Abstract
Improving the sensitivity of parasite detection is an urgent task for global public health. The traditional manual microscopy method still has some shortcomings in fecal parasite detection. This study aimed to evaluate the clinical application value of the KU-F40 fully automated fecal analyzer in fecal parasite detection by comparing the fecal detection results of the manual microscopy method with those of the KU-F40 instrumental method. Fecal test results were collected from January to June 2023 and January to June 2024, and divided into manual microscopy group (n = 51,627) and KU-F40 instrumental group (n = 50,606) according to methodology. The detection levels and species of parasites detected by the two methods were retrospectively analyzed. The KU-F40 instrumental group had a higher parasite detection level (8.74%) than the manual microscopy group (2.81%), with statistically significant differences between groups (χ2 = 1661.333, P < 0.05). Five species of parasites were detected by the manual microscopy method and nine species of parasites were detected by the KU-F40 instrumental method, of which the detection levels of Clonorchis sinensis eggs, hookworm eggs and Blastocystis hominis were higher than those of the manual microscopy method, with statistically significant differences (P < 0.05). Although the detection levels of tapeworm eggs and Strongyloides stercoralis were also higher than the manual microscopy method, the differences were not statistically significant (P > 0.05). The KU-F40 fully automatic fecal analyzer has a higher sensitivity to parasite detection and holds significant clinical application value in the detection of fecal parasites. With the combination of manual re-examination, the accuracy of test results can be significantly improved.
Similar content being viewed by others
Background
Parasitic diseases are widespread and difficult to control in China due to its large population and vast land and unbalanced socioeconomic development across regions, which pose a serious threat to patients’ health and cause damage to the society and economy1. Stool examination is the gold standard for diagnosis and identification of intestinal parasitic infections2. Currently, a majority of the medical institutions still differentiate fecal parasite infections via manual microscopy, which is cumbersome to operate, low in detection level, high in biosecurity risk and prone to result in large discrepancies due to the subjective consciousness of the inspectors3. With the continuous development of automation and standardization of fecal testing and the increasing clinical demand, the KU-F40 fully automated fecal analyzer was introduced to our hospital in November 2023, provided by Zhuhai Keyu Biological Engineering Co., Ltd., China. In this study, we conducted a large-sample retrospective comparative analysis of the fecal test results using manual microscopy method from January to June 2023 and instrumental method from January to June 2024 in our hospital to explore the clinical application value of the KU-F40 in fecal parasite detection.
Materials and methods
Source of specimens
51,627 samples that conducted fecal analysis from January to June 2023 in the First Affiliated Hospital of Guangxi Medical University and 50,606 samples that conducted fecal analysis from January to June 2024 in our hospital.
Instruments and reagents
A KU-F40 fully automatic fecal analyzer with its corresponding sample collection cups and reagents from Zhuhai Keyu Biological Engineering Co., Ltd. were used. Saline at a concentration of 0.9% was used for manual microscopy with a LEICA biomicroscope.
Manual microscopy
The operational procedures were strictly adhered to the “National Clinical Laboratory Operating Procedures” (4th edition)4. One to two drops of saline were added to a sterile slide. Take a fresh fecal sample around match-head size (approximately 2 mg) with a wooden applicator stick and mix with the saline on the slide to prepare a uniform suspension. If the fecal sample contains abnormal components such as mucus, pus or blood, prioritize taking samples from the areas containing these abnormal components. The slide thickness ensured that newspaper print underneath was legible. A coverslip was then placed on top. Initially, a 10 × 10 low-power objective was used to observe the entire slide (more than 10 fields of view), followed by a 10 × 40 high-power objective to examine and identify suspected parasitic elements (more than 20 fields of view). All samples were tested within 2 h of collection.
