Abstract
Elevated serum IgG4 is a key diagnostic marker for type 1 autoimmune pancreatitis (AIP), but some patients lack IgG4 elevation, complicating diagnosis. This study investigated the clinicopathological features of AIP without elevated serum IgG4 levels. A total of 323 patients diagnosed with AIP at Hiroshima University Hospital and affiliated institutions were analyzed. Patients were categorized into IgG4-positive (≥ 135 mg/dL), IgG4-negative (< 135 mg/dL with “definite” or “probable” diagnosis), and possible (with “possible” diagnosis) groups. Comparative analysis was performed between the IgG4-positive (n = 281) and IgG4-negative (n = 20) groups. Segmental or focal narrowing of the main pancreatic duct (MPD) and retroperitoneal fibrosis were significantly more common in the IgG4-negative group (90.0% vs. 58.7%, P = 0.011; and 35.0% vs. 12.5%, P = 0.012, respectively). Although the number of pathological findings was comparable, the rate of surgical intervention was significantly higher in the IgG4-negative group (P < 0.001). No significant differences were observed in relapse rates or relapse sites between the two groups. These findings suggest that MPD narrowing and retroperitoneal fibrosis may be characteristic of AIP without elevated IgG4. Vigilant monitoring for relapse is warranted, regardless of serum IgG4 levels.
Similar content being viewed by others
Introduction
Type 1 autoimmune pancreatitis (AIP) is a pancreatic lesion of immunoglobulin G4 (IgG4)-related disease and is characterized by elevated serum IgG4 levels and significant infiltration of IgG4-positive plasma cells in pancreatic and extra-pancreatic lesions (such as sclerosing cholangitis, sclerosing dacryoadenitis and sialadenitis, retroperitoneal fibrosis, abdominal and hilar lymphadenopathy, chronic thyroiditis, and interstitial nephritis)1,2,3,4,5. The diagnosis of AIP is based on a combination of findings, including pancreatic enlargement, irregular narrowing of the main pancreatic duct (MPD), elevated serum IgG4 levels, pathological findings, extra-pancreatic lesions, and responsiveness to glucocorticoid therapy1. Among these findings, elevated serum IgG4 levels are an important parameter, exhibiting high sensitivity and specificity of 73–76% and 93–94%, respectively6,7,8. However, in some cases of AIP, serum IgG4 levels are not elevated, which poses diagnostic challenges9,10,11,12,13. In particular, cases showing focal enlargement of the pancreas without elevated serum IgG4 levels are often difficult to differentiate from pancreatic cancer, which may lead to unnecessary surgery11.
In this study, we retrospectively investigated the clinical characteristics of type 1 AIP patients without elevated serum IgG4 levels to diagnose AIP more accurately.
Methods
Patients
Patients diagnosed with type 1 AIP at Hiroshima University Hospital and its affiliated hospitals between April 2003 and December 2022 were enrolled in this study. All patients were diagnosed based on the Japanese Clinical Diagnostic Criteria for AIP 2018 (JPS 2018)14 and were classified into “definite diagnosis,” “probable diagnosis,” or “possible diagnosis.” The JPS 2018 criteria require characteristic imaging findings of the pancreas and the exclusion of other pancreatic diseases, including pancreatic cancer. Patients with primary sclerosing cholangitis (PSC), cholangiocarcinoma, or other pancreatobiliary diseases that could interfere with the diagnosis of AIP were excluded. In particular, in cases presenting with sclerosing cholangitis of the extra-pancreatic bile ducts, careful differentiation of IgG4-related sclerosing cholangitis from PSC and cholangiocarcinoma was essential. These differential diagnoses were thoroughly evaluated using ERCP or MRCP findings, with histopathological confirmation obtained when necessary. Image evaluations were conducted by gastroenterologists at each institution, all with over 15 years of experience in interpreting pancreatic imaging.
This study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of Hiroshima University (approval number: E-246). The need for informed consent was waived due to the retrospective nature of this study.
