearctic H. corti46 taxon. That the sequences of our two Hysteromorpha isolates from Germany cluster together with those from Brazil, Mexico, Canada, Italy and Denmark reflects the low variability caused by high evolutionary conservation of the rDNA, as already referred to by Locke et al.46. Anyhow, according to the biogeographical subdivision, individuals collected in Germany are to be designated as H. triloba. Their morphology and morphometric characters were similar to previous descriptions of H. triloba46,47,48, and molecular analysis confirmed this identification. Likewise, the low pairwise distances among all sequences of Hysteromorpha included in the dataset agree with reported intraspecific variation46,49,50.
Cyathocotylids isolated from tench agreed with the original description of metacercaria of Paracoenogonimus ovatus51, and the body size of our largest specimens corresponded approximately to that of the specimen described therein. The ventral sucker was not visible in all specimens isolated from tench, suggesting different developmental degrees of the metacercariae. According to Komiya52, the ventral sucker is clearly visible only in older metacercaria, while it is not developed in metacercariae younger than five weeks. In the phylogenetic tree of ITS sequences, our cyathocotylid metacercariae clustered with metacercariae assigned as Holostephanus sp. (Hungary and Italy), and adults and a metacercaria, identified as Paracoenogonimus ovatus (Russia). The smaller body size of Holostephanus spp. and morphological characteristics distinguishing the genera Paracoenogonimus (subfamily Prohemistominae) and Holostephanus (subfamily Cyathocotylinae)47,53 suggest that the cyathocotylid metacercariae isolated from tench belong to P. ovatus. These include Brandes organ, which in Paracoenogonimus is smaller in relation to the body size and located in the posterior part of the body, and the position of the ventral sucker in the middle of the body, at a clear distance from the intestinal furcation. Furthermore, the low genetic divergence between our cyathocotylid metacercariae, those from Hungarian and Italian fishes54 and adult specimens of P. ovatus of Sokolov et al.55 further support that all belong unequivocally to P. ovatus. However, additional molecular data of the species along its distribution range and from different host species are needed to address the question whether the range of variation observed between some specimens still falls within intraspecific levels, especially as P. ovatus may represent a species complex55,56.
The occurrence of P. truncatum in tench constitutes a new host record in Germany. In Europe, hitherto metacercariae of two opisthorchiid species have been detected in tench: P. truncatum in UK31 and Italy9, and O. felineus in Italy35. The prevalences of P. truncatum metacercariae in tench from natural waterbodies from 46 to 100% were similar to prevalences reported for roach in the Gulf of Finland with 46%57 and in the River Shannon in Ireland with 89%58 and higher than prevalences of opisthorchiids infecting roach from the Berlin area (River Havel, Lake Müggelsee and Teltowkanal) with values of 1.3% for P. truncatum and 28.8% for metacercariae of the “O. felineus-M. bilis” type22.
Paracoenogonimus ovatus, which was originally described from metacercariae collected in Germany52, is known as a parasite of tench54, and co-infections with metacercariae of the P. truncatum and P. ovatus were also observed by Simakova et al.59 in the middle Ob River basin. Tench harbouring metacercaria of P. ovatus have been reported in Poland60,61 with a prevalence of 19%60. Similar prevalences to those of our study have been observed in roach and bleak in natural waters of Poland62 and in the Mykolaiv Region of Ukraine63. High prevalences of metacercariae of P. ovatus (classified as Cyathocotylidae gen. sp.) were found in carp from fish farms in Hungary54.
Metacercariae of H. triloba have been previously reported in tench and other cyprinids in Germany20,64. The only known final host for H. triloba in Europe is the black cormorant (Phalacrocorax carbo)42,47, and it is not suspected of infecting mammals. The different prevalences of H. triloba in tench probably reflect different degrees of occurrences of cormorants, suggesting larger populations around Lake Müggelsee and the fish farm in Brandenburg.
The zoonotic potential of P. truncatum detected in tench may be inferred from several reports that show fish-eating mammals as their final hosts44,58,65, and that it can also infect humans66. In contrast, the zoonotic potential of P. ovatus is discussed controversially. Several publications indicate possible infections not only of avian hosts but also in humans60,61. Mice and laboratory rats have also been successfully infected experimentally51,62. However, the experiments conducted by Sándor et al.67 with metacercariae of P. ovatus (misidentified as Holostephanus sp.) were unsuccessful, which led Sokolov et al.55 to suggest that P. ovatus found in mammals and birds could probably belong to different species. This latter example shows how important unequivocal taxonomic identification is for human health assessment.
