Abstract
Mango kernel meal (MKM) is an affordable and widely available industrial byproduct, commonly used as an aquaculture feed supplement due to its immuno-physiological properties. This study represents a novel effort to evaluate the dietary effect of MKM on growth, feed utilization, haemato-biochemical attributes, and expression of growth and immune genes in Gibelion catla. G. catla were subjected to four treatments in triplicate (n = 40 fish/replicate), and fed diets supplemented with 0 (control group), 5, 10, 20 g/Kg (T1-T3, respectively) of MKM over a 90-days trial. The results revealed a significant enhancement in the growth indices and reduced feed conversion ratio in the T1 group than control and other treatment groups. Fish fed 5 g/kg MKM had significantly (P < 0.05) higher RBC, Hb, and Hct than the control and other tested diets. Inclusion of MKM at 5 g/kg in the diet significantly reduced haemato-biochemical stress biomarkers (glucose, and cortisol) as well as hepatic transaminase levels. Frequencies of erythrocytes cellular abnormalities (ECA) and erythrocytes nuclear abnormalities (ENA) were higher at 20 g/Kg of MKM inclusion. The relative mRNA expression profile of examined growth and immune-related genes were upregulated at 5 g/kg of the MKM supplementation diet than the control group. The principal component analysis results demonstrated that supplementation with 5 g/kg of MKM significantly improved nutritional and haemato-biochemical parameters. These findings suggest that the inclusion of 5 g/kg of MKM in the basal diet as a dietary supplement for G catla exerts a positive influence on growth performance, and immune-physiological status, highlighting its potential as a beneficial feed additive.
Similar content being viewed by others
Introduction
Aquaculture is one of the most significant and rapidly expanding sectors globally, playing an influential role in producing nutritionally enriched food1. The expansion of aquaculture is vital for addressing the rising demand for fish as well as alleviating pressure on declining capture fisheries2. The intensification of aquaculture has resulted in increased disease outbreaks, posing a significant threat to sustainable and resilient production systems3. Antibiotics and chemotherapeutics are frequently used as prophylactic measures to prevent disease outbreaks, but their application can adversely affect the environment and compromise food security systems4,5. In recent years, there has been increasing emphasis on eco-friendly and sustainable strategies for aquaculture disease management, with a particular focus on utilizing medicinal plants and their extracts for anti-stress, pharmacological, and immunomodulatory properties6,7,8,9,10,11,12. Several recent studies reported that fruit by-products improve the immune function and growth performance of farm fish, offering a safe, cost-effective, and eco-friendly food additive13,14,15,16.
Mangifera indica L. is a large, evergreen species belonging to the Anacardiaceae family, and native to tropical and subtropical regions of South and Southeast Asia17. Different parts of the mango possess a diverse array of medicinal properties, including antimicrobial, anti-inflammatory, antioxidant, and immunomodulatory activities18,19,20,21,22. Among all the by-products of mango, the mango kernel is the most cost-effective and readily accessible, making it a valuable and sustainable source of alternative animal feed additives. It can be regarded as a promising energy-rich feed ingredient owing to its high content of oil, starch, and antioxidants with low crude protein content23,24. It has been reported that mango kernel exhibits potential as a natural antibiotic, demonstrating antimicrobial activity against a wide range of pathogenic microorganisms25. According to the previous report, mango kernel is utilized as an unconventional feed additive in broiler chicken, rat, and rabbit26,27,28,29. In aquaculture, mango kernel is utilized as feed to modulate growth, nutritional indices, antioxidant activity, and non-specific immunity in fish and shellfish30,31,32.
G. catla is a fast-growing and commercially valued aquaculture fish species among Indian major carps in Asian countries. Disease outbreaks caused by infectious and non-infectious pathogens increase mortality and reduce production efficiency in G. catla aquaculture33. The use of subtherapeutic antibiotics and chemotherapeutics to control these diseases raises concerns about antibiotic resistance, environmental contamination, and consumer aversion to antibiotic residues in fish34. Supplementation of mango kernel meal in fish feeds serves as a potential alternative to antibiotics by enhancing immunity35. To the best of our knowledge, no studies have been conducted to evaluate the potential applications of mango kernel as a nutritional supplement in G. catla. Therefore, the present study aimed to determine the dietary effect of mango kernel on growth response, haemato-biochemical parameters, and gene expressions related to growth and immunity in G. catla.
Results
Water quality attributes
The mean values of hydro-ecological parameters obtained from the experimental trial as presented in Table 1. The results indicate that MKM supplementation in the diet had no significant effect on the hydro-ecological attributes of the plastic tank water, suggesting that MKM supplementation does not influence on the suitability of water quality.
Evaluation of growth performance, nutritional indices, and survivability
The growth performance, feed utilization parameter, condition factor, and survival of G. catla fed on varying concentrations of MKM feed based on reared in triplicate treatments are displayed in Table 2. Growth attributes including FW, WG, and SGR%, were significantly augmented in the T1 group than control and other treatment groups. A significant reduction in the FCR was recorded in the T1 group compared to the control group. However, FCR showed no significant differences among examined feeding groups. PER was significantly higher in treatment T1 followed by T2, control, and T3 groups. At the end of the feeding trial, a significantly increased condition factor was observed in the T1 group than other dietary groups. The survival rate of fish did not differ significantly across dietary treatments after the 90-day feeding trial. Quadratic regression analysis of WG and SGR identified the optimal inclusion levels of MKM for maximal growth performance in G. catla as 8.77%, and 8.46%, respectively (Fig. 1).