Study design
All patients were divided into two groups based on diagnostic criteria: those with a definite or probable diagnosis and those with a possible diagnosis (“possible group”). The possible diagnosis category included patients showing pancreatic enlargement, irregular narrowing of the MPD, and response to glucocorticoid therapy. Patients with a definite or probable diagnosis were further divided into two groups based on their serum IgG4 levels. A serum IgG4 level of ≥ 135 mg/dL was considered the threshold to define elevated IgG4 levels, in accordance with the JPS 2018. Patients with serum IgG4 levels ≥ 135 mg/dL were categorized as the “IgG4-positive group,” and those with serum IgG4 levels < 135 mg/dL were categorized as the “IgG4-negative group.” Clinicopathological characteristics at diagnosis, relapse rates, and sites were compared between the IgG4-positive and IgG4-negative groups. The response to glucocorticoid therapy was determined using computed tomography, endoscopic retrograde cholangiopancreatography, or magnetic resonance cholangiopancreatography to evaluate improvements in pancreatic enlargement and narrowing of the MPD. Relapse was defined as re-swelling of the pancreas or re-narrowing of the MPD; development or reappearance of extra-pancreatic lesions, such as extra-pancreatic sclerosing cholangitis, retroperitoneal fibrosis, sclerosing dacryoadenitis/sialadenitis; or the appearance of renal lesions on imaging studies, regardless of serum IgG4 levels. Sclerosing cholangitis was evaluated using ERCP or MRCP. Other extra-pancreatic lesions were evaluated using computed tomography.
Outcomes
The primary endpoint was the clinicopathological difference between the IgG4-positive and IgG4-negative groups. The following clinical variables were analyzed: age, sex, serological findings at diagnosis (serum IgG level, serum IgG4 level, antinuclear antibody positivity, and rheumatoid factor positivity), pancreatic enlargement, irregular narrowing of the MPD, extra-pancreatic lesions (extra-pancreatic sclerosing cholangitis, sclerosing dacryoadenitis/sialadenitis, retroperitoneal fibrosis, and renal lesions), and glucocorticoid therapy. Pathological findings included the number of positive results among the following four features obtained by endoscopic ultrasound-guided fine needle aspiration (EUS-FNA), percutaneous pancreatic biopsy, and surgical resection: prominent infiltration of lymphocytes and plasma cells along with fibrosis, more than 10 IgG4-positive plasma cells per high-power microscopic field, storiform fibrosis, and obliterative phlebitis.
The secondary endpoint was the cumulative relapse rate for all patients in the two groups, including those who received glucocorticoid therapy.
Statistical analyses
Statistical analyses were performed using JMP Pro 16.0.0 (SAS Institute Inc., Cary, North Carolina, USA). Continuous variables were compared using the Wilcoxon rank-sum test, and categorical variables were compared using the chi-square test or Fisher’s exact test. Multivariate logistic regression analyses were performed to identify clinical features independently associated with IgG4-negative status. Parameters with a p value < 0.05 in the univariate analysis were included in the multivariate analysis. Relapse rates were calculated using the Kaplan–Meier method, and the log-rank test was used for comparison. Univariate Cox proportional hazards regression analyses were performed to identify significant factors associated with relapse in patients without elevated serum IgG4 levels. The cutoff value for each quantitative variable was determined using the Youden index derived from the receiver operating characteristic (ROC) curve. P values < 0.05 were considered statistically significant.
Results
Patient characteristics and grouping based on serum IgG4 levels
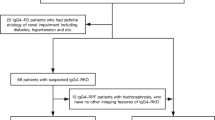
In total, 323 patients with type 1 AIP were enrolled in this study. The clinical characteristics of the 323 patients are presented in Table 1. The median serum IgG4 level was 351 mg/dL (interquartile range [IQR], 174–651), and elevated serum IgG4 levels (≥ 135 mg/dL) were observed in 281 (87.0%) patients. The diagnosis, according to the JPS 2018, was “definite” in 281 (87.0%) patients, “probable” in 20 (6.2%), and “possible” in 22 (6.8%). Figure 1 illustrates the grouping process based on diagnostic criteria and serum IgG4 levels. Initially, all patients were categorized into the following two groups: 301 patients with a “definite” or “probable” diagnosis according to the JPS 2018 criteria (93.2%) and 22 patients with a “possible” diagnosis (possible group, 6.8%). The 301 patients diagnosed as definite or probable were further divided into two groups based on their serum IgG4 levels. 281 patients with serum IgG4 levels ≥ 135 mg/dL were classified as the IgG4-positive group, and 20 patients with serum IgG4 levels < 135 mg/dL were categorized into the IgG4-negative group.