The presence of common haplotypes of P. truncatum in different European countries suggests gene flow between populations, possibly promoted by the high dispersal capacities of mobile hosts across Europe and are corresponding to low nucleotide diversity68. The significant negative values of Tajima’s D indicate an excess of rare genetic variants that could be associated with population expansion, which is in agreement with the emergence of P. truncatum in various final hosts throughout Europe44,58,65. There are no discernible limits for the spread of P. truncatum, as its snail and cyprinid intermediate hosts are widely distributed in Europe31.
The high prevalence of P. truncatum in tench, especially from natural waters, clearly shows that zoonotic trematodes can have a serious impact on food safety and public health in Central Europe. In addition, the zoonotic potential of the widespread P. ovatus cannot yet be ruled out. Our study indicates that P. truncatum is more prevalent in natural waters than in aquaculture ponds. However, it also shows that the presence of zoonotic opisthorchiid trematodes cannot be excluded in pond culture. So far, the risk of opisthorchiasis in Germany has been regarded as low because the consumption of raw or undercooked cyprinids has no tradition here20, and reported cases in humans are rare, with the most recent case in 200969. Compared to the main species rainbow trout and carp, tench plays only a minor role in German aquaculture production70. However, as assumed by Schuster et al.20, anglers in particular could run the risk of becoming infected with opisthorchiids, e.g. by eating cold-smoked fish, especially as they target and consume also other fish species such as roach (Rutilus rutilus), bream (Abramis brama) and rudd (Scardinius erythrophthalmus)26, which play no major role in the fish trade, and can also serve as fish hosts for the parasite20,42,43. Another group with an increased risk of opisthorchiasis are immigrants, especially from eastern countries with different eating traditions20. In addition, repeated outbreaks of opisthorchiasis in Italy, which are attributed to Opisthorchis felineus, clearly show the risk of infection through the consumption of corresponding fish delicacies in a restaurant27,28,34,71 and revealed the possible fish substitutions using tench as whitefish for food fraud34. The general lack of research on zoonotic parasites specifically in European freshwater fish, and the trend towards consumption of raw or semi-marinated fish contrasts with a lack of surveillance data on fish-borne zoonotic parasites72. It should be noted here that more parasites in freshwater fish than those mentioned in this article have zoonotic potential, including Clinostomum complanatum (Trematoda), Contracaecum rudolphii, Eustrongylides excisus (Nematoda), Dibothriocephalus latus (Cestoda)73. Our study shows that the parasites are present, and it will only depend on the spread of both traditional and new trends in food preparation whether infective larvae are ingested, and human infections may occur. In conclusion, more and improved surveillance across more freshwater fish species and in multiple waterbodies is a requirement of zoonotic disease prevention in Central Europe.
Materials and methods
Sample collection
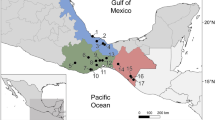
From March to September 2022, tench were obtained from three fish farms (1–3) located in Brandenburg (1), Saxony (2), and Bavaria (3) and from three natural waterbodies (4–6) in Berlin and Brandenburg, namely Lake Müggelsee (4), Lake Blankensee (5), and River Spree (6) near Mönchswinkel (Fig. 6).
Location of the sampled natural waters (triangles) and fish farms (circles) in Germany. Figure was created using QGIS version 3.10.6 (https://qgis.org/). Germany’s administrative map VK2500 was obtained from GeoBasis-DE under the license “dl-de/by-2-0” (www.govdata.de/dl-de/by-2-0).
Weight and total length of the fish were measured prior to dissection, to the nearest of 1 g or 1 mm, respectively. Fish fillets were examined individually through the compression method according to Waikagul & Thaenkham1. Cysts were gently isolated using dissection needles and placed into Petri dishes with 0.7% saline. Living metacercariae within cysts and after their mechanical excystment were individually and without pressure examined on microscopic slides with a drop of 0.7% saline and photographed at 50x, 100x and 200x magnification. Morphological and morphometric characters of the metacercariae including size, shape and number of layers of the cyst, length and width of the body, size of oral and ventral suckers, and of other taxonomically relevant structures were recorded. All measurements were taken to an accuracy of 1 μm.
The morphological identification of the metacercariae followed the taxonomic keys of Bykhovskaya-Pavlovskaya et al.42, Bauer43, and Niewiadomska47. The identification of individual specimens was followed by their preservation in 96% ethanol for further molecular analyses. The selection criteria of metacercaria specimens used in molecular analyses included a relative uncertainty level in preliminary identification based on morphology, the representation of intraspecific morphological variation and different sampling locations.
