Abstract
The Early Aptian Oceanic Anoxic Event 1a (OAE 1a) marks a period of global marine anoxia linked to volcanic activity and climate changes during the Cretaceous. This study analyzes a ~ 40-m-thick Lower Aptian succession from the Kazhdumi Intrashelf Basin, Iran, using I/(Ca + Mg) ratios, Ce anomalies, trace elements, δ¹³C, and REY parameters. Results reveal persistent suboxic to anoxic bottom-water conditions, with increased oxygenation in segments C3 and upper C5 + C6, driven by weathering and sea-level changes. Minimal diagenetic overprinting is indicated by C-isotope trends and low Mn/Sr ratios. The Kazhdumi Basin serves as a high-resolution archive of redox evolution during OAE 1a, emphasizing the utility of I/(Ca + Mg) and Ce anomalies as proxies in ancient carbonates.
Similar content being viewed by others
Introduction
The Cretaceous period witnessed multiple episodes of intense volcanic activity, which are hypothesized to have acted as catalysts for global climate warming, shifts in ocean circulation patterns, and increased marine productivity. These oceanic anoxic events (OAEs)1,2 led to the widespread marine anoxia and the formation of organic-rich, black mudstone and shale deposits (total organic carbon (TOC) > 0.5 wt%). The Early Aptian OAE 1a is a notable example characterized by a significant increase in δ13C due to extensive accumulation of organic sediments in marine settings3,4. At the onset of the OAE 1a, an abrupt negative excursion in the δ13C curve (C3 segment sensu3) is commonly interpreted as a sign of isotopically light carbon entering the ocean-atmosphere system from marine volcanic emissions and the release of methane gas hydrates4,5. This negative spike is followed by a prolonged positive excursion in both organic and inorganic δ13C, which is recorded globally accross marine and terrestrial systems3,6.
During the OAE 1a, the Tethyan realm experienced various paleoenvironmental changes, including a warm climate interspersed with cooler periods7,8,9,10,11, enhanced chemical weathering6,12, carbonate platform drowning (northern Tethys13,14), the occurrence of Bacinella encrusters (central and southern Tethys15,16,17), and the development of restricted epicontinental seas (western Tethys18). These factors collectively contributed to to the development of OAE 1a, one of the most pronounced episodes of marine anoxia during the Cretaceous period19.
While the sedimentary, δ13C, and biogeochemical record of the OAE 1a is well documented globally, the connections between redox conditions and organic carbon burial remain poorly constrained20. Based on the analogy with modern oxygen-depleted marine environments, the redox-sensitive trace elements (RSTE) and rare earth elements plus yttrium (REY) are utilized as a proxy for the redox status of ambient waters21,22,23. They are commonly associated with organic matter, incorporated into sulfides, or bioconcentrated by planktonic fauna24,25,26,27,28. Since no single proxy can inarguably indicate a redox change and/or development of anoxia, it becomes imperative to adopt multiproxy strategies to unravel the paleoenvironmental evolution of OAE 1a. Furthermore, the majority of commonly utilized redox proxies are connected to redox conditions in bottom water and porewater.
To explore the climate sensitivity of ocean oxygen levels, both currently and across geological periods, a geochemical method to measure water oxygen content is essential. Iodine has emerged as a promising candidate due to its similarity to O2/H2O redox potential and its changing forms (iodide and iodate) in response to oxygen variations29. Iodate (IO3−) and iodide (I−) are the two thermodynamically favored species of inorganic iodine in seawater30,31. In the presence of oxygen, iodine is predominantly present as IO3−, and its conversion to I− occurs progressively with the reduction of dissolved oxygen29,32,33. Empirical investigations demonstrate the exclusive integration of IO3− into the crystalline structure of carbonate minerals, whereas I− is excluded32,34. The I/Ca ratio observed in contemporary planktonic foraminifera, approximately 5 µmol/mol, signifies adequately oxygenated water, while in oxygen-depleted regions, the I/Ca ratio significantly diminishes to 0.5 to 2.5 µmol/mol35,36. Consequently, the ratio of iodine to the combined concentrations of Ca and Mg in carbonates, denoted as I/(Ca + Mg) or I/Ca, serves as an indicator for assessing IO3− content in seawater, and thus dissolved oxygen concentration, during carbonate mineral formation. Elevated I/(Ca + Mg) values in carbonate rock samples correlate with higher oxygen concentrations in seawater32. However, this ratio can be diminished by diagenetic alterations, making I/(Ca + Mg) ratios in carbonate rocks conservative indicators, providing a lower limit estimation of IO3− presence (and free O2) during deposition37,38,39,40,41.
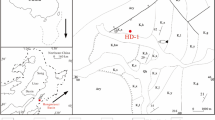
The Lower Dariyan Formation of the Kazhdumi Intrashelf Basin, Zagros Mountains, Iran offers a well-preserved archive of paleoenvironmental conditions during the Early Aptian OAE 1a along the NE margin of the Arabian Plate42. Throughout the Early Aptian, the basin, with maximum water depths of up to 135 m43, experienced continuous deposition of shallow-marine to hemipelagic facies, likely minimally impacted by meteoric diagenesis42,44 (Supplementary Information). The basin maintained a direct connection to the open ocean via a relatively narrow northern seaway43,45 (Fig. 1). Consequently, the Kazhdumi Basin is hypothesized to serve as a repository for a high-resolution record of regional paleoenvironmental changes, along with insights into climatic conditions42,44. This study investigates the environmental and diagenetic controls on I/(Ca + Mg) ratios in carbonate samples from the Lower Aptian Kazhdumi Basin. By integrating this proxy with other geochemical indicators (δ¹³C, Ce/Ce*, Mn/Ca, Fe/Ca), we aim to reconstruct paleoredox conditions during the OAE 1a. We hypothesis that, although diagenetic overprinting introduces variability, the I/(Ca + Mg) ratios—when interpreted alongside redox-sensitive trace elements and cerium anomalies—primarily record shifts in seawater oxygenation. These trends reveal a complex interplay among basin restriction, organic matter burial, and continental weathering as key drivers of paleoenvironmental change during the OAE 1a.