Effect on haematological response
Results of haematological parameters of G. catla fed different inclusions of dietary MKM following 90-days of experimental trial are shown in Table 3. The results indicate that dietary inclusion of MKM significantly influences the analyzed haematological indices. Fish fed the diet at a 5% inclusion rate had significantly (P < 0.05) higher erythrocyte count (RBC), hemoglobin (Hb) content, and haematocrit (Hct) than control and other examined diets. The RBC indices (MCV, MCH, and MCHC) of fish are significantly modulated in those fed an MKM supplemented diet compared to the control group. The mean values of peripheral leukocytes (WBC) and lymphocytes (lym) demonstrated significant elevation in fish fed a diet containing 20% MKM. However, no significant effects were observed on monocytes (MON), and eosinophils (EOS) in response to the different levels of MKM supplementation.
Effect on stress biomarkers
The effect of G. catla fed dietary MKM supplementation on circulating stress related biomarkers (blood glucose, and serum cortisol) is presented in Fig. 2. Among the treatment groups, G. catla fed with 5 g/Kg of MKM supplementation demonstrated the lowest glucose concentration. Dietary MKM supplementation exerted pronounced and statistically significant effects on the serum cortisol level. In comparison to the control and other treatment group, dietary 5 g/Kg of MKM inclusion resulted in a significant decrease in cortisol levels (P < 0.05).
Effect on hepatic transaminase variables
The modulation of hepatic transaminase variables in G. catla fed with MKM supplemented and control diets is depicted in Fig. 3. The group fed a 5 g/Kg of MKM inclusion diet exhibited significantly lower plasma levels of ALT and AST compared to the control group. No significant differences were detected between the control group and the T3 group.
Erythrocytic nuclear and cellular abnormalities
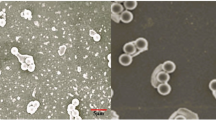
Leishman staining of blood smears was employed to assess cellular and nuclear abnormalities of erythrocytes between the control and treatment groups. The group treated with MKM at doses of 5 g/Kg in the basal diet exhibited significant decreases in the number of erythrocytes with elongated, notched, and twin nuclei (Fig. 4). Although the percentage of elongated erythrocytic cells was higher in all treatment groups, no significant differences were observed between the control and treatment groups. The groups that received MKM at doses of 20 g/Kg in the basal diet displayed the highest percentage of erythrocytic cellular and nuclear abnormalities.
(A) Various erythrocytic cellular and nuclear abnormalities (ECAs) observed in blood smears of G. catla. Red arrows indicate notched nuclei, yellow arrows indicate elongated nuclei, and black arrows indicate twin-shaped nuclei. (B) Assessment of MKM supplementation on cellular and nuclear abnormalities in erythrocytes of G. catla.
Effect on mRNA expression of growth related gene
The mRNA expression levels of growth gene in G. catla fed MKM supplemented diets are shown in Fig. 5. G. catla fed a diet supplemented with 5 g/kg of MKM exhibited significantly upregulated expression of growth-related gene transcripts compared to the control group. In contrast, fish fed a diet supplemented with 20 g/kg of MKM showed no significant differences in the mRNA expression levels of the examined growth genes than control group.
The expression levels of growth-related genes (A) growth hormone and (B) early growth response 2B in the liver of G. catla fed diets with varying inclusions of MKM powder. The data were compared to the control value, which was assigned a relative value of 1. Significant differences are indicated with an asterisk (P < 0.05).
Effect on mRNA expression of immune related gene
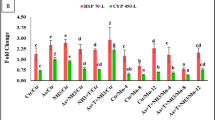
The expression levels of immune gene (interferon γ and interleukin 1β gene) are presented in Fig. 6. Our results indicate that the expression of the interferon γ gene was significantly higher in the T1 group compared to the control group, Although, the expression of the interleukin 1β gene was upregulated in the treated group, but no significant differences were observed between the control and treated groups.
Principal Component Analysis (PCA)
The effects of varying levels of MKM inclusion in the diet on growth, feed utilization, and haemato-biochemical indices of G. catla were analyzed using PCA. The first two principal components (PC1 = 57.1% and PC2 = 14.4%) accounted for 71.5% of the cumulative variance (Fig. 7). The PCA plot revealed that MKM supplemented groups with 5 g and 10 g inclusion levels were positively correlated with growth, feed utilization, and haemato-biochemical attributes compared to the control group. However, parameters such as FCR, glucose, AST, ALT, and cortisol exhibited a negative correlation (Fig. 7A, B).
The PCA scores and biplot effectively represent the relationships among various treatment groups and examined parameters at the conclusion of the experimental trial. The graph provides insights into positive and negative associations across treatment groups (A) The biplot of variables, illustrating their contributions to the principal components, (B) Loadings of variables in each group.
Discussion
The intensification of aquaculture has led to stress-induced health issues in fish, prompting interest in natural feed additives as alternatives to antibiotics. This study evaluates the potential role of MKM as a functional feed additive, supporting sustainable aquaculture nutrition strategies.
The mean values of the hydro-ecological parameters remained consistent across feeding frequencies throughout the experimental trial. These recorded values remain within acceptable ranges, ensuring for physiological performance, growth, and immune competence of G. catla36.