DNA extraction
DNA was extracted from whole individual excysted metacercaria using the QIAamp DNA mini kit (Qiagen, Hilden, Germany) with slight modifications to the manufacturer’s protocol as follows: 90 µl of Buffer ATL and 10 µl of Proteinase K were added to the tubes, mixed, and incubated at 56 °C for 2 h. The lysis was followed by the addition of 100 µl of Buffer AL and incubation for 10 min at 70 °C. One hundred microliters of molecular grade ethanol were incorporated to the mixture and pipetted onto the QIAamp Mini spin column placed in a 2 ml collection tube and centrifuged (8000 rpm, 1 min). After centrifugation, the spin column was placed into a new 2 ml collection tube and the flow-through discarded. The same procedure was repeated in the next two steps after adding 500 µl of Buffer AW1 (8000 rpm, 1 min) and subsequently 500 µl of Buffer AW2 (14000 rpm, 3 min). Finally, 35 µl of Buffer AE was added to the tube, incubated at 37 °C for 5 min and centrifuged (10000 rpm, 1 min). The concentration of DNA yielded was estimated in a microvolume spectrophotometer (NanoDrop ND-1000, Marshall Scientific, Hampton, USA). The DNA samples were stored at −20 °C until PCRs were run.
PCR amplification
All PCR-amplifications were conducted on a Mastercycler Nexus GSX1 (Eppendorf, Hamburg, Germany). Negative and positive controls were included in each amplification run.
New degenerate primers targeting an 837 bp fragment of the mitochondrial cox1 region of opisthorchiids were designed using available sequences of the family Opisthorchiidae from the GenBank database, namely the primers cox1_opist_F (5´-CDATGGATCAYAAGCGTATAGG-3´) and cox1_opist_R (5´- TGATGAGCTCAAACMACMCT-3´). The PCR had a total volume of 25 µl and included 5 µL 5x PCR AllTaq Buffer (Qiagen), 0.5 µL dNTP, 1.25 µL of each primer, 0.5 µL AllTaq Polymerase (Qiagen), 6 µl of DNA template and 10.5 µL H2O. PCR conditions were 93 °C for 3 min, 40 cycles of 93 °C for 20 s, 55 °C for 30 s, and 72 °C for 1 min, with a final extension at 72 °C for 5 min. The nuclear ITS1-region of opisthorchiids was amplified with the forward primer (5´-CAAGGTTTCCGTAGGTGA-3´) and reverse primer (5-CTGCGTTCTTCATCGACAC-3´)41. The PCR-reactions included 2.5 µL 5x PCR AllTaq Buffer (Qiagen), 1.25 µL MgCl2, 0.5 µL dNTP, 1.25 µL of each primer, 0.5 µL AllTaq Polymerase (Qiagen), 6 µl of DNA template, and 11.75 µL H2O for a total volume of 25 µl. PCR conditions were 95 °C for 2 min, 40 cycles of 94 °C for 1 min, 58 °C for 45 s, and 72 °C for 45 s, with a final extension at 72 °C for 5 min.
A segment of the rDNA (1065 bp) including the complete ITS1–5.8S–ITS2 region of diplostomids and cyathocotylids was amplified with the primers D1 (5′-AGGAATTCCTGG TAAGTGCAAG-3′) and D2 (5′- CGTTACTGAGGGAATCCTGGT-3′)74. The PCR had a total volume of 25 µl and included 5 µL 5x PCR AllTaq Buffer (Qiagen), 3 µL MgCl2, 1 µL dNTP, 1.25 µL of each primer, 0.5 µL AllTaq Polymerase (Qiagen), 6 µl of DNA template, and 7 µL H2O – PCR grade. PCR conditions were 94 °C for 2 min, 40 cycles of 94 °C for 1 min, 56 °C for 1 min, and 72 °C for 2 min, with a final extension at 72 °C for 5 min.
Sequence generation, alignment, and species assignment
PCR products were visualized using electrophoresis with 1.5% TAE agarose gels stained with ethidium bromide. Amplified PCR-products were sent to Eurofins Genomics (Ebersberg, Germany) for purification and Sanger-sequencing. The electropherograms of the generated sequences were evaluated and their ends trimmed using Finch TV v1.4.0 (Geospiza, Inc.; Seattle, WA, USA). Consensus sequences were constructed using SeaView v. 5 software75.