Results and discussion
Environmental and diagenetic controls on iodine-to-calcium-magnesium ratios
The measured I/(Ca + Mg) ratios range from 0.005 to 1.30 µmol/mol (mean value 0.318 µmol/mol; Table 1). Most samples (> 80%) exhibit I/(Ca + Mg) values below 0.5 µmol/mol, whereas a small subset shows ratios above 0.6 µmol/mol. A positive correlation exists between I/(Ca + Mg) ratios and the manganese and iron contents (Figs. 2 and 3). The published δ13C values of the samples range from − 0.8 to 2.89‰ VPDB, with an average of 1.73‰ VPDB42,44. The negative δ13C values are not in phase with positive shifts in I/(Ca + Mg), nor do they correlate with Mn and Fe enrichment (Fig. 2). Mg/Ca and Mn/Sr values are mainly in the range of 0.01 to ~ 0.16 (mean 0.03 mol/mol), and ~ 0.02 to 1.5 (mean 0.16 ppm/ppm), respectively (Table 1). There is no correlation between I/(Ca + Mg) ratios and Mg/Ca, Mn/Sr, δ18O, Al, CaCO3, and TOC contents (Fig. 3).
Lithologic log of the Lower Aptian succession, Kazhdumi Intrashelf Basin showing microfacies and depositional environments, depositional sequences42, C-isotopic segments (after3), carbon and oxygen isotopes42, TOC, I/(Ca + Mg) ratios, Fe/Ca and Mn/Ca (mmol/mol) values, and the evolution of key REY (rare earth plus yttrium) parameters. Red dashed line at 0.5 µmol/mol is considered the foundational baseline for I/(Ca + Mg) ratios in marine Proterozoic carbonates, based on numerous data points obtained from these carbonates38,39,48. MDST = lime mudstone, WKST = wackestone, PKST = packstone, FLST = floatstone, RDST = rudstone.
Cross-plots of geochemical results from the studied succession. (A) δ13C vs. δ18O, (B) δ13C vs. microfacies, (C) I/(Ca + Mg) vs. Mg/Ca, (D) I/(Ca + Mg) vs. δ18O, (E) I/(Ca + Mg) vs. Mn/Sr, (F) I/(Ca + Mg) vs. Al, (G) I/(Ca + Mg) vs. CaCO3, (H) I/(Ca + Mg) vs. TOC, (I)/(Ca + Mg) vs. I, and (J) Mn/Ca vs. Fe/Ca.
The pre-OAE 1a interval (upper C2 segment) exhibits a stepwise increasing trend of rather low I/(Ca + Mg) ratios that correspond to relatively high Ce/Ce* values (> 0.9), which suggests that the basinal waters were depleted in oxygen even before the onset of the global event. The latter is evident in the basin by a major negative δ13Ccarb excursion, and a decrease in I/(Ca + Mg) and Ce/Ce* ratios (segment C3, Fig. 2). Relatively uniform I/(Ca + Mg) ratios (mean 0.23 µmol/mol) characterize segments C4 and lower part of C5 + C6, suggestive of low oxygenation levels. A prominent stepwise increase in I/(Ca + Mg) ratios (from ~ 0.2 to ~ 1.3 µmol/mol) in the upper C5 + C6 segment is compatible with an increase in dissolved oxygen, which also matches an increase in Mn/Ca and Fe/Ca, and less pronounced increase in Ce/Ca ratios. The increase of these elements in basinal waters likely reflects an increase in continental weathering associated with relative sea-level fall at that stage (regressive systems tract of depositional sequence 2 sensu42). That continental weathering likely played an important role in increasing the amount of trace elements in basinal waters is also suggested by the relatively higher Mn/Ca, Fe/Ca, and Ce/Ca values in the upper C2 and C3 segments both of which form the regressive systems tract of the depositional sequence 1.
The range of I/(Ca + Mg) ratios from the Lower Aptian Kazhdumi Basin is similar to those recorded from the Proterozoic successions, albeit lower than the values from numerous Phanerozoic successions46, as well as from the modern well-oxygenated environments showing > 2.6 µmol/mol35,36. The values < 2.5 µmol/mol indicate the water-column iodate concentration of < 250 nM, which is commonly observed in marine environments, specifically within the oxycline situated directly above anoxic waters29,36. As a semi-quantitative guideline, an I/(Ca + Mg) value exceeding 0 µmol/mol may suggest the accumulation of iodate (IO3−) within seawater and an O2 level greater than 1–3 µM38,47. Conversely, the I/(Ca + Mg) value > 0.5 µmol/mol, representing a value higher than the baseline observed in the Precambrian time, signifies relatively well-oxygenated conditions39,48. But when the I/(Ca + Mg) ratio exceeds 2.6 µmol/mol, it indicates the absence of aquatic environments with O2 levels below 20–70 µM in contemporary shallow seawater36,38. Except for a small number of samples showing values near zero (Table 1), all the analyzed samples exhibit I/(Ca + Mg) ratios > 0 µmol/mol, suggesting porewater O2 conditions of at least 1–3 µM. Thus, the low but non-zero (mostly < 0.5 µmol/mol) I/(Ca + Mg) ratios of the studied samples are compatible with modern and ancient carbonates from suboxic and anoxic depositional environments.