KU-F40 fully automatic fecal analyzer
The KU-F40 fully automatic fecal analyzer employs the principle of fecal formed element image analysis. Using a soybean-sized (approximately 200 mg) fecal specimen collected in a clean, sterile container, the instrument automatically dilutes, mixes, filters, subsequently draws 2.3 ml of the diluted fecal sample into a flow counting chamber, and allows for precipitation over a certain period of time. Artificial intelligence is utilized to identify the types of parasites (eggs) and other formed elements through high-definition cameras. Suspected parasites (eggs) detection was then manually reviewed by laboratory personnel before outputting a report. All procedures were strictly adhered to the instrument’s SOP (Standard Operation Protocol). All samples were tested within 2 h of collection.
Statistical analysis
The results were compiled using Microsoft Excel and statistical analysis was conducted with SPSS 23.0. The detection level, which was a count data and either presented as a percentage [n (%)] and was analyzed using the chi-square (χ²) test. A P-value of less than 0.05 was considered statistically significant.
Results
Comparison of parasite detection level
In the first half of 2023, via manual microscopy, 1450 out of 51,627 cases of parasites (eggs) were detected, resulting in a detection level of 2.81%. While in the first half of 2024, using the KU-F40, 4424 out of 50,606 cases of parasites (eggs) were detected, yielding a detection level of 8.74%. The results indicate that the detection level of the KU-F40 instrumental method is higher than that of the manual microscopy method, with a statistically significant difference (χ² = 1661.333, P < 0.05) as shown in Table 1.
Comparison of parasite type detection
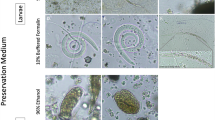
The manual microscopy method identified a total of 5 types of parasites (eggs), whereas the use of the KU-F40 instrumental method detected 9 types (Table 2; Fig. 1). Compared to the manual microscopy method, the KU-F40 instrumental method showed higher detection levels for Clonorchis sinensis eggs, hookworm eggs and Blastocystis hominis, with statistically significant differences (all P < 0.05).
Tornado diagram for parasite (egg) species detection levels by manual microscopy and KU-F40. Note: Due to the significant difference in detection levels between Clonorchis sinensis eggs and other parasites (eggs), an open section is used to represent the omitted portion of the actual detection level
.
Discussion
Global public health exigency in improving the sensitivity of parasite detection
Intestinal parasites reside in the human intestine. Among the wide variety of species, more than 300 types of parasites can be transmitted to human5, including but not limited to Clonorchis sinensis, hookworms, tapeworms, pinworms, whipworms, Ascaris lumbricoides, Entamoeba and Giardia lamblia. These parasites cause diseases through toxic effects, nutrient depletion from the host, mechanical damage and excessive immune activation6, leading to malnutrition, microecological imbalance, anemia, growth and development disorders and impaired intelligence in the host7. Such conditions severely compromise human health.
The results of the 3rd national survey in 2019 on the current major human parasitic diseases in China showed that the infection rate of C. sinensis in the population was 0.47%, with the most severe prevalence in Guangdong, Guangxi and the Northeast regions of China8, which corroborates the higher detection level of C. sinensis eggs found in this study. Located in the plains and river basins, Guangxi has a warm and humid climate, a prevalent custom of consuming raw fish, an increasing number of pet owners and issues related to food hygiene, all of which contribute to a higher risk of parasitic infection9.
Fecal parasite examination is the gold standard for the diagnosis and differential diagnosis of intestinal parasitic infections2. Medical institutions have traditionally relied on manual microscopy, which is time-consuming, poses high biosafety and cross-contamination risks, is prone to subjective judgment errors, has a low detection level and low work efficiency, and let alone the unpleasant odor that leads to resistance among testing personnel. Early fecal automatic instruments were plagued by immature technology, high failure rates, low recall rates (i.e., the proportion of true positive samples detected out of the total number of actual positive samples) for formed components (such as cells, parasites, crystals, fungi, etc.) and low detection levels10. Therefore, improving the detection level of parasites is not only an urgent need for the Guangxi region but also an urgent task for the global public health sector. The introduction of automatic fecal analyzers with high sensitivity, convenience, efficiency and compliance with biosafety standards is a future trend.