In order to corroborate preliminary morphological identification, a first assessment with searches of the nucleotide sequences generated using the Basic Alignment Search Tools (BLAST) of the National Center for Biotechnology Information (NCBI) (https://www.ncbi.nlm.nih.gov/) was complemented with phylogenetic analyses, per family and molecular marker, to verify the position of the sequenced individuals in the phylogenetic tree. Alignments of the generated sequences were built separately using MUSCLE76 as implemented in MEGA 11.077 with similar sequences retrieved by BLAST searches or previous phylogenetic studies of the families (Supplementary table S1). ML analyses were performed using PhyML3.078 on the ATGC bioinformatics portal (http://www.atgc-montpellier.fr/). The Bayesian Information Criterion was used to determine the best-fit nucleotide substitution model for each dataset, namely the HKY85 + G + I for the alignment of Opisthorchiidae (cox1), HKY85 + G for Opisthorchiidae (ITS1), GTR + G for Diplostomidae (ITS), and K80 + G for Cyathocotylidae (ITS). Pairwise genetic divergence within taxon was determined using uncorrected distances (p-distance) calculated in MEGA 11.0 with default settings. The program PopArt (Population Analysis with Reticulate Trees) v1.779 was used to construct unrooted statistical parsimony TCS haplotype networks and to estimate the number of segregating sites, the number of parsimony informative sites, nucleotide diversity (Pi), and the Tajima’s D test.
Statistical analyses
The Kruskal-Wallis H-test followed by Dunn’s multiple comparison tests was applied to test for significant differences between localities in the mass of tench and infection intensities of metacercariae. The differences between localities in prevalence of infection were evaluated using pairwise Chi-square tests with Bonferroni correction for multiple comparisons. For all analyses, significance was accepted when P ≤ 0.05. Confidence intervals were calculated according to Newcombe & Soto80. Statistical analyses were performed using R Statistical Software (v 4.4.1)81.
Data availability
Sequences were submitted to GenBank under the accession numbers PV426866-PV426895 and PV482927-PV482968 (Pseudamphistomum truncatum); PV482969-PV482970 (Hysteromorpha triloba), and PV482971-PV482973 (Paracoenogonimus ovatus). All raw sampling and measurement data generated are available from the corresponding author on reasonable request.
References
Waikagul, J. & Thaenkham, U. Approaches To Research on the Systematics of Fish Borne Trematodes1–16 (Academic, 2014).
Chai, J. & Jung, B. Epidemiology of trematode infections: an update. Adv. Exp. Med. Biol. 1154, 359–409 (2019).
Toledo, R., Esteban, J. G. & Fried, B. Current status of food-borne trematode infections. Eur. J. Clin. Microbiol. Infect. Dis. 31, 1705–1718 (2012).
Keiser, J. & Utzinger, J. Food-borne trematodiases. Clin. Microbiol. Rev. 22, 466–483 (2009).
Murell, K. & Pozio, E. The Liver Flukes: Clonorchis sinensis, Opisthorchis spp, and Metorchis spp. in Global Water Pathogen Project (eds. Rose, J. B. & Jiménez-Cisneros) (2017). https://www.waterpathogens.org/book/liver-flukes
IARC. Infection with liver flukes (Opisthorchis viverrini, Opisthorchis felineus and Clonorchis sinensis). IARC Monogr. Eval Carcinog. Risks Hum. 61, 121–175 (1994).
World Health Organization. Control of foodborne trematode infections. World Health Organ. Tech. Rep. Ser. 849, 1–157 (1995).
Pozio, E., Armignacco, O., Ferri, F. & Gomez Morales, M. A. Opisthorchis felineus, an emerging infection in Italy and its implication for the European union. Acta Trop. 126, 54–62 (2013).
Caffara, M., Gustinelli, A., Mazzone, A. & Fioravanti, M. L. Multiplex PCR for simultaneous identification of the most common European opisthorchiid and heterophyid in fish or fish products. Food Waterborne Parasitol. 19, e00081. https://doi.org/10.1016/j.fawpar.2020.e00081 (2020).
Duflot, M., Setbon, T., Midelet, G., Brauge, T. & Gay, M. A review of molecular identification tools for the opisthorchioidea. J. Microbiol. Methods. 187, 1–12 (2021).
Moszczynska, A., Locke, S. A., McLaughlin, J. D., Marcogliese, D. J. & Crease, T. J. Development of primers for the mitochondrial cytochrome c oxidase I gene in digenetic trematodes (Platyhelminthes) illustrates the challenge of barcoding parasitic helminths. Mol. Ecol. Resour. 9, 75–82 (2009).
Selbach, C., Soldánová, M., Feld, C. K., Kostadinova, A. & Sures, B. Hidden parasite diversity in a European freshwater system. Sci. Rep. 10 https://doi.org/10.1038/s41598-020-59548-5 (2020).
Thaenkham, U., Chaisiri, K. & Chan, A. Molecular Systematics of Parasitic Helminths. 396 ppSpringer,. (2022).