The lack of field evidence for pervasive meteoric cementation, increased leaching, and/or dolomitization is supported by the δ13C values which do not suggest any pronounced impact of diagenesis in either the shallow or deeper-water facies of the Lower Dariyan Formation42 (Fig. 3A). Furthermore, there is no systematic variation of δ13C values (against microfacies), which mainly span from 0.6‰ to 2.80‰ VPDB (Table 1; Fig. 3B). This may point to the independency of δ13C values from the sedimentary (environmental) control. The weak correlation between Mg/Ca and I/(Ca + Mg) ratios (Fig. 3C) is compatible with the negligible impact of dolomitization on the I/(Ca + Mg) ratios. During the progression of meteoric diagenesis, a reduction in Sr and δ18O contents is typical, accompanied by concurrent increases in Mn and Fe concentrations49,50. The low δ18O values (Fig. 2) suggest that the original values underwent diagenetic alteration due to dissolution and the precipitation of micro-scale cement within a context of elevated temperatures in the deep burial environment42,50. The δ18O values generally vary between − 7.11‰ and − 4.44‰ VPDB and exhibit no distinct correlation with I/(Ca + Mg) ratios (Fig. 3D). This may suggest that, while a degree of (meteoric) diagenetic alteration is likely, the I/(Ca + Mg) values have not undergone substantial modification and consequently, these values can still serve as indicators of shifts in contemporary seawater redox conditions. The impact of meteoric diagenesis on I/(Ca + Mg) ratios becomes most evident when plotting I/(Ca + Mg) against Mn/Sr ratios (Fig. 3E). The majority of samples derived from the Lower Dariyan Formation exhibit indications of constrained meteoric diagenetic modification, characterized by Mn/Sr ratios < 1.5 (mean 0.16). The instances with the highest I/(Ca + Mg) ratios are observed in samples where Mn/Sr is < 1. Given the typical iodine concentration disparity between freshwater and seawater, where freshwater exhibits concentrations about one order of magnitude lower than seawater51,52, the admixture of freshwater and seawater would result in a reduction of iodine concentration53. This pattern implies that subsequent alterations likely contributed to variable decreases in I/(Ca + Mg) ratios. On a contrasting note, it is important to highlight that samples featuring notably high Mn/Sr content do not consistently exhibit correspondingly elevated I/(Ca + Mg) ratios (Table 1). The I/(Ca + Mg) values, albeit low, remain nonzero and are mainly distributed within the range of ≤ 0.5 µmol/mol (Table 1; Fig. 2; mainly from C4 to middle C5 + C6 segments sensu3), and suggest that specific instances of low I/(Ca + Mg) ratios might indeed represent original seawater signatures. In a broader context, our collected data support the fact that diagenetic processes may have fostered increased variability in I/(Ca + Mg) ratios, generally leading to lower values in some samples. However, the diagenetic alterations do not adequately explain occurrences of high I/(Ca + Mg) ratios38,39,40,53.
Finally, the presence of clay minerals and organic content is not likely to be a significant contributor to iodine in the studied carbonate samples. Our experimental approach involving the use of diluted acid (3% nitric acid32) helped to mitigate the liberation of iodine from organic matter and consequently, the I/(Ca + Mg) ratios of the samples are anticipated to be minimally influenced by potential organic matter contamination. This conclusion is supported by the weak correlations observed between I/(Ca + Mg) and aluminum content (R2 = 0.006; Fig. 3F), as well as between I/(Ca + Mg) and CaCO3 (R2 = 0.06; Fig. 3G) and TOC (R2 = 0.03; Fig. 3H). In contrast, a strong correlation exists between I/(Ca + Mg) and I content (R2 = 0.86; Fig. 3I), indicating that these values primarily capture the original seawater characteristics and/or the I/(Ca + Mg) ratio is regulated by the iodine content within the carbonates, rather than being dictated by the carbonate and TOC contents of the specimens39,54.
Cerium anomalies as possible redox proxies
Cerium (Ce) is distinguished from other rare earth elements (REEs) by its ability to adopt a tetravalent [Ce(IV)] state under oxidizing conditions, whereas other REEs remain trivalent55. This redox sensitivity allows Ce anomalies (Ce/Ce*) to serve as powerful paleoredox proxies. In modern oxygenated seawater, Ce3+ undergoes oxidation to Ce+4, mediated by manganese oxides and/or bacteria56. The insoluble Ce+4 is subsequently scavenged by Fe-Mn nodules and crusts, particularly in the deep ocean57. This process depletes seawater in cerium, creating a negative Ce anomaly (Ce/Ce* < 1), while concurrently enriching Fe-Mn oxides, resulting in a positive Ce anomaly. In anoxic waters, Ce remains primarily as soluble Ce³⁺, resulting in minimal fractionation, and potentially leading to weak or even positive Ce anomalies in associated sediments, especially when authigenic minerals preserve the seawater signal58,59. Consequently, lower Ce/Ce* values in our carbonates likely record incorporation of Ce-depleted seawater under oxic to suboxic conditions. Higher values (> 1.0), however, may signify either Ce retention under anoxic water column conditions or diagenetic modification within reducing porewaters. Overall, our interpretation of redox conditions relies on relative changes in Ce/Ce* values within the stratigraphic context, rather than absolute thresholds alone.
The basal part of the section (segment C2) shows uniform Ce/Ce* values (~ 0.85), followed by a stepwise decreasing trend into lower C4 where it reaches 0.67 (Fig. 2). This suggests an increase in seawater dissolved oxygen content before and at the onset of the OAE 1a, which is recorded in a predominantly carbonate interval with abundant benthic biota (ostreids, orbitolinids), extensive bioturbation, low productivity-sensitive trace elements (PSTE) and RSTE44. The Ce/Ce* values are highly variable in the middle and upper C4 and lower C5 + C6, reaching a maximum value of 1.17, i.e., the peak value of anoxia, in the upper Radiolarian Flood Zone (RFZ) (Fig. 2). The latter interval, represented by organic-rich deep open-marine and intrashelf basin pelagic dark gray shales and chert bands (TOC 4.5–9.0%60), is the intrashelf basin analog to the marine black shales. In the shallower Bab Basin and surrounding carbonate platform areas of the Arabian Peninsula, the coeval deposits record a significant expansion of the microencruster Bacinella that continued through C615,61. The remaining C5 + C6 segment overlying the RFZ exhibits a stepwise increase in Ce/Ce* values, suggesting a decrease in seawater-dissolved oxygen content up section. Coupled with relatively high δ13C values, lithology (dark to light gray deeper-water limestone and marl; Supplementary Information) with variable TOC, RSTE, and PSTE44, this is compatible with recurrent suboxic-oxic water conditions. Overall, the minimum Ce/Ce* value of the studied samples is 0.56, which is significantly higher than the modern-day Ce anomaly of 0.4 observed in the oxygenated upper ocean55 and close to the arbitrary cut-off value of 0.6 for poorly oxygenated Hauterivian-Aptian upper oceanic seawater62. Given the global distribution of Lower Aptian organic-rich shales2, the cerium anomaly observed in hemipelagic sediments of the Vocontian Trough62 likely reflects redox changes in water masses at a global scale. Although Ce has a short residence time in modern oceans (< 200 years63) and Ce anomalies are typically interpreted as indicators of local to regional redox conditions64, the anomaly recorded in Lower Aptian sediments of the Kazhdumi Basin was likely shaped by both regional and global deoxygenation during OAE 1a. The vast majority (97%) of the studied samples exhibit a Ce anomaly > 0.6 (mean: 0.84), indicating low oxygenation of the Aptian Kazhdumi Basin seawater (Fig. 2).