Significant clinical value of the KU-F40 in parasite detection
With the continuous development of automation, rapidity and standardization in hospital clinical laboratories, our hospital has introduced the KU-F40 fully automatic fecal analyzer. This study conducted a retrospective comparative analysis of the detection levels and types of fecal parasites using the manual microscopy method from January to June 2023 and the KU-F40 instrumental method from January to June 2024. The population included in this study was from the same season and hospital to improve data comparability and reduce the impact of seasonal and population differences on parasite detection levels11,12. The results showed that compared to the manual microscopy method, the KU-F40 instrumental method increased the sensitivity of parasite detection by 3.11 times. In the first half of 2024, a total of 9 types of parasites (eggs) were detected using the KU-F40 instrumental method, which was more than the 5 types detected by the manual microscopy method in the first half of 2023. In the early stage, our research group conducted a prospective study13 using the same set of specimens (n = 1030), which demonstrated that the normal mode of KU-F40 outperformed the manual microscopy method in terms of parasite detection level (16.3% vs. 13.1%, P < 0.05) and sensitivity (71.2% vs. 57.2%), and the normal mode of KU-F40 demonstrated superior concordance (90.78%, Kappa = 0.633, 95% CI 0.566-0.70) and diagnostic accuracy. Additionally, the preliminary research results have shown that the high specificity of the KU-F40 method was 94.7% in detecting parasites. And the dominant parasite in the Guangxi region—C. sinensis—in the same batch of samples, KU-F40 achieved an accuracy rate of 93.40%. Therefore, the KU-F40 has significant advantages in both the detection of fecal parasites (eggs) and the identification of species.
The KU-F40 fully automatic fecal analyzer effectively addresses the shortcomings of the traditional manual microscopy method, boasting prominent advantages such as compliance with biosafety standards, an ability to capture multi-field images and a high degree of automation and standardization. On one hand, the traditional manual microscopy method is prone to sample cross-contamination and poses a high risk of biosafety issues14,15. In contrast, the KU-F40 processes fecal specimens before, during and after testing in a completely enclosed environment, evidently improving biosafety. The KU-F40 instrument also features a well-designed pipeline system and an automatic cleaning function for the inner and outer walls of the sampling needle, which as much as possible eliminate the risk of sample cross-contamination.
On the other hand, due to the limitations of labor costs and testing timeliness, the manual microscopy method often cannot observe all fields of view in the fecal smears, and prolonged reading can lead to fatigue among the testing personnel, easily resulting in missed detection16. The KU-F40 is equipped with an automatic microscope and a high-definition camera, which can locate formed elements such as parasites (eggs) under low magnification and then automatically track and capture images with high magnification. Through the layered scanning imaging technology, the KU-F40 is capable of taking multiple images under different fields of view with high clarity. This allows the testing personnel to observe multiple fields of view, reducing the chance of missed detection and saving labor costs. Furthermore, KU-F40 significantly reduces analysis time. Manual microscopy typically requires 5–10 min per sample for completing parasite detection, for inexperienced operators, time will be longer. Whereas KU-F40 completes the automated analysis process in approximately 5 min per sample, including specimen handling, microscope photography, AI recognition, and analysis of parasites (eggs). The substantial increase in throughput is particularly advantageous for high-volume clinical laboratories.
Additionally, the manual microscopy method is often constrained by the testing personnel’s work experience, knowledge base, skill level and varying fields of view, leading to difficulties in standardizing and normalizing the operational process and significant subjective differences in test results17,18. In contrast, the KU-F40 features an automatic detection system and a standardized testing procedure, including a specific mode for detecting parasite eggs. It can perform artificial intelligence-based identification of parasite (egg) species from the captured images, reducing human error, enhancing the standardization level of testing and improving the sensitivity and accuracy of species identification for parasites (eggs).
Between May and June 2023, our research group collected 1030 fecal specimens from patients in our hospital and conducted a comparative study on parasite detection using the KU-F40 fully automatic fecal analyzer (normal mode and flotation-precipitation mode), acid-ether centrifugal sedimentation and manual microscopy. The study results indicated that the detection level of parasites using the KU-F40 normal mode was higher than that of the manual microscopy method, with increased sensitivity and specificity13. Based on these findings, this study further compared and analyzed the fecal test results from January to June 2023 and January to June 2024, before and after the implementation of the KU-F40. The results showed that, compared to the manual microscopy method, the KU-F40 instrumental method not only had a higher sensitivity for parasite detection but also identified a broader variety of parasite species. Integrating the results of both studies, KU-F40 demonstrated significant advantages over manual microscopy in terms of improving sensitivity in detecting parasites and increasing the diversity of detected parasite egg species.