Sándor, D. et al. An investigation of the host-specificity of metacercariae of species of Apophallus in freshwater fishes using morphological, experimental and molecular methods. Parasitol. Res. 116, 3065–3076 (2017).
Chan, A. H. E., Kittipong, C., Saralamba, S., Morand, S. & Thaenkham, U. Assessing the suitability of mitochondrial and nuclear DNA genetic markers for molecular systematics and species identification of helminths. Parasites Vectors. 14, 233 (2021).
Dayrat, B. Towards integrative taxonomy. Biol. J. Linn. Soc. 85, 407–415 (2005).
Gibson, G. & Reed, L. K. Cryptic genetic variation. Curr. Biol. 18, R989–R990. https://doi.org/10.1016/j.cub.2008.08.011 (2008).
Nadler, S. Pérez-Ponce de león, G. Integrating molecular and morphological approaches for characterizing parasite cryptic species: implications for parasitology. Parasitology 138, 1688–1709 (2011).
Hering-Hagenbeck, S. & Schuster, R. A focus of opisthorchiidosis in Germany. Appl. Parasitol. 37, 260–265 (1996).
Schuster, R., Wanjek, C. & Hering-Hagenbeck, S. Untersuchung von Karpfenfischen (Cyprinidae) auf Metazerkarien der familie Opisthorchiidae. Mitt Österr Ges Tropenmed Parasitol. 20, 123–130 (1998).
Schuster, R., Bonin, J., Staubach, C. & Heidrich, R. Liver fluke (Opisthorchiidae) findings in red foxes (Vulpes vulpes) in the Eastern part of the federal state brandenburg, Germany - A contribution to the epidemiology of opisthorchiidosis. Parasitol. Res. 85, 142–146 (1999).
Schuster, R., Wanjek, K. & Schein, E. Untersuchungen zum Vorkommen von muskelmetazerkarien Bei Plötzen (Rutilus rutilus) Aus Berliner gewässern. Ein beitrag Zur lebensmittelhygienischen relevanz einheimicher Süßwasserfische. Arch. Lebensm Hyg. 52, 102–104 (2001).
Schuster, R. et al. Seroepidemiological survey on the occurrence of opisthorchiid liver flukes in red foxes (Vulpes vulpes) in berlin, Germany. Parasitol. Res. 90, 400–404 (2003).
Schuster, R., Specht, P. & Rieger, S. On the helminth fauna of the muskrat (Ondatra zibethicus) (Linnaeus, 1766)) in the Barnim district of Brandenburg state/germany. Animals 11, 2444. https://doi.org/10.3390/ani11082444 (2021).
Kocour et al. Performance of different tench, Tinca tinca (L.), groups under semi-intensive pond conditions: it is worth Establishing a coordinated breeding program. Rev. Fish. Biol. Fisheries. 20, 345–355. https://doi.org/10.1007/s11160-009-9140-3 (2010).
Arlinghaus, R., Mehner, T. A. & Management-Orientated Comparative analysis of urban and rural anglers living in a metropolis (Berlin, Germany). Environ. Manage. 33, 331–344. https://doi.org/10.1007/s00267-004-0025-x (2004).
Crotti, D., D’Annibale, M. L. & Crotti, S. Opisthorchiasi autoctona al Lago Trasimeno (Perugia): Descrizione Di due episodi epidemici Da Opisthorchis felineus e problematiche Diagnostiche Differenziali. Microbilologia Med. 22, 36–41 (2007).
Armignacco, O. et al. Human illnesses caused by Opisthorchis felineus flukes, Italy. Emerg. Infect. Dis. 14, 1902–1905 (2008).
Pikalov, E. Fischparasiten in Mecklenburg-Vorpommern und nordeuropäischen Süßgewässern. Dissertation, University of Rostock, 1–411. (2017). https://rosdok.uni-rostock.de/file/rosdok_disshab_0000001816/rosdok_derivate_0000038042/Dissertation_Pikalov_2017.pdf
Sokolov, S., Kalmykov, A., Frolov, E. & Atopkin, D. Taxonomic Myths and phylogenetic realities in the systematics of the Opisthorchiidae (Trematoda). Zoolog. Scr. 51, 232–245 (2022).
Sherrard-Smith, E. et al. Distribution and molecular phylogeny of biliary trematodes (Opisthorchiidae) infecting native Lutra lutra and alien Neovison vison across Europe. Parasitol. Int. 65, 163–170 (2016).