The Ce/Ce* vs. Pr/Pr* cross-plots are a useful tool when assessing whether Ce anomalies are genuine or were created artificially under the potential influence of lanthanum65,66,67 (Fig. 4). In the current study, only two samples, both from segment C5 + C6, exhibit positive Ce anomalies indicative of a genuine Ce enrichment (field IIIa in Fig. 4). Conversely, the majority of the samples exhibit negative Ce anomalies due to genuine Ce depletion (field IIIb in Fig. 4), which is similar to the coeval dataset from the Vocontian Trough of SE France62. Overall, the predominance of negative Ce anomalies suggests that the environmental conditions during the deposition were more conducive to Ce depletion rather than enrichment, assuming that the REY values incorporated into carbonate preserved an authigenic seawater signal59.
Lanthanum anomaly diagram (Ce/Ce* vs., Pr/Pr*) of the Lower Aptian succession, Kazhdumi Intrashelf Basin. La anomaly fields I-III after65.
The alternating paleoredox conditions during the deposition of the Lower Dariyan Formation are also evident from the overall REY enrichment when compared to the modern oxic seawater68,69 and the overall positive Ce anomalies. In addition, the consistent and long-term decrease of the Ce/Ca ratio from an average value of 6.3 (before C3) to 3.5 µmol/mol (C3 and beyond) further suggests the shifting from the oxic-suboxic condition before OAE 1a into a more anoxic condition during OAE 1a, as indicated by other works on this proxy32.
The Y anomaly (Fig. 2) ranges from ~ 35 to 73.3 (mean 48.4), which suggests that the samples preserved an unaltered seawater REY signal, which is associated with Y/Ho > 3669. The Y anomaly is consistently low before and at the onset of the OAE 1a, and shows poor correlation with Ce anomaly, similar to modern seawater70. The Eu anomaly exhibits a broad range of values (Fig. 2), fluctuating between ~ 0.9 and 1.69 (mean 1.2), with high values (> 1.3) occurring in segments C4 and C5 + C6. These positive Eu anomalies possibly indicate periodically reducing conditions where Eu2+ was more soluble and mobile71. Shale-normalized Nd/Yb are consistently around 1, except for the three outliers in segment C3 that show values from 2 to > 3 (Fig. 2). This, along with a similar trend observed with shale-normalized Sm/Yb values, is suggestive of a significant periodic enrichment of LREEs compared to HREEs likely associated with scavenging of HREEs by organic matter72.
Redox sensitive elements
The Fe/Ca values range between 0.08 and 3.24 mmol/mol (mean 0.08 mmol/mol), while the Mn/Ca values vary from 0.02 to 0.4 mmol/mol (mean 0.02 mmol/mol), with both elements showing a notable positive correlation (R2 = 0.5; Fig. 3J). This correlation highlights their shared response to changes in redox states, as both manganese and iron are redox-sensitive elements.
Due to the redox-sensitive nature of both Mn and Fe elements, their covariation signifies a congruent reaction to changes in redox states73,74. In the initial stages of marine diagenesis, using oxygen and nitrate during the bacterial remineralization of organic matter coincides with the reduction of manganese. In dysoxic to anoxic environments, manganese depletion in sediments is attributed to the reductive dissolution of manganese oxyhydroxides, resulting in the mobilization of soluble manganese (Mn2+)75. Conversely, in oxygen-rich conditions, manganese predominantly forms less soluble compounds, adopting trivalent or tetravalent forms in the solid phase such as oxyhydroxides or oxides73. In extensively stratified redox settings, manganese is selectively deposited as Mn-oxyhydroxides in sediments located at or above the redoxcline. However, beneath this boundary, in sediments positioned lower, the element will experience reductive dissolution75,76. Consequently, fluctuations in Mn concentrations can transpire independently of sedimentary facies, sequence surfaces, and systems tracts, reflecting alterations in redox conditions. Hence, the enrichment of manganese within sediments serves as a potentially robust indicator for assessing paleoredox conditions77,78.
The relatively low Mn/Ca ratios in the studied succession suggest the absence of primary manganese-bearing minerals or the diffusion of soluble Mn²⁺ into the overlying water column28,79. The negative correlation between δ13C and Mn content (Fig. 2) supports the presence of authentic marine signatures, consistent with precipitation from primary marine fluids80. The simultaneous increase in Fe and Mn values, along with heightened Mn/Fe ratios, indicates precipitation within a reducing environment during burial diagenesis81,82 (Fig. 3J). This positive relationship also underscores a common source for Fe and Mn, reinforcing the idea of a shared origin for these elements.
Summary of redox evolution
In segment C2, geochemical signals indicate an initial phase of ocean deoxygenation (Table 2). The δ¹³C values begin to slightly increase, while I/(Ca + Mg) ratios remain low but show a stepwise increase, and Ce anomalies are relatively high (> 0.9). Periodically high Mo values suggest short periods of Mo removal into sediments under reducing conditions42. These trends are consistent with a transition from oxic to suboxic, likely driven by enhanced continental weathering and nutrient delivery to the basin. This phase sets the stage for the onset of Oceanic Anoxic Event 1a (OAE 1a).
A sharp negative δ¹³C excursion defining segment C3 marks a major perturbation in the global carbon cycle, associated with large-scale volcanic CO₂ input and the release of methane gas hydrates4,5 (Table 2). During this interval, both I/(Ca + Mg) and Ce/Ce* ratios decline, indicating deterioration in preceding periodic suboxic conditions and return to well-oxigenated setting.