In the present study, the inclusion of MKM at a supplementation level of 5 g/kg in the basal diet significantly incremented prime growth performance indicators (FW, WG, SGR%, and PER) compared to the control group (p < 0.05). These enhancements in zootechnical performance suggest that MKM exerts a growth-promoting effect, potentially due to its rich profile of bioactive compounds, and essential nutrients that may enhance nutrient utilization and metabolic efficiency37. A significant improvement in growth metrices was observed in Oreochromis niloticus fed a diet supplemented with MKM at 5 g/kg31. Incorporation of fermented mango seed kernel meal as a 50% replacement for maize in the diet significantly improved the growth indices of Clarias batrachus38. A previous research reported that 5% inclusion of MKM in diet demonstrated a higher growth and survival rate of Macrobrachium rosenbergii39. They also demonstrated that the proteins and carbohydrates present in mango seed kernels are digestible by post-larvae. Digestibility assay indicated that these macronutrients in MKM are efficiently metabolized; supporting improved nutrient uptake and assimilation during early development stages40.This positive growth-promoting effect could be attributed to the inherent nutrients that enhanced feed utilization, ultimately contributing to improved growth performance41. Studies have shown that MKM can enhance the activity of digestive enzymes leading to increased feed conversion efficiency and SGR. Additionally, the fibrous components in MKM act as prebiotics, fostering the growth of beneficial gut bacteria, which in turn helps in better nutrient assimilation42. On the contrary, the higher inclusion of MKM at a supplementation level of 20 g/kg in the diet led to a decrease in growth performance and feed utilization efficiency. This could be attributed to the presence of anti-nutritional factors (tannins, phytates, and oxalates), which may reduce the palatability of the diets as their inclusion levels increase. These anti-nutritional factors reducing the bioavailability of minerals, impairing digestion, disrupt intestinal function, and also hinder the availability of carbohydrates and proteins43. Quadratic regression model has been used as a powerful tool in aquaculture nutritional studies to determine optimal dietary incorporation levels due to their ability to apprehend the diminishing returns beyond the peak feeding rate44.Based on quadratic regression analysis, the optimal feeding rate should focus on growth attributes, particularly WG and SGR. Our findings suggest that the optimum dietary inclusion levels for this species are 8.77 to 8.46%, which promotes the most effective growth, immunity, and other physiological parameter. Thus, the identified optimal range for G. catla feeding provides a typical guideline for maximizing yield while ensuring sustainability and fish welfare. Such experimental results are in agreement with earlier studies that reveal the crucial role of dietary optimization in fish species to increase both productivity and fish welfare45,46.
The condition factor is a vital tool for assessing fish health, reflecting the physical well-being of a fish population through measurements of their relative plumpness or fatness47. Significantly higher values were observed in fish fed a basal diet supplemented with 5 g/kg MKM. Elevated KF values generally reflect favorable somatic development and indicate that the internal and external environments supported optimal fish growth. The positive modulation between MKM supplementation and condition factor also aligns with earlier studies demonstrating the efficacy of agro-industrial by-products in fish nutrition when used at moderate inclusion levels48. Therefore, the elevated K values observed in the current study confirm that the fish were in good condition and that MKM at 5 g/kg can be a viable inclusion of sustainable aqua-feeds.
No significant mortality was recorded across all treatment groups during the experimental period, suggesting that the inclusion of MKM did not exert any acute or chronic toxic effects on the fish. Similarly, the survival rate of Clarias gariepinus juveniles was not significantly influenced by the inclusion of fermented MKM at various substitution levels for maize in their diets38.
Haematological markers are affected by dietary nutrition and environmental stressors, and they serve as important indicators for assessing fish health and physiological conditions49. Haematological techniques, including the assessment of RBC count, Hb concentration, Hct value, and WBC count, have proven to be valuable tools for fishery biologists in evaluating fish health50. The current results showed significant increases in RBC, Hb, Hct, and RBC indices (MCV, MCH, and MCHC) at 5 g/Kg MKM inclusion in the diet. The inclusion of 5 g/kg MKM in the diet may enhance erythropoiesis by supplying iron, essential amino acids, and antioxidants that support RBC synthesis and hemoglobin formation. Improved antioxidant status reduces oxidative stress on hematopoietic tissues, increasing hematocrit and boosting RBC indices, indicating larger and more hemoglobin-rich red cells. This observation suggests that MKM contains constituents that effectively stimulate the immune system and support the proper functioning of organs involved in blood cell formation51. Additionally, bioactive constituents in MKM can positively modulate immune and hematopoietic functions through the regulation of cytokines and gene expression involved in erythroid lineage development. The results of this study are in agreement with the findings of52who reported that the haematological indices in broiler chickens were significantly influenced by the differently processed mango seed kernel meal at varying inclusions level. Thus, MKM supplementation may serve as a functional feed additive to enhance both hematological health and systemic immunity in aquafarm species.
WBC count is a critical biomarker for assessing fish health, as it reflects the immune system’s response to various physiological and environmental factors53,54,55. An elevation in leucocyte counts in this study suggests that the fish have enhanced cellular defenses against infections by activating nonspecific immunity55. MKM is rich in polyphenolic compounds and bioactive phytochemicals such as gallic acid, tannins, and flavonoids, which possess strong antioxidant and anti-inflammatory properties56.These bioactives can mitigate oxidative stress by scavenging reactive oxygen species, thereby reducing lipid peroxidation and cellular damage. The antioxidant activity of MKM may modulate leukocyte dynamics by protecting immune cells from oxidative injury and maintaining homeostasis in immune signaling pathways57. As a result, MKM supplementation may influence total and differential leucocyte counts by modulating both oxidative stress and the inflammatory status of the organism.
Serum biochemical parameters serve as an excellent indicators of animal’s health status, nutritional status, physiological condition, metabolic functions, and potential clinical issues58. Examining specific serum indices is valuable for evaluating feeding adequacy, detecting metabolic disorders, assessing rearing conditions, and identifying acute or chronic stressors59. Glucose and cortisol are widely recognized physiological biomarkers of stress in fish, with elevated levels typically indicating heightened stress60,61. In this study, a significant reduction in glucose and cortisol levels was observed in the MKM treated groups compared to the control group, which exhibited the highest serum glucose levels. These findings are consistent with the observations of32who demonstrated that dietary supplementation with herbal immunostimulants effectively reduced stress biomarkers in aquatic animals. A previous study reported that diets supplemented with mango kernel flour significantly (P < 0.05) modulated fasting blood glucose levels in rats62. The reduction of glucose and cortisol levels in the diet is attributed to bioactive molecules (flavonoids and phenols), which exhibit stress-relief effects in animals63. Bioactive compounds in MKM may influence the hypothalamic-pituitary-adrenal (HPA) axis and reduce cortisol levels. A study demonstrated that mangiferin administration in rats mitigated HPA axis dysregulation, oxidative damage, and neuroinflammation following stress exposure64. The lower levels of glucose and cortisol in fish fed the supplemented diets suggest that G. catla is maintained in optimal physiological conditions. The significantly lower plasma glucose and cortisol levels observed in G. catla fed with 5 g/kg supplemented diets indicate a reduction in metabolic and environmental stress. The observed decline in these parameters following dietary inclusion indicated improved homeostatic regulation and enhanced health status, which may contribute to better growth and immune function.