Locke, S. A., Daniel McLaughlin, J. & Marcogliese, D. J. DNA barcodes show cryptic diversity and a potential physiological basis for host specificity among Diplostomoidea (Platyhelminthes: Digenea) parasitizing freshwater fishes in the st. Lawrence river, Canada. Mol. Ecol. 19 (13), 2813–2827. https://doi.org/10.1111/j.1365-294X.2010.04713.x (2010).
Locke, S. A., McLaughlin, J. D., Lapierre, A. R., Johnson, P. T. & Marcogliese, D. J. Linking larvae and adults of Apharyngostrigea cornu, Hysteromorpha triloba, and Alaria mustelae (Diplostomoidea: Digenea) using molecular data. J. Parasitol. 97 (5), 846–851. https://doi.org/10.1645/GE-2775.1 (2011).
Cacciò, S. M., Chalmers, R. M., Dorny, P. & Robertson, L. J. Foodborne parasites: outbreaks and outbreak investigations. A meeting report from the European network for foodborne parasites (Euro-FBP). Food Waterb Parasitol. 10, 1–5. https://doi.org/10.1016/j.fawpar.2018.01.001 (2018).
De Liberato, C. et al. Investigation on Opisthorchis felineus occurrence and life cycle in Italy. Vet. Parasitol. 177, 67–71 (2011).
Besprozvannykh, V., Tatonova, Y. & Shumenko, P. Life cycle, morphology of developmental stages of Metorchis ussuriensis sp. Nov. (Trematoda: Opisthorchiidae), and phylogenetic relationships with other opisthorchiids. J. Zool. Syst. Evol. Res. 57, 24–40 (2019).
Sitko, J. et al. Integrative taxonomy of European parasitic flatworms of the genus Metorchis looss, 1899 (Trematoda: Opisthorchiidae). Parasitol. Int. 65, 258–267 (2016).
Vilas, R., Criscione, C. & Blouin, M. A comparison between mitochondrial DNA and the ribosomal internal transcribed regions in prospecting for cryptic species of platyhelminth parasites. Parasitology 131, 839–846 (2005).
Duan, Y., Al-Jubury, A., Kania, P. & Buchmann, K. Trematode diversity reflecting the community structure of Danish freshwater systems: molecular clues. Parasites Vectors. 14, 43. https://doi.org/10.1186/s13071-020-04536-x (2021).
Kang, S. et al. Molecular identification and phylogenetic analysis of nuclear rDNA sequences among 43 three opisthorchid liver fluke species (Opisthorchiidae: Trematoda). Parasitol. Int. 57, 191–197 (2008).
Xiao, J. et al. Genetic variation among Clonorchis sinensis isolates from different hosts and geographical locations revealed by sequence analysis of mitochondrial and ribosomal DNA regions. Mitochondrial DNA. 24, 559–564 (2013).
Bykhovskaya-Pavlovskaya, I. et al. Key to Parasites of Freshwater Fish of the U.S.S.R. Translated from the Russian (Israel Program for Scientific Translations 1964).
Bauer, O. N. in Key To the Parasites of Freshwater Fish of the U.S.S.R., Volume 3. Parasitic Metazoa (Part 2). 77–198 (eds Russian) (Akademiya Nauka S.S.S.R., 1987).
Skov, J., Kania, P. W., Jørgensen, T. R. & Buchmann, K. Molecular and morphometric study of metacercariae and adults of Pseudamphistomum truncatum (Opisthorchiidae) from Roach (Rutilus rutilus) and wild American Mink (Mustela vison). Vet. Parasitol. 155, 209–216 (2008).
Gaevskaya, A. The World of Human Parasites. I. Trematodes and Trematodes of Food Origin (ECOSI-Hydrophysics, 2015).
Locke, S. et al. Validity of the Diplostomoidea and diplostomida (Digenea, Platyhelminthes) upheld in phylogenomic analysis. Int. J. Parasitol. 48, 1043–1059 (2018).
Niewiadomska, K. Przywry (Trematoda). Część Ogólna; Część systematyczna – Aspidogastrea, Digenea: Strigeida (Wydawnictwo Uniwersytetu Łódzkiego, 2010).
Ciurea, I. Contributions à l’étude morphologique et biologique de Quelques strigeidés des Oiseaux ichtyophages de La faune de Roumanie (recherches expérimentales). Arch. Roum Pathol. Expérimentale Microbiol. 3, 277–323 (1930).
López-Hernández, D. et al. Molecular, morphological and experimental-infection studies of cercariae of five species in the superfamily Diplostomoidea (Trematoda: Digenea) infecting Biomphalaria straminea (Mollusca: Planorbidae) in Brazil. Acta Trop. 199, 105082. https://doi.org/10.1016/j.actatropica.2019.105082 (2019).