In segment C4 and the lower part of C5 + C6, δ¹³C values are characterized by a major positive excursion followed by relatively constant but high values, signifying increased burial of organic matter (Table 2). The low but non-zero (mostly < 0.5 µmol/mol) I/(Ca + Mg) ratios and periodically high Ce/Ce* ratios, coupled with high RSTE, PSTE, and TOC42 indicate suboxic to anoxic conditions, with little evidence for recovery.
Finally, in the upper portion of C5 + C6, the geochemical trends shift markedly (Table 2). δ¹³C values display little vriability, whereas I/(Ca + Mg) increases significantly—from around 0.2 to over 1.3 µmol/mol—in a stepwise manner and Ce/Ce* shows a slight rise. These changes suggest improving oxygenation, likely transitioning from anoxic or suboxic toward more dysoxic conditions. This redox recovery may reflect a combination of sea-level fall and enhanced weathering, promoting greater ventilation of the basin waters.
Methods
A 39.5-m-thick continuous hillside section of the Lower Dariyan Formation from the Kazhdumi Intrashelf Basin margin was studied near the village of Dareh Sefid, ~ 65 km north of Shiraz City, Fars Province. The major lithofacies types are summarized in Supplementary Information. A total of 62 bulk rock samples were collected every 0.6 to 1.3 m along the section (Fig. 2) and prepared for inductively coupled plasma mass spectrometry (ICP-MS) and inductively coupled optical emission spectrometry (ICP-OES). We also utilized recently published stable carbon and oxygen isotope data from this Sect42.
For iodine analysis (n = 62), ∼24 mg of MQ water-rinsed dry powders were utilized. 4 mL nitric acid (3%) was added for dissolution and then centrifuged to obtain supernatant. To stabilize iodine, 3% tertiary amine solution was added to the supernatant, and then diluted to 0.1% with MQ water. The iodine content was measured within 48 h to avoid any iodine loss, using an ICP-MS (Thermo iCAPTM TQ) at the National Research Center of Geoanalysis, Beijing. The sensitivity of iodine was tuned to ~ 50 kcps for a 1 ppb standard. Analytical uncertainties for iodine were monitored by a reference material JDo-1 (dolostone) and the detection limit of iodine is typically below 0.1 µmol/mol. For calcium (Ca), magnesium (Mg), manganese (Mn), and strontium (Sr) concentrations, supernatant was used and then diluted to 1:100.000 with 3% HNO3, were calibrated using JDo-1 and measured via ICP-MS (Thermo iCAPTM TQ) (Table 1). Rare earth elements and yttrium (REY) were analyzed by an Agilent 7700e inductively coupled plasma mass spectrometry (ICP-MS) on 55 samples at ALSGLOBAL Laboratory, China. Reproducibility was better than 5% for all REYs. Following the previous studies54,83, the powders of each (marl/shale) sample were rinsed with deionized water for two times to remove clay minerals and soluble materials, and then separated into aliquots for analysis. Their concentrations were standardized against the Post Archean Australian Shale (PAAS)84 (Table S1) to provide a comparative baseline. Ce anomalies were derived using the formula Ce/Ce* = [Ce/(Pr2/Nd)]SN85, while Pr anomalies were calculated as Pr/Pr* = [Pr/(0.5 Ce + 0.5 Nd)]SN86. Eu anomalies were determined by Eu/Eu* = [Eu/(0.67Sm + Nd)]SN65. The enrichment of middle rare earth elements (MREE) and light rare earth elements (LREE) was assessed by evaluating the ratios of (Sm/Yb)SN and (Nd/Yb)SN, respectively. Elemental analysis (no = 62; Mn/Ca and Fe/Ca) was carried out by inductively coupled optical emission spectrometry (ICP-OES; Table 1) using an Agilent 5110 VDV at University of Exeter, United Kingdom, following the methodology outlined in detail in Ullmann87. Bulk powders were weighed to 1 µg precision and 320 to 770 µg transferred into a 15 mL centrifuge tube. Samples were then digested in 2% v/v HNO3 with a target of achieving 25 µg/g nominal Ca concentration and dilution factors gravimetrically controlled. Ca concentrations and element/Ca ratios were calibrated against a set of a blank solution and three matrix-matched multi-element standards prepared from certified single-element plasma standards. Internal consistency of the measurements was checked with a quality control solution made of the same single-element plasma standards and the bias of the measurements was 1% or less for all element/Ca ratios. Repeat analyses of a stock solution of JLs-1 (n = 24; 2 s.d.) provided further reproducibility control yielding Mn/Ca of 0.032 ± 0.001 mmol/mol, and Fe/Ca of 0.17 ± 0.01 mmol/mol.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Schlanger, S. O. & Jenkyns, H. C. Cretaceous oceanic anoxic events: causes and consequences. Geol. Mijnbouw. 55, 179–184 (1976).
Arthur, M. A., Jenkyns, H. C., Brumsack, H. J. & Schlanger, S. O. Stratigraphy, geochemistry, and paleoceanography of organic carbon-rich Cretaceous sequences. In Cretaceous Resources, Events and Rhythms: Background and Plans for Research (eds Ginsburg, R. N. & Beaudoin, B.) 75–119 (Kluwer, 1990).
Menegatti, A. P. et al. High-resolution δ13C stratigraphy through the early aptian Livello selli of the alpine Tethys. Paleoceanography 13, 530–545 (1998).
Méhay, S. et al. A volcanic CO2 pulse triggered the cretaceous oceanic anoxic event 1a and a biocalcification crisis. Geology 37, 819–822 (2009).
Jahren, A. H., Arens, N. C., Sarmiento, G., Guerrero, J. & Amundson, R. Terrestrial record of methane hydrate dissociation in the early cretaceous. Geology 29, 159–162 (2001).
van Breugel, Y. et al. Synchronous negative carbon isotope shifts in marine and terrestrial biomarkers at the onset of the early aptian oceanic anoxic event 1a: evidence for the release of 13C-depleted carbon into the atmosphere. Paleoceanography 22, PA1210 (2007).