AST and ALT are plasma enzymes released into the bloodstream, providing critical information about nutritional status, liver function, and circulatory system condition53,65. The elevated levels of these hepatic enzymes indicate damage to the plasma membrane and cellular destruction in the liver66. In this study, the AST and ALT levels significantly decreased in fish fed a diet supplemented with 5 g/kg MKM. MKM contains polyphenols, and flavonoids that scavenge free radicals, reducing oxidative stress and liver cell damage. MKM also helps control inflammation and supports lipid metabolism, preventing fat accumulation in the liver. Additionally, its nutritional content aids in liver repair and overall function, leading to decreased leakage of liver enzymes into the bloodstream67. This suggests potential plasma membrane stabilization and harmful, toxic damage caused by free radicals affecting hepatic cell liver protection. Aligned with our findings, MKM dietary supplementation markedly reduced hepatic transaminase activity in Nile tilapia31.
In the present investigation, fish fed diets supplemented with 20 g/kg of MKM exhibited a significantly elevated frequency of both cellular and nuclear abnormalities in erythrocytes compared to the control group. This finding indicates that the incorporation of MKM at this concentration may exert cytotoxic effects, potentially distress the structural integrity and physiological function of erythrocytes in fish, thereby posing a risk hematological health and systemic homeostasis.These abnormalities in blood erythrocytes serve as important indicators of oxidative stress, providing critical insights into cellular and systemic health68. The incorporation of 5 g/kg MKM into the diet resulted in a reduced frequency of erythrocytic abnormalities, indicating the beneficial role of MKM in alleviating the effects of oxidative stress69.
Molecular tools are crucial in aquaculture research, offering valuable insights into fish responses to stress and environmental changes70. This study explores the relationship between gene expression profile and the inclusion of MKM in the diets of G. catla. To date, the mRNA expression profile of G. catla in response to varying inclusion levels of MKM remains unexplored. In the present study, dietary inclusion of MKM at a concentration of 5 g/kg resulted in a significant upregulation of growth-related genes in G. catla. This suggests that MKM may exert a growth-promoting effect, possibly through modulation of the somatotropic axis and increment of muscle cell proliferation and differentiation. The observed effect could be attributed to the presence of bioactive compounds which have been reported to improve nutrient utilization and promote anabolic activity in fish71. Similar findings were observed by72who reported that optimimum inclusion levels of plant-based feed ingredients can enhance growth performance and gene expression associated with somatic growth in fish species.
This study also analyzed the expression levels of immune-related genes, specifically interferon γ and interleukin 1β, to assess their involvement in immune response regulation. The expression of the interferon γ gene was significantly upregulated in the group fed a diet with 5 g/kg MKM inclusion. Although the expression level of interleukin 1β was higher in the MKM treated group, no significant differences were observed between the treatment and control groups. Flavonoids found in mango kernels have the potential to induce the upregulation of immune-related genes, highlighting their possible role as immunomodulatory agents and/or compounds capable of restoring immune function. Mangiferin has been shown to modulate endocrine and immune signaling pathways, directly influencing gene expression related to growth, development, and immunity73. It exerts its effects through the regulation of key transcription factors, critical in governing the expression of genes related to immune defense and cellular protection73. This integrated modulation of immune and endocrine networks implies that MKM may serve as promising feed ingredients for enhancing immune function and maintaining immunological homeostasis74.
PCA was used to evaluate the impact of MKM inclusion on fish growth, feed utilization, and haemato-biochemical parameters. The plot revealed that G. catla showed improved growth performance when fed a diet with 5 g/kg MKM. In a previous study, PCA analysis was used to assess the impact of Moringa oleifera on the growth, feed utilization, and haemato-biochemical indices of Asian catfish75. The PCA analysis suggests that adding 5 g/Kg MKM to the basal diet may improve physiological activity by affecting nutritional attributes.
In conclusion, this study revealed that incorporating 5 g/kg MKM in the diet of G. catla positively influenced growth performance, blood parameters, and the expression of genes related to growth and immunity. Using this feed additive at a 5 g/kg inclusion level offers a promising approach to boost G. catla production and promote the economic sustainability of aquaculture by minimizing feed expenses.
Materials and methods
Collection and Preparation of Mango Kernel Meal (MKM)
Mango fruits were collected from the local markets, and brought to the Molecular Biology and Conservation Laboratory, Faculty of Fisheries, Patuakhali Science and Technology University. The seed kernels were obtained from each fruit by cutting the seed coat using a sharp knife, washed thoroughly with distilled water, and air-dried separately. Subsequently, the kernels were removed from the seeds, grinding, and boiled to eradicate most anti-nutritional factors. They were transferred to the refrigerator at −80 °C, dried carefully in a freeze dryer to remove excessive moisture, and stored at 4 °C for subsequent use.