Limitations and vision
While this study demonstrates the KU-F40’s superior detection capability in real-world clinical practice, several limitations warrant consideration. Most critically, this retrospective design fundamentally limited our ability to assess the instrument’s specificity – defined as its ability to correctly identify parasite-negative samples. This constraint stems from two interrelated factors: (1) the absence of prospective planning for false-positive quantification in historical clinical workflows, and (2) the lack of systematic retention of critical data required for such analysis, including detailed false-positive records, raw AI decision logs, and misclassified images. To illustrate this gap, we refer to findings from our research team’s prior prospective study13, wherein controlled evaluation of the same set of specimens (n = 1030) demonstrated robust for KU-F40’s specificity which was 94.7% in normal mode, with a 93.40% precision rate for C. sinensis egg recognition and the recall rate was 89.40%.
Nevertheless, despite this evidence of core performance, persistent challenges in parasite (egg) detection merit acknowledgment. For starters, the type of specimen can affect the detection level of parasite eggs. When the specimen is loose stool due to diarrhea, the instrument’s automatic dilution may affect the accuracy of the test results19. Furthermore, the instrument is susceptible to interference from food residue and the incomplete nature of the parasite (egg) image database13, which is easy to mistakenly identify certain pollen, starch granules and amorphous sediments with similar shapes to parasite eggs as eggs20, resulting in false positives. It is evident that laboratory personnel cannot rely entirely on the instrument’s report results. It is necessary to manually review the results of the specimens that are reported positive by the instrument and, if necessary, use the concentration method for re-examination to reduce false-negative and false-positive rates. Building on the advantages of the instrument, this ‘AI screening-manual microscopy confirmation’ hierarchical structure critically bridges the sensitivity-specificity trade-off, ensuring the reliability of diagnosis. Therefore, the core of continuously improving the performance of automatic fecal analysers lies in two aspects. First and foremost, it is crucial to improve the parasite (egg) image database and AI recognition capabilities to reduce false negatives and false positives at the source during the screening phase. Moreover, future advancements should incorporate common false positive examples as training and validation data, and implement a standardised error logging system to enable continuous performance monitoring and validation. These measures collectively form the key pathway to enhancing sensitivity in detecting parasite eggs.
Conclusion
In summary, the KU-F40 fully automatic fecal analyzer boasts high sensitivity in detecting parasites, high clarity in captured images and the ability to intelligently identify a variety of parasite egg types. With high work efficiency, it complies with biosafety standards and has a low risk of cross-contamination, meeting the clinical testing demands for large sample volumes. It has high clinical application value in the detection of fecal parasites. For positive or suspected positive specimens, laboratory personnel still need to perform manual confirmation or re-examination before issuing the test report.
Data availability
The datasets generated and analysed during the current study are not publicly available as the ethical approval from the hospital restricts the use of the data to this study only, but a sharing protocol will be worked out in consultation with the hospital if there is interest from researchers.
References
Wenhua, G. & Tingting, Y. Research progress on clinical laboratory examination methods for intestinal parasites. Trace Elem. Health Res. 41(5), 83–86 (2024).
Lee, Y. J. et al. Utility of an automatic Vision-Based examination system (AVE-562) for the detection of Clonorchis sinensis eggs in Stool. Ann. Lab. Med. 41(2), 221–224. https://doi.org/10.3343/alm.2021.41.2.221 (2021).
Zehong, Q. & Yanqin, W. Comparison of the application of automatic stool analyzer and manual microscopy in fecal specimen detection. Smart Health. 8(35), 20–23 (2022).
Hong, S., Yusan, W. & Ziyu, S. National Clinical Laboratory Testing Procedures. 4th Edition. Beijing: People’s Health Publishing House pp.175–177 (2015).