Price, G. D. The evidence and implications of Polar ice during the mesozoic. Earth Sci Rev. 48, 183–210 (1999).
Matthews, R. K. & Frohlich, C. Maximum flooding surface and sequence boundaries: comparisons between observation and orbital forcing in the cretaceous and jurassic (65–190 Ma). GeoArabia 7, 503–538 (2002).
Husinec, A. & Jelaska, V. Relative sea-level changes recorded on an isolated carbonate platform: tithonian to cenomanian succession, Southern Croatia. J Sediment Res. 76, 1120–1136 (2006).
Husinec, A. et al. Sequence development influenced by intermittent cooling events in the cretaceous aptian greenhouse, Adriatic platform, Croatia. AAPG Bull. 96, 2215–2244 (2012).
Bover-Arnal, T., Salas, R., Guimera, J. & Moreno-Bedmar, J. A. Eustasy in the aptian world: a vision from the Eastern margin of the Iberian plate. Global Planet Change. 214, 103849 (2022).
Weissert, H. Siliciclastics in the early cretaceous Tethys and North Atlantic oceans: documents of periodic greenhouse climate conditions. Mem Soc Geol Ital. 44, 59–69 (1990).
Weissert, H., Lini, A., Föllmi, K. B. & Kuhn, O. Correlation of early cretaceous carbon isotope stratigraphy and platform drowning events: a possible link? Palaeogeogr. Palaeoclimatol Palaeoecol. 137, 189–203 (1998).
Föllmi, K. B., Godet, A., Bodin, S. & Linder, P. Interactions between environmental change and shallow-water carbonate build-up along the Northern Tethyan margin and their impact on the early cretaceous carbon-isotope record. Paleoceanography 21, PA4211 (2006).
Immenhauser, A., Hillgärtner, H. & van Bentum, E. Microbial–foraminiferal episodes in the early aptian of the Southern Tethyan margin: ecological significance and possible relation to oceanic anoxic event 1a. Sedimentology 52, 77–99 (2005).
Huck, S. et al. Latitudinally different responses of Tethyan shoal-water carbonate systems to the early aptian oceanic anoxic event (OAE 1a). Sedimentology 57, 1585–1614 (2010).
Rameil, N., Immenhauser, A., Warrlich, G., Hillgärtner, H. & Droste, H. J. Morphological patterns of aptian Lithocodium-Bacinella geobodies: relation to environment and scale. Sedimentology 57, 883–911 (2010).
Bralower, T. J. et al. Timing and paleoceanography of oceanic dysoxia/anoxia in the late barremian to early aptian. Palaios 9, 335–369 (1994).
Jenkyns, H. C. Transient cooling episodes during cretaceous oceanic anoxic events with special reference to OAE 1a (Early Aptian). Philos Trans R Soc Lond. 376, 20170073 (2018).
Bauer, K. W., McKenzie, N. R., Bottini, C., Erba, E. & Crowe, S. A. Carbon pump dynamics and limited organic carbon burial during OAE1a. Geobiology 21, 341–354 (2023).
Brumsack, H. J. Geochemistry of recent TOC-rich sediments from the Gulf of California and the black sea. Geol Rundsch. 78, 851–882 (1989).
Anderson, T. F. & Raiswell, R. Sources and mechanisms for enriching highly reactive iron in euxinic black sea sediments. Am J Sci. 304, 203–233 (2004).
Brumsack, H. J. The trace metal content of recent organic carbon-rich sediments: implications for cretaceous black shales formation. Palaeogeogr Palaeoclimatol Palaeoecol. 232, 344–361 (2006).
Brumsack, H. J. The inorganic geochemistry of cretaceous black shales (DSDP leg 41) in comparison to modern upwelling sediments from the Gulf of California and the black sea. Geol Rundsch. 78, 851–882 (1986).
Hatch, J. R. & Leventhal, J. S. Relationship between inferred redox potential of the depositional environment and geochemistry of the upper Pennsylvanian (Missourian) Starck shale member of the Dennis limestone, Wabaunsee county, kansas, USA. Chem Geol. 99, 65–82 (1992).
Turgeon, S. & Brumsack, H. J. Anoxic vs dysoxic events reflected in sediment geochemistry during the Cenomanian–Turonian boundary event (Cretaceous) in the Umbria–Marche basin of central Italy. Chem Geol. 234, 321–339 (2006).
Algeo, T. J. & Tribovillard, N. Environmental analysis of paleoceanographic systems based on molybdenum–uranium covariation. Chem Geol. 268, 211–225 (2009).
Hetzel, A., Böttcher, M. E., Wortmann, U. G. & Brumsack, H. J. Paleo-redox conditions during OAE 2 reflected in demerara rise sediment geochemistry (ODP leg 207). Palaeogeogr Palaeoclimatol Palaeoecol. 273, 302–328 (2009).
Rue, E. L., Smith, G. J., Cutter, G. A. & Bruland, K. W. The response of trace element redox couples to suboxic conditions in the water column. Deep Sea Res Part I. 44, 113–134 (1997).
Tsunogai, S. & Sase, T. Formation of iodide-iodine in the ocean. Deep-Sea Res Oceanogr Abstr. 16, 489–496 (1969).
Chance, R., Baker, A. R., Carpenter, L. & Jickells, T. D. The distribution of iodide at the sea surface. Environ Sci Process Impacts. 16, 1841–1859 (2014).
Lu, Z., Jenkyns, H. C. & Rickaby, R. E. M. Iodine to calcium ratios in marine carbonate as a paleo-redox proxy during oceanic anoxic events. Geology 38, 1107–1110 (2010).
Chapman, P. & Truesdale, V. W. Preliminary evidence for iodate reduction in bottom waters of the Gulf of Mexico during a hypoxic event. Aquat Geochem. 17, 671–695 (2011).
Kerisit, S. N., Smith, F. N., Saslow, S. A., Hoover, M. E. & Lawter, A. R. Qafoku, N. P. Incorporation modes of iodate in calcite. Environ Sci Technol. 52, 5902–5910 (2018).