Experimental layout and trial experiment
Four hundred and eighty G. catla fingerlings were procured from the commercial fish hatchery (Jessore, Bangladesh), and acclimatized for 2 weeks in hapa with a commercial diet prior to the experimental initiation. Afterwards, the fingerlings were transferred to rectangular white plastic tanks (66 × 36 × 36 cm with a capacity of 85 L) filled with freshwater, and equipped with individual biofilters, and aeration. The basal diet was prepared as per our previously published article75. The healthy fingerlings of uniform size were randomly distributed and divided into four equal groups with three biological replications (40 fish/replicate). The MKM powder was incorporated into the basal diet at 0, 5 g, 10 g, and 20 g per Kg, and designated as control, T1, T2, and T3 treatment, respectively. The fish were fed at 5% body weight twice a day for a period of 90-days.
Determination of water quality parameter
To analyze water quality parameters, pH, dissolved oxygen (DO), water temperature, electric conductivity, and ammonia were measured daily using a water quality multiparameter (HI9813-61, Hanna Instruments, USA) during the experimental period.
Assessments of growth, feed and nutrient utilization, and survival of experimental fish
Biometric attributes, including total length (TL) and total weight (TW) were measured at the beginning and at fifteen day intervals throughout the experimental period. The following growth metrics, feed utilization indices, survival, and condition factor of experimental fish were calculated using the following formulae:
Fulton’s condition factor (KF) was estimated by using the equation-
.
Where, BW = body weight in g and L = standard length in cm
.
Blood sample collection, and determination of haemato-biochemical parameters
At the end of the trial, five fishes from each replicate were collected and anesthetized for 2–3 min with sodium bicarbonate-buffered tricaine methane sulphate (MS-222, 0.1 g/l, Sigma-Aldrich). Subsequently, the blood sample was collected from the caudal peduncle of the fish using a 1 ml hypodermic syringe (23-gauge needle). For haematological analysis, a portion of the blood sample was placed into a 2 ml heparin-coated tube. The remaining blood was put into 1.5 ml serum separating eppendorf tube without anticoagulant and then centrifuged at 3000 rpm for 15 min for the serum biochemistry analysis. The haemato-biochemical parameters of fish feed with dietary inclusion of MKM and control groups was determined following the previous protocol75,76. Serum cortisol was analyzed by enzyme-linked immunosorbent assay method using a kit following the manufacturer’s instructions (Monobind, Lake forest, CA, USA).
Assessment of Erythrocytes Cellular Abnormalities (ECA) and Erythrocytes Nuclear Abnormalities (ENA)
After collection of blood from fish vain, blood was immediately smeared on the glass slides. The blood drop was dispersed onto a slide using the end of another slide. After ten minutes to dry, the blood smear was fixed with methanol. Subsequently, Leishman stain solution was applied to the slides. Stain-filled slides were allowed to air dry after being washed with distilled water. Prior to the investigation of ENA and ECA, the slides were maintained at room temperature and mounted with Dibutylphthalate polystyrene xylene (DPX). ENA and ECA were inspected using an electron microscope (Euromex IS.1153-EPL, iScope Brightfield Biological Trinocular Microscope). The ECAs and ENAs of blood smears in H. fossilis were assessed in eight randomly selected regions and were presented as the average percentage of abnormal cells per total number of cells in each section.
Expression analysis of growth and immune related gene
Growth factor and immune response genes was retrieved from the NCBI database based on their growth and immunological function (Table 4). Liver samples of three fish from each experimental group were collected, placed in RNAlater, and stored at −80 °C for subsequent analysis. Total RNA extraction, cDNA synthesis, and quantitative real time PCR (qRT-PCR) assay were conducted following the previously published protocol77,78,79. Elongation factor-1(EF-1) gene was used as an internal reference based on its stability. Each assay was conducted in triplicate for maintaining accuracy, and the relative level of examined gene expression was determined using 2−ΔΔct method.
Statistical analysis
Shapiro–Wilk and Levene tests were applied to confirm the normality and homogeneity of variance of the experimental data. Following the test, one-way ANOVA was performed for the data analysis, and variances between control and treated fish groups were determined using Tukey’s (HSD) post hoc test. Statistical data analyses and visualization were done using GraphPad Prism 9.0.2. PCA was done with the help of an open-source R environment (version 4.2.1). All values are expressed as mean ± standard error, and significant levels are considered at P \(\:\le\:\)0.05.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Tadese, D. A. et al. The role of currently used medicinal plants in aquaculture and their action mechanisms: A review. Rev. Aquac. 14, 816–847 (2022).
Tacon, A. G. J. & Metian, M. Feed matters: satisfying the feed demand of aquaculture. Rev. Fish. Sci. Aquac. 23, 1–10 (2015).
Chowdhury, S. et al. Antibiotics usage practices in aquaculture in Bangladesh and their associated factors. One Heal. 15, 100445 (2022).
Alagawany, M. et al. Curcumin and its different forms: A review on fish nutrition. Aquaculture 532, 736030 (2021).
Harikrishnan, R. et al. Effect of plant active compounds on immune response and disease resistance in cirrhina Mrigala infected with fungal fish pathogen, Aphanomyces invadans. Aquac Res. 40, 1170–1181 (2009).
Khan, T. et al. Quercetin supplementation in the diet of Labeo rohita: effects on growth, proximate composition, antioxidative indices and immunity. Anim. Feed Sci. Technol. 303, 115699 (2023).
Ali, W., Fatima, M., Shah, S. Z. H., Khan, N. & Naveed, S. Black cardamom (Amomum subulatum) extract improves growth potential, antioxidant status, immune parameters and response to crowding stress in Catla catla. J. Anim. Physiol. Anim. Nutr. (Berl). 108, 274–284 (2024).
Ali, W., Fatima, M., Shah, S. Z. H., Khan, N. & Naveed, S. Star Anise extract supplementation improved growth performance, hepatic-antioxidant enzyme status, hematology, serum biochemistry, and survival against crowding stress in Catla catla. Aquaculture 584, 740674 (2024).