Król, G. et al. Toxicity of parasites and their unconventional use in medicine. Ann. Agric. Environ. Med. 26(4), 523–531. https://doi.org/10.26444/aaem/109665 (2019).
Meizhen, C. Analysis of stool parasite examination results in outpatient population and characteristics of parasitic infections . Med. Equip. 31(12), 50–51 (2018).
Boonyong, S. et al. High-throughput detection of parasites and Ova in stool using the fully automatic digital feces analyzer, orienter model fa280. Parasit. Vectors. 17(1), 13. https://doi.org/10.1186/s13071-023-06108-1 (2024).
Jiaxu, C. et al. Current status and challenges of prevention and control of important human parasitic diseases in China. Lab. Med. 36(10), 993–1000 (2021).
Tingting, Z., Yueyi, F. & Yingsi, L. Estimation of the disease burden of clonorchiasis in China and analysis of its trend. Chin. J. Schistosomiasis Control. 33(2), 162–168 (2021).
Wei, Y. & Jie, M. Performance evaluation of AVE-562 automatic fecal analyzer in fecal testing. Int. J. Lab. Med. 38(21), 3038–3040 (2017).
GL, L. et al. Analysis on clonorchiasis surveillance in Guangxi Zhuang autonomous region from 2016 to 2020. Trop. Dis. Parasitol. 19(03), 121–126 (2021).
Zhu, Y. Y. et al. Determinants of Clonorchis sinensis infection and subsequent treatment: a qualitative study in Guangxi, China. Acta Trop. 267, 107664. https://doi.org/10.1016/j.actatropica.2025.107664 (2025).
Wang, Z., Liao, L., Huang, X., Tang, J. & Lin, F. Evaluation of alarm notification of artificial intelligence in automated analyzer detection of parasites. Med. (Baltim). 103(39), e39788. https://doi.org/10.1097/MD.0000000000039788 (2024).
Mn, C. & Jinya, D. Application of AVE-562 automatic fecal analyzer in parasite detection. Lab. Med. Clin. 14(12), 1790–1791 (2017).
Cheng, W. et al. Clinical application evaluation of Wowent F280 automatic fecal analyzer. Contemp. Med. 28(8), 18–20 (2022).
Hualiang, C. et al. Application research of FA180 fully automatic fecal parasite (egg) identification system. Chin. J. Health Lab. Technol. 33(16), 1934–1936 (2023).
Shuang, D. et al. Analysis of the clinical application value of AVE-562 fully automatic fecal analyzer. Lab. Med. Clin. 14(17), 2599–2601 (2017).
Lichun, W. & Yong, L. Comparison and analysis of fecal test results between automatic fecal analyzer and manual review method. J. Practical Lab. Physicians. 16(1), 69–72 (2024).
Song, Y. et al. Clinical application evaluation of automated fecal analyzers. Beijing Med. 39(5), 540–541 (2017).
Yihong, D. et al. Comparison of test results between AVE-562 and KU-F10 automated fecal analyzers. Chin. Foreign Med. Res. 18(19), 82–84 (2020).
Acknowledgements
The authors would like to express their sincere gratitude to their colleagues and mentors for their guidance and strong support throughout this research. Their invaluable contributions have been instrumental in the completion of this work.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Chu Huang: Study design, case data organization, manuscript writing; Yunhua Huang: Data collection, figure creation, paper review; Faquan Lin: Data analysis and statistics, paper review; Lin Liao: Research guidance, paper review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical standards
The research protocol was approved by the Medical Ethics Committee of the First Affiliated Hospital of Guangxi Medical University (Approval Number: 2024-E0831). All methods were carried out in accordance with relevant guidelines and regulations.
Informed consent
Informed consent was obtained from all subjects or their legal guardians.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Huang, C., Huang, Y., Lin, F. et al. The clinical application value of the KU-F40 fully automatic fecal analyzer for the detection of fecal parasites: A large-sample retrospective study. Sci Rep 15, 24172 (2025). https://doi.org/10.1038/s41598-025-09935-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-09935-7