Glock, N., Liebetrau, V. & Eisenhauer, A. I/Ca ratios in benthic foraminifera from the Peruvian oxygen minimum zone: analytical methodology and evaluation as a proxy for redox conditions. Biogeosciences 11, 7077–7095 (2014).
Lu, Z. et al. Oxygen depletion recorded in upper waters of the glacial Southern ocean. Nat Commun. 7, 11146 (2016).
Zhou, X. et al. Upper ocean oxygenation dynamics from i/ca ratios during the Cenomanian-Turonian OAE 2. Paleoceanography 30, 510–526 (2015).
Hardisty, D. S. et al. Perspectives on proterozoic surface ocean redox from iodine contents in ancient and recent carbonate. Earth Planet Sci Lett. 463, 159–170 (2017).
Shang, M. et al. A pulse of oxygen increase in the early mesoproterozoic ocean at ca. 1.57–1.56 Ga. Earth Planet. Sci. Lett. 527, 115797 (2019).
Wörndle, S., Crockford, P. W., Kunzmann, M., Bui, T. H. & Halverson, G. P. Linking the bitter springs carbon isotope anomaly and early neoproterozoic oxygenation through I/[Ca + Mg] ratios. Chem Geol. 524, 119–135 (2019).
Wei, B. et al. A pulsed oxygenation in the terminal paleoproterozoic ocean: evidence from the transition between the Chuanlinggou and Tuanshanzi formations, North China. Geochem Geophys Geosyst 22, (2021). e2020GC009612.
Jafarian, A. et al. Aptian oceanic anoxic event 1a in the shallow, carbonate-dominated intrashelf Kazhdumi basin, Zagros mountains. Sedimentology 70, 1981–2014 (2023).
van Buchem, F. S. P. et al. Barremian-Lower Albian sequence stratigraphy of Southwest Iran (Gadvan, Dariyan and Kazhdumi formations) and its comparison with oman, Qatar and the united Arab Emirates. GeoArabia Spec Publ. 4, 503–548 (2010).
Jafarian, A. et al. Intrashelf basin record of redox and productivity changes along the Arabian margin of Neo-Tethys during oceanic anoxic event 1a. Palaeogeogr Palaeoclimatol Palaeoecol. 636, 111975 (2024).
van Buchem, F. S. P. et al. Sequence-stratigraphic synthesis of the Barremian-Aptian of the Eastern Arabian plate and implications for the petroleum habitat. GeoArabia Spec Publ. 4, 9–48 (2010).
Lu, W. et al. Late inception of a resiliently oxygenated upper ocean. Science 361, 174–177 (2018).
Hardisty, D. S. et al. An iodine record of paleoproterozoic surface ocean oxygenation. Geology 42, 619–622 (2014).
Lu, W. et al. Iodine proxy evidence for increased ocean oxygenation during the bitter springs anomaly. Geochem Perspect Lett. 5, 53–57 (2017).
Brand, U. & Veizer, J. Chemical diagenesis of a multicomponent carbonate system-1: trace elements. J Sediment Res. 50, 1219–1236 (1980).
Swart, P. K. The geochemistry of carbonate diagenesis: the past, present and future. Sedimentology 62, 1233–1304 (2015).
Küpper, F. C. et al. Commemorating two centuries of iodine research: an interdisciplinary overview of current research. Angew Chem Int Ed. 50, 11598–11620 (2011).
Fehn, U. Tracing crustal fluids: applications of natural 129I and 36Cl. Annu Rev Earth Planet Sci. 40, 45–67 (2012).
He, R., Jiang, G., Lu, W. & Lu, Z. Iodine records from the Ediacaran Doushantuo cap carbonates of the Yangtze block, South China. Precambrian Res. 347, 105843 (2020).
Ding, Y. et al. Low oxygen levels with high redox heterogeneity in the late Ediacaran shallow ocean: constraints from I/(Ca + Mg) and ce/ce** of the Dengying formation, South China. Geobiology 20, 790–809 (2022).
Nozaki, Y. Rare earth elements and their isotopes in the ocean. In Encyclopedia of Ocean Sciences (2nd edn) (eds Steele, J. H., Turekian, K. K. & Thorpe, S. A.) 653–665 (2008).
Tanaka, K. et al. A specific Ce oxidation process during sorption of rare Earth elements on biogenic Mn oxide produced by Acremonium sp. strain KR21-2. Geochim. Cosmochim Acta. 74, 5463–5477 (2010).
Bau, M., Koschinsky, A., Dulski, P. & Hein, J. R. Comparison of the partitioning behaviours of yttrium, rare Earth elements, and titanium between hydrogenetic marine ferromanganese crusts and seawater. Geochim. Cosmochim Acta. 60, 1709–1725 (1996).
Kamber, B. S. & Webb, G. E. The geochemistry of late Archaean microbial carbonate: implications for ocean chemistry and continental erosion history. Geochim Cosmochim Acta. 65, 2509–2525 (2001).
Cao, C., Liu, X. M., Bataille, C. P. & Liu, C. What do Ce anomalies in marine carbonates really mean? A perspective from leaching experiments. Chem Geol. 532, 119413 (2020).
Bordenave, M. L. & Hegre, J. A. Current distribution of oil and gas fields in the Zagros fold belt of Iran and contiguous offshore as the result of the petroleum systems. Geol Soc Am Spec Publ. 330, 291–353 (2010).
Yamamoto, K. et al. Early aptian paleoenvironmental evolution of the Bab basin at the Southern Neo-Tethys margin: response to global carbon-cycle perturbations across ocean anoxic event 1a. Geochem Geophys Geosyst. 14, 1104–1130 (2013).
Bodin, S., Godet, A., Westermann, S. & Föllmi, K. B. Secular change in Northwestern Tethyan water-mass oxygenation during the late Hauterivian – early aptian. Earth Planet Sci Lett. 374, 121–131 (2013).
Alibo, D. S. & Nozaki, Y. Rare Earth elements in seawater: particle association, shale-normalization, and Ce oxidation. Geochim Cosmochim Acta. 63, 363–372 (1999).
Zhang, K. & Shields, G. A. Sedimentary Ce anomalies: secular change and implications for paleoenvironmental evolution. Earth-Sci Rev. 229, 104015 (2022).