Elgendy, M. Y. et al. Alternative therapies recently applied in controlling farmed fish diseases: mechanisms, challenges, and prospects. Aquac Int. 32, 9017–9078 (2024).
Liao, W. et al. Review of medicinal plants and active pharmaceutical ingredients against aquatic pathogenic viruses. Viruses 14, 1281 (2022).
Zhang, W., Zhao, J., Ma, Y., Li, J. & Chen, X. The effective components of herbal medicines used for prevention and control of fish diseases. Fish. Shellfish Immunol. 126, 73–83 (2022).
Dadras, F., Velisek, J. & Zuskova, E. An update about beneficial effects of medicinal plants in aquaculture: A review. Vet. Med. (Praha). 68, 449 (2023).
Abdel Rahman, A. N. et al. Dietary Prunus armeniaca augments antioxidant-immune-capacity, absorptive function, and growth and upregulates nutrient transporters and immune-regulatory genes of Oreochromis niloticus. Aquaculture 596, 741820 (2025).
Outama, P. et al. Modulation of growth, immune response, and immune-antioxidant related gene expression of nile tilapia (Oreochromis niloticus) reared under Biofloc system using Mango Peel powder. Fish. Shellfish Immunol. 131, 1136–1143 (2022).
Kari, Z. A. et al. Palm date meal as a non-traditional ingredient for feeding aquatic animals: A review. Aquac Rep. 25, 101233 (2022).
Chekani, R., Akrami, R., Ghiasvand, Z., Chitsaz, H. & Jorjani, S. Effect of dietary dehydrated lemon Peel (Citrus limon) supplementation on growth, hemato-immunolological and antioxidant status of rainbow trout (Oncorhynchus mykiss) under exposure to crowding stress. Aquaculture 539, 736597 (2021).
Shah, K., Patel, M., Patel, R. & Parmar, P. Mangifera Indica (Mango). Pharmacogn Rev. 4, 42–48 (2010).
Saviano, A. et al. Anti-inflammatory and Immunomodulatory activity of Mangifera indica L. reveals the modulation of COX-2/mPGES-1 axis and Th17/Treg ratio. Pharmacol. Res. 182, 106283 (2022).
Anila, L. & Vijayalakshmi, N. R. Antioxidant action of flavonoids from Mangifera indica and emblica officinalis in hypercholesterolemic rats. Food Chem. 83, 569–574 (2003).
Scartezzini, P. & Speroni, E. Review on some plants of Indian traditional medicine with antioxidant activity. J. Ethnopharmacol. 71, 23–43 (2000).
Garrido, G. et al. In vivo and in vitro anti-inflammatory activity of Mangifera indica L. extract (VIMANG®). Pharmacol. Res. 50, 143–149 (2004).
Kabuki, T. et al. Characterization of novel antimicrobial compounds from Mango (Mangifera indica L.) kernel seeds. Food Chem. 71, 61–66 (2000).
Akhter, M. J., Sarkar, S., Rayhanujjaman, M., Kabir, M. S. & Hosain, M. M. Characterization of Mango seed kernel starch: extraction and analysis. Food Chem. Adv. 5, 100806 (2024).
Soong, Y. Y. & Barlow, P. J. Antioxidant activity and phenolic content of selected fruit seeds. Food Chem. 88, 411–417 (2004).
Mutua, J. K., Imathiu, S. & Owino, W. Evaluation of the proximate composition, antioxidant potential, and antimicrobial activity of Mango seed kernel extracts. Food Sci. Nutr. 5, 349–357 (2017).
Saleh, N. & Bello, K. M. Effects of replacing maize with Mango seed kernel meal on performance, carcass characteristics and economic of production of weaner rabbits. Sci. J. Zool. 6, 15–19 (2015).
Beriso, Y., Tamir, B. & Tesfaye, E. Growth performance and feed utilization of Hubbard classic chickens fed on boiled and sundried Mango (Mangifera indica Linn.) seed kernel. Ethiop. J. Sci. Technol. 15, 277–292 (2022).
Odunsi, A. A. Response of laying hens and growing broilers to the dietary inclusion of Mango (Mangifera indica L.) seed kernel meal. Trop. Anim. Health Prod. 37, 139–150 (2005).
Okai, D. B. & Aboagye, J. The effects of Mango seed kernel meal (MSKM) on the performance of growing rats. Biol. Wastes. 34, 171–175 (1990).
Aarumugam, P., Bhavan, P. & Muralisankar, T. Growth of macrobrachium rosenbergii fed with Mango seed kernel, banana Peel and Papaya Peel incorporated feeds. Int. J. Appl. Biogy Pharm. Technol. 4, 12–25 (2013).
El-Houseiny, W., El-Murr, A. & El-Sayed, B. M. Evaluation of dietary inclusion of Mango kernel meal and oat extract on performance and immunity of Oreochromis niloticus. Zagazig Vet. J. 45, 118–125 (2017).
Sahu, S. et al. Effect of magnifera indica kernel as a feed additive on immunity and resistance to Aeromonas hydrophila in Labeo rohita fingerlings. Fish. Shellfish Immunol. 23, 109–118 (2007).
Irshath, A. A., Rajan, A. P., Vimal, S., Prabhakaran, V. S. & Ganesan, R. Bacterial pathogenesis in various fish diseases: recent advances and specific challenges in vaccine development. Vaccines 11, 470 (2023).
Ljubojević Pelić, D. et al. Antibiotic residues in cultured fish: implications for food safety and regulatory concerns. Fishes 2024. 9, 484 (2024).
Fontana, C. M. et al. Effects of Mango seed (Mangifera indica) powder on growth performance, immune response, gut morphology, and gene expression of nile Tilapia (Oreochromis niloticus). Fishes 2024. 9, 514 (2024).