Sereno-Uribe, A., López-Jimenez, A., Andrade-Gómez, L. & García-Varela, M. A morphological and molecular study of adults and metacercariae of Hysteromorpha triloba (Rudolpi, 1819), Lutz 1931 (Diplostomidae) from the Neotropical region. J. Helminthol. 93, 91–99 (2019).
Katsurada, F. Studien Über Trematodenlarven Bei süßwasserfischen, Mit besonderer berücksichtung der Elb- und Alsterfische. Zbl Bakt Hyg. 1 Abt Orig A. 73, 304–314 (1914).
Komiya, Y. Die entwicklung des exkretionssystems Einiger Trematodenlarven Aus Alster und elbe, Nebst bemerkungen Über ihren entwicklungszyklus. Z. Parasitenkunde. 10, 625–654 (1938).
Niewiadomska, K. Pasożyty Ryb Polski (klucze Do oznaczania): Przywry - Digenea (Polskie Towarzystwo Parazytologiczne, 2003).
Cech, G. et al. Digenean trematodes in Hungarian freshwater aquacultures. Food Waterb Parasitol. 22, e00101. https://doi.org/10.1016/j.fawpar.2020.e00101 (2020).
Sokolov, S. G., Vlasenkov, S. A., Bugmyrin, S. V., Kalmykov, A. P. & Lebedeva, D. I. Phylogeny and morphology of some European cyathocotylid digeneans (Trematoda: Diplostomoidea). J. Helminthol. 98, e44. https://doi.org/10.1017/S0022149X24000348 (2024).
Voronin, V. et al. Distribution of Paracoenogonimus ovatus katsurada, 1914 in fishes of Russia. Bull. Scand. -Nord Soc. Parasitol. 10, 136 (2000).
Näreaho, A. et al. High prevalence of zoonotic trematodes in Roach (Rutilus rutilus) in the Gulf of Finland. Acta Vet. Scand. 59, 75 (2017).
Hawkins, C., Caffrey, J., Stuart, P. & Lawton, C. Biliary parasite Pseudamphistomum truncatum (Opistorchiidae) in American Mink (Mustela vison) and Eurasian otter (Lutra lutra) in Ireland. Parasitol. Res. 107, 993–997 (2010).
Simakova, A. V. et al. Abundance of Opisthorchis felineus Metacercariae in cyprinid fish in the middle Ob River basin (Tomsk region, Russia). Food Waterb. Parasitol. 22, e00113 10.1016/j.fawpar.2021.e00113 (2021).
Adamczyk, L. B. Nowe i Rzadkie w Faunie Polski Gatunki Pasożytów Ryb [New and rare species of fish parasites in Polish fauna]. Ann. Univ. Mariae Curie-Skłodowska Lub -Pol C. 29, 107–117 (1974).
Linowska, A. & Sobecka, E. Health risks associated with freshwater fish consumption. Eur. J. Nutr. Food Saf. 5, 275–280 (2015).
Ostrowska, K., Wiśniewski, G. & Piasecki, W. Spatial distribution of skin and muscle metacercariae (Digenea) of roach, Rutilus rutilus, and bleak, Alburnus alburnus (Actinopterygii: cypriniformes: Cyprinidae), from an estuary lake in central Europe. Acta Ichthyol. Piscat. 49, 421–429 (2019).
Goncharov, S. & Soroka, N. The occurrence of Paracoenogonimus ovatus (Trematoda, Cyathocotylidae) in fish of natural reservoirs of Mykolaiv region. Vestnik Zoologii. 49, 421–426 (2015).
Pietrock, M., Wolber, J. E. & Schreckenbach, K. Untersuchungen zur Notwendigkeit und zu Möglichkeiten einer wirksamen Entkeimung des Zuflusswassers der Satzfischanlage Sproitz. Abschlussbericht. Fachmaterial Sächsische Landesanstalt für Landwirtschaft. (2006). https://www.landwirtschaft.sachsen.de/download/Abschlussbericht_Zerkarien.pdf
Neimanis, A. S. et al. Emergence of the zoonotic biliary trematode pseudamphistomum truncatum in grey seals (Halichoerus grypus) in the Baltic sea. PLOS ONE. 11, e0164782. https://doi.org/10.1371/journal.pone.0164782 (2016).
Chai, J. & Jung, B. General overview of the current status of human foodborne trematodiasis. Parasitology 149, 1262–1285 (2022).