Bau, M. & Dulski, P. Distribution of yttrium and rare-earth elements in the Penge and Kuruman iron-formations, Transvaal supergroup, South Africa. Precambrian Res. 79, 37–55 (1996).
Shields, G. & Stille, P. Diagenetic constraints on the use of cerium anomalies as Palaeoseawater redox proxies: an isotopic and REE study of cambrian phosphorites. Chem Geol. 175, 29–48 (2001).
Hueter, A. et al. Central Tethyan platform-top anoxia during oceanic anoxic event 1a. Clim Past. 15, 1327–1344 (2019).
Kamber, B. S. Archean mafic-ultramafic volcanic landmasses and their effect on ocean-atmosphere chemistry. Chem Geol. 274, 19–28 (2010).
Tostevin, R. et al. Effective use of cerium anomalies as redox proxy in carbonate-dominated marine settings. Chem Geol. 438, 146–162 (2016).
Lin, P. C. & Catling, D. C. The rare Earth element distribution in marine carbonates as a potential proxy for seawater ph on early Earth. Am J Sci. 324, 10 (2024).
MacRae, N., Nesbitt, H. & Kronberg, B. Development of a positive Eu anomaly during diagenesis. Earth Planet Sci Lett. 109, 585–591 (1992).
Deng, Y. et al. Rare Earth element geochemistry characteristics of seawater and Porewater from deep sea in Western Pacific. Sci Rep. 7, 1–13 (2017).
Tribovillard, N., Algeo, T. J., Lyons, T. & Riboulleau, A. Trace metals as paleoredox and paleoproductivity proxies: an update. Chem Geol. 232, 12–32 (2006).
Johnson, J. E., Webb, S. M., Ma, C. & Fischer, W. W. Manganese mineralogy and diagenesis in the sedimentary rock record. Geochim Cosmochim Acta. 173, 210–231 (2016).
Calvert, S. & Pedersen, T. Sedimentary geochemistry of manganese; implications for the environment of formation of manganiferous black shales. Econ Geol. 91, 36–47 (1996).
Neumann, T., Heiser, U., Leosson, M. A. & Kersten, M. Early diagenetic processes during Mn-carbonate formation: evidence from the isotopic composition of authigenic Ca-rhodochrosites of the Baltic sea. Geochim Cosmochim Acta. 66, 867–879 (2002).
Tsikos, H., Matthews, A., Erel, Y. & Moore, J. M. Iron isotopes constrain biogeochemical redox cycling of iron and manganese in a palaeoproterozoic stratified basin. Earth Planet Sci Lett. 298, 125–134 (2010).
Hetzel, A., März, C., Vogt, C. & Brumsack, H. J. Geochemical environment of Cenomanian-Turonian black shale deposition at Wunstorf (northern Germany). Cretac. Res. 32, 480–494 (2011).
Jenkyns, H. C. Geochemistry of oceanic anoxic events. Geochem Geophys Geosyst. 11, Q03004 (2010).
Banner, J. L. & Hanson, G. N. Calculation of simultaneous isotopic and trace element variations during water-rock interaction with applications to carbonate diagenesis. Geochim Cosmochim Acta. 54, 3123–3137 (1990).
Tucker, M. E. Formerly Aragonitic limestones associated with tillites in the late proterozoic of death valley, California. J Sediment Petrol. 56, 818–830 (1986).
Gilleaudeau, G. J., Sahoo, S. K., Kah, L. C., Henderson, M. A. & Kaufman, A. J. Proterozoic carbonates of the Vindhyan basin, india: chemostratigraphy and diagenesis. Gondwana Res. 57, 10–25 (2018).
Tang, D., Shi, X., Wang, X. & Jiang, G. Extremely low oxygen concentration in mid-Proterozoic shallow seawaters. Precambrian Res. 276, 145–157 (2016).
McLennan, S. Rare Earth elements in sedimentary rocks; influence of provenance and sedimentary processes. Rev Mineral Geochem. 21, 169–200 (1989).
Lawrence, M. G., Greig, A., Collerson, K. D. & Kamber, B. S. Rare Earth element and yttrium variability in South East Queensland waterways. Aquat Geochem. 12, 39–72 (2006).
Webb, G. E. & Kamber, B. S. Rare Earth elements in holocene reefal microbialites: a new shallow seawater proxy. Geochim Cosmochim Acta. 64, 1557–1565 (2000).
Ullmann, C. V. et al. Warm afterglow from the toarcian oceanic anoxic event drives the success of deep-adapted brachiopods. Sci Rep. 10, 1–11 (2020).
Stampfli, G. M. & Borel, G. D. A plate tectonic model for the paleozoic and mesozoic constrained by dynamic plate boundaries and restored synthetic oceanic isochrons. Earth Planet Sci Lett. 196, 17–33 (2002).
Acknowledgements
Key Research and Development Project of the Xinjiang Uygur Autonomous Region (Social Development), Project Number: 2024B03013-1.
Author information
Authors and Affiliations
Contributions
A.J. conceived the study (investigation, methodology), collected the samples, performed pre-measurements, draw figures, wrote the original draft. A.H. draw figures, wrote the original draft. A.K. wrote the original draft. X.C. performed inductively coupled plasma mass spectrometry (ICP-MS; Thermo iCAPTM TQ), wrote the related method section, reviewed the manuscript. M.W. performed inductively coupled plasma mass spectrometry (ICP-MS; Agilent 7700e), wrote the related method section, reviewed the manuscript. C.V.U. performed inductively coupled optical emission spectrometry (ICP-OES; Agilent 5110 VDV), wrote the related method section, reviewed the manuscript. A.S. draw figures, reviewed the manuscript. K.H. reviewed the manuscript. C.W. supervised the study, reviewed the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jafarian, A., Husinec, A., Koeshidayatullah, A. et al. Cerium anomalies and iodine track nonuniform paleoredox conditions during the Aptian Oceanic Anoxic Event 1a. Sci Rep 15, 23802 (2025). https://doi.org/10.1038/s41598-025-09471-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-09471-4