Hossain, M. B. et al. Growth, yield and profitability of major carps culture in coastal homestead ponds stocked with wild and hatchery fish seed. Agric. 2022. 12, Page 1131 (12), 1131 (2022).
Ebenebe, C. I., Onunkwo, D. N., Amaduruonye, W. & Daniel-Igwe, G. Effects of sun-dried Mango seed kernel meal on growth performance of giant African snail(Archachatina marginata). Niger J. Anim. Prod. 45 (201-), 189 (2018).
FALAYE, A., SULE, S. O. & KOUROUMA, M. Performance of Clarias gariepinus juveniles fed fermented Mango seed kernel diet at different inclusion level. Acta Aquat. Turc. 17, 214–220 (2021).
Belsare, S. & Singh, H. Effect of diets containing different levels of Mango seed Karnel on growth and survival of Postlrvae of Macrobrachium rosenbergii. Asian J. Anim. Sci. 2, 59–63 (2007).
Omoregie, E. Utilization and nutrient digestibility of Mango seeds and palm kernel meal by juvenile Labeo senegalensis (Antheriniformes: Cyprinidae). Aquac Res. 32, 681–687 (2001).
Baba, E., Acar, Ü., Öntaş, C., Kesbiç, O. S. & Yilmaz, S. The use of Avena sativa extract against Aeromonas hydrophila and its effect on growth performance, hematological and immunological parameters in common carp (Cyprinus carpio). Ital. J. Anim. Sci. 15, 325–333 (2016).
Lubis, A. R. et al. Impact of Mango Peel pectin and Bacillus iranensis on growth, immunity, and gene expression in nile tilapia (Oreochromis niloticus). Aquac Rep. 41, 102661 (2025).
Azaza, M. S. et al. Evaluation of faba beans (Vicia faba L. Var. minuta) as a replacement for soybean meal in practical diets of juvenile nile tilapia Oreochromis niloticus. Aquaculture 287, 174–179 (2009).
Khieokhajonkhet, A., Aeksiri, N., Rojtinnakorn, J., Van Doan, H. & Kaneko, G. Sacha Inchi meal as a fish-meal replacer in red hybrid tilapia (Oreochromis niloticus × O. mossambicus) feeds: effects on dietary digestibility, growth metrics, hematology, and liver and intestinal histology. Aquac Int. 30, 677–698 (2022).
Imtiaz, A. Effects of feeding levels on growth performance, feed utilization, body composition, energy and protein maintenance requirement of fingerling, rainbow trout, Oncorhynchus mykiss (Walbaum 1792). Iran. J. Fish. Sci. 17, 745–762 (2018).
Hussein, E. E. et al. Effect of dietary Sage (Salvia officinalis L.) on the growth performance, feed efficacy, blood indices, non-specific immunity, and intestinal microbiota of European sea bass (Dicentrarchus labrax). Aquac Rep. 28, 101460 (2023).
Datta, S. N., Kaur, V. I., Dhawan, A. & Jassal, G. Estimation of length-weight relationship and condition factor of spotted Snakehead Channa punctata (Bloch) under different feeding regimes. Springerplus 2, 1–5 (2013).
Serra, V., Pastorelli, G., Tedesco, D. E. A., Turin, L. & Guerrini, A. Alternative protein sources in aquafeed: current scenario and future perspectives. Vet. Anim. Sci. 25, 100381 (2024).
Fazio, F. Fish hematology analysis as an important tool of aquaculture: A review. Aquaculture 500, 237–242 (2019).
Witeska, M., Kondera, E. & Bojarski, B. Hematological and Hematopoietic Analysis in Fish Toxicology—A Review. Anim. Vol. 13, Page 2625 13, 2625 (2023). (2023).
Ma, X. et al. Polyphenolic compounds and antioxidant properties in Mango fruits. Sci. Hortic. (Amsterdam). 129, 102–107 (2011).
Jatutu, S. S., Bello, K. M., & Kalla, D. J. U. Heamatological and carcass characteristics of broiler chickens fed differently processed mango seed kernel meal. Niger J. Anim. Prod. 45, 1539–1545 (2020).
Kumar, V., Makkar, H. P. S. & Becker, K. Nutritional, physiological and haematological responses in rainbow trout (Oncorhynchus mykiss) juveniles fed detoxified Jatropha curcas kernel meal. Aquac Nutr. 17, 451–467 (2011).
Khieokhajonkhet, A. et al. Effects of long-term exposure to high temperature on growth performance, chemical composition, hematological and histological changes, and physiological responses in hybrid catfish [♂Clarias gariepinus (Burchell, 1822) ×♀C. macrocephalus (Günther, 1864)]. J. Therm. Biol. 105, 103226 (2022).
Clauss, T. M., Dove, A. D. M. & Arnold, J. E. Hematologic disorders of fish. Vet. Clin. North. Am. Exot Anim. Pract. 11, 445–462 (2008).
Duarte, L. G. et al. Mango (Mangifera indica L.) By-products in food processing and health promotion. Nutr. Rev. https://doi.org/10.1093/NUTRIT/NUAE214 (2025).
Abdel-Aty, A. M., Salama, W. H., Hamed, M. B., Fahmy, A. S. & Mohamed, S. A. Phenolic-antioxidant capacity of Mango seed kernels: therapeutic effect against Viper venoms. Rev. Bras. Farmacogn. 28, 594–601 (2018).
Faggio, C. et al. Metabolic response to monthly variations of Sparus aurata reared in mediterranean On-Shore tanks. Turkish J. Fish. Aquat. Sci. 14, 567–574 (2014).