Sándor, D. et al. Digenean Holostephanus (Trematoda: digenea: Cyathocotylidae) metacercariae in common carp (Cyprinus Carpio linnaeus, 1758) muscle: zoonotic potential and sensitivity to physico-chemical treatments. J. Helminthol. 94, e117. https://doi.org/10.1017/s0022149x1900110x (2020).
Enabulele, E. E., Awharitoma, A. O., Lawton, S. P. & Kirk, R. S. First molecular identification of an agent of diplostomiasis, Diplostomum Pseudospathaceum (Niewiadomska 1984) in the united Kingdom and its genetic relationship with populations in Europe. Acta Parasitol. 63 (3), 444–453. https://doi.org/10.1515/ap-2018-005 (2018).
Melling, N., Hohenberger, W. & Yedibela, S. Opisthorchiasis mimicking primary biliary cirrhosis as an indication for liver transplantation. J. Hepatol. 50 (5), 1057–1059. https://doi.org/10.1016/j.jhep.2008.11.029 (2009).
FIZ. Fischwirtschaft – Daten und Fakten 20224 Fisch-Informationszentrum e.V. Hamburg (FIZ). (2024). Available at: https://www.aquakulturinfo.de/sites/default/files/media-files/download-files/FIZ_DF_2024.pdf (accessed on 17.03.2025).
Papalini, C. et al. A new human opisthorchiasis outbreak in central italy: a never-ending story. Infection 53, 175–181. https://doi.org/10.1007/s15010-024-02340-8 (2025).
EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards). Re-evaluation of certain aspects of the EFSA scientific opinion of April 2010 on risk assessment of parasites in fishery products, based on new scientific data. Part 1: ToRs1–3. EFSA J. 22, e8719. https://doi.org/10.2903/j.efsa.2024.8719 (2024).
Menconi, V. et al. The occurrence of freshwater Fish-Borne zoonotic helminths in Italy and neighbouring countries: A systematic review. Animals 13, 3793. https://doi.org/10.3390/ani13243793 (2023).
Galazzo, D., Dayanandan, S., Marcogliese, D. & McLaughlin, J. Molecular systematics of some North American species of Diplostomum (Digenea) based on rDNA-sequence data and comparisons with European congeners. Can. J. Zool. 80, 2207–2217 (2002).
Gouy, M., Tannier, E., Comte, N. & Parsons, D. P. Seaview version 5: A multiplatform software for multiple sequence alignment, molecular phylogenetic analyses, and tree reconciliation in Multiple sequence alignment. Methods Mol. Biology. 2231, 241–260 (2021).
Edgar, R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797 (2004).
Tamura, K., Stecher, G. & Kumar, S. MEGA11: molecular evolutionary genetics analysis 11. Mol. Biol. Evol. 38, 3022–3027 (2021).
Guindon, S. et al. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 59 (3), 307–321. https://doi.org/10.1093/sysbio/syq010 (2010).
Leigh, J. W. & Bryant, D. Popart: full-feature software for haplotype network construction. Methods Ecol. Evol. 6, 1110–1116 (2015).
Newcombe, R. & Soto, C. Intervalos de confianza Para Las estimaciones de proporciones y Las diferencias entre Ellas. Interdisciplinaria 23, 141–154 (2006).
R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. (2021). https://www.R-project.org/
Acknowledgements
We are grateful to Wibke Kleiner and Viola Schöning for their valuable assistance in the laboratory and Mathias Kunow for his help in collecting the fishes.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
K.K. and M.S. conceived and supervised the study. C.M.-M. conducted the sampling, laboratory work and data analyses and drafted the manuscript. All authors reviewed and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
Fish were sacrificed by severing the spinal cord behind the head after sedation by percussive blow in the head with approval of the institute’s animal welfare officer. According to the German Animal Welfare Act (Tierschutzgesetz, article 7(2) and article 8), ethics approval by an institutional and/or authorising committee is not necessary if the killing of an animal is carried out solely for the purpose of using the animal’s organ or tissues for scientific purposes without prior experimental treatment. For the killing of the fish, the relevant regulations, i.e. the Directive on the Protection of Animals Used for Experimental or Other Scientific Purposes ('Verordnung zum Schutz von zu Versuchszwecken oder zu anderen wissenschaftlichen Zwecken verwendeten Tiere’ - Tierschutz-Versuchstierverordnung, Annex 2) and the superordinate Directive 2010/63/EU (Annex 2) were followed in full.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mata-Marcano, C., Stöck, M. & Knopf, K. Morphological and molecular assessment of muscle metacercariae infecting tench Tinca tinca from fish farms and wild populations in Germany. Sci Rep 15, 23700 (2025). https://doi.org/10.1038/s41598-025-09396-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-09396-y