Eslamloo, K., Falahatkar, B. & Yokoyama, S. Effects of dietary bovine lactoferrin on growth, physiological performance, iron metabolism and non-specific immune responses of Siberian sturgeon Acipenser Baeri. Fish. Shellfish Immunol. 32, 976–985 (2012).
Khieokhajonkhet, A. et al. Effects of dietary supplementation of turmeric (Curcuma longa) extract on growth, feed and nutrient utilization, coloration, hematology, and expression of genes related immune response in goldfish (Carassius auratus). Aquac Rep. 32, 101705 (2023).
Pelusio, N. F. et al. Interaction between dietary lipid level and seasonal temperature changes in Gilthead sea Bream Sparus aurata: effects on growth, fat deposition, plasma biochemistry, digestive enzyme activity, and gut bacterial community. Front. Mar. Sci. 8, 664701 (2021).
Irondi, E. A., Oboh, G. & Akindahunsi, A. A. Antidiabetic effects of Mangifera indica kernel Flour-supplemented diet in streptozotocin‐induced type 2 diabetes in rats. Food Sci. Nutr. 4, 828 (2016).
Choudhary, P. et al. Mango Seed Kernel: A Bountiful Source of Nutritional and Bioactive Compounds. Food Bioprocess Technol. 16, 289–312 (2022). (2022).
Márquez, L., García-Bueno, B., Madrigal, J. L. M. & Leza, J. C. Mangiferin decreases inflammation and oxidative damage in rat brain after stress. Eur. J. Nutr. 51, 729–739 (2012).
Lemaire, P. et al. Changes with different diets in plasma enzymes (GOT, GPT, LDH, ALP) and plasma lipids (cholesterol, triglycerides) of sea-bass (Dicentrarchus labrax). Aquaculture 93, 63–75 (1991).
Davern, T. J. Drug-Induced liver disease. Clin. Liver Dis. 16, 231–245 (2012).
Nithitanakool, S., Pithayanukul, P. & Bavovada, R. Antioxidant and hepatoprotective activities of Thai Mango seed kernel extract. Planta Med. 75, 1118–1123 (2009).
Harabawy, A. S. A. & Mosleh, Y. Y. I. The role of vitamins A, C, E and selenium as antioxidants against genotoxicity and cytotoxicity of cadmium, copper, lead and zinc on erythrocytes of nile tilapia, Oreochromis niloticus. Ecotoxicol. Environ. Saf. 104, 28–35 (2014).
Araújo, L. R. S. et al. Dietary ethanol extract of mango increases antioxidant activity of pork. animal 15, 100099 (2021).
Panserat, S. & Kaushik, S. J. Regulation of gene expression by nutritional factors in fish. Aquac Res. 41, 751–762 (2010).
Ojha, P., Raut, S., Subedi, U. & Upadhaya, N. Study of nutritional, phytochemicals and functional properties of Mango kernel powder. J. Food Sci. Technol. Nepal. 11, 32–38 (2019).
El-Sayed, A. F. M. et al. Dietary effect of a Plant-Based mixture (Phyto AquaMeric) on growth performance, biochemical analysis, intestinal histology, gene expression and environmental parameters of nile Tilapia (Oreochromis niloticus). Fishes 2024. 9, 358 (2024).
Imran, M. et al. Mangiferin: a natural miracle bioactive compound against lifestyle related disorders. Lipids Health Dis. 16, 1–17 (2017).
Chaudhary, M. & Chhabra, S. Potential significance of Mango seed kernel in boosting the immune system. Int. J. Food Sci. 8, 1–3 (2022).
Sharker, M. R. et al. Effects of moringa (Moringa oleifera) leaf powder supplementation on growth performance, haematobiochemical parameters and gene expression profile of stinging catfish, Heteropneustes fossilis. Aquac Rep. 39, 102388 (2024).
Hassan, A., Ahmed, I. & Wani, G. B. Effect of Supplementation of Vitamin A on Growth, Haemato-Biochemical Composition, and Antioxidant Ability in Cyprinus carpio var. communis. Aquac. Nutr. 8446092 (2022). (2022).
Sharker, M. R. et al. Carbonic anhydrase in Pacific abalone Haliotis discus hannai: characterization, expression, and role in biomineralization. Front. Mol. Biosci. 8, 655115 (2021).
Sharker, M. R. et al. Molecular characterization of carbonic anhydrase II (CA II) and its potential involvement in regulating shell formation in the Pacific abalone, Haliotis discus hannai. Front Mol. Biosci. 8, 1–11 (2021).
Sukhan, Z. P. et al. Thermal stress affects gonadal maturation by regulating gnrh, GnRH receptor, apgwamide, and serotonin receptor gene expression in male Pacific abalone, Haliotis discus hannai during breeding season. Front Mar. Sci. 8, 1–16 (2021).
Author information
Authors and Affiliations
Contributions
Md. Rajib Sharker: Conceptualization, Methodology, Validation, Sample analysis, Data curation, Supervision, Investigation. Kanij Rukshana Sumi: Supervision, Sample analysis, Data curation, Writing-Original Draft. Mariya Afroz: Data Curation, Sample analysis, Writing-Original Draft. Syed Ariful Haque: Methodology, Data Curation, Sample analysis. Md Fakhrul Islam: Data Curation, Sample analysis. Md. Mokhlasur Rahman: Sample analysis. Writing-Review and Editing. A.N.M. Musfiques Shalehin: Methodology, Sample analysis, Writing-Original Draft. Sang Duk Choi: Investigation, Writing-Review and Editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sharker, M.R., Sumi, K.R., Afroz, M. et al. Effects of dietary Mango Kernel Meal (MKM) inclusion on growth indices, haemato-biochemical profiles, and growth and immune-regulatory genes of Gibelion catla. Sci Rep 15, 23789 (2025). https://doi.org/10.1038/s41598-025-09786-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-09786-2