Comparison of clinical characteristics
Table 1 presents the comparison of clinical characteristics between the IgG4-positive and IgG4-negative groups. The median serum IgG4 level was 407 mg/dL (IQR, 257–704) in the IgG4-positive group and 77 mg/dL (IQR, 36–110) in the IgG4-negative group (P < 0.001). The incidence of jaundice was significantly higher in the IgG4-positive group than in the IgG4-negative group (29.5% vs. 5.0%, P = 0.018). The incidence of abdominal pain did not differ significantly between the two groups. The extent of irregular narrowing of the MPD differed significantly between the two groups (P = 0.011); segmental or focal narrowing of the MPD was significantly more common in the IgG4-negative group than in the IgG4-positive group (90.0% vs. 58.7%). Although the extent of pancreatic enlargement did not differ significantly between the two groups, the IgG4-negative group tended to show segmental or focal pancreatic enlargement more frequently than the IgG4-positive group (75.0% vs. 52.0%, P = 0.062). The complication rate of extra-pancreatic lesions did not differ significantly between the two groups (P = 0.086). Sclerosing dacryoadenitis/sialadenitis was observed in 42 (14.9%) patients in the IgG4-positive group but was not observed in any of the patients in the IgG4-negative group. In contrast, retroperitoneal fibrosis was significantly more common in the IgG4-negative group than in the IgG4-positive group (35.0% vs. 12.5%, P = 0.012). The rate of steroid therapy initiation was significantly lower in the IgG4-negative group than in the IgG4-positive group (65.0% vs. 87.9%, P = 0.010). Multivariate logistic regression analysis revealed that the absence of jaundice (OR = 7.092, 95% CI: 1.400–129.4, p = 0.013), segmental/focal irregular narrowing of the MPD (OR = 6.208, 95% CI: 1.714–39.89, p = 0.003), and retroperitoneal fibrosis (OR = 3.438, 95% CI: 1.181–9.406, p = 0.025) were independently associated with IgG4-negative AIP.
Comparison of pathological findings
A comparison of the pathological findings between the two groups is shown in Table 2. Pathological examinations were performed in 203 (72.2%) patients in the IgG4-positive group and 17 (85.0%) in the IgG4-negative group. The number of patients who underwent EUS-FNA was 194 (69.0%) in the IgG4-positive group and 13 (65.0%) in the IgG4-negative group, with no significant difference (P = 0.803). The number of pathological findings also did not differ significantly between the two groups. A total of 21 patients (7.5%) in the IgG4-positive group and two (10.0%) in the IgG4-negative group underwent percutaneous pancreatic biopsy, with no significant differences between the groups (P = 0.382). Furthermore, no significant differences were observed in the pathological findings between the two groups. The number of patients undergoing surgery was significantly higher in the IgG4-negative group than in the IgG4-positive group (8 (2.8%) vs. 7 (35.0%), P < 0.001). Among the 15 patients who underwent surgery in both groups, 14 (93.3%) had focal pancreatic enlargement or focal narrowing of the MPD. Three or more findings were observed in all eight patients in the IgG4-positive group who underwent surgery. Of the seven patients in the IgG4-negative group who underwent surgery, two had only two findings, and five had three or more findings.
Relapse rates and sites in the two groups
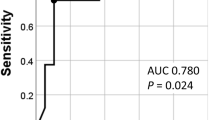
A comparison of the relapse rates and sites between the two groups is shown in Table 3. The overall relapse rates were 33.5% (94/281) in the IgG4-positive group and 30.0% (6/20) in the IgG4-negative group, with no significant differences between the groups (P = 0.358). In addition, the relapse rates did not differ significantly, regardless of whether glucocorticoid therapy was administered. The cumulative relapse rate did not differ between the two groups, both in all patients and in those who received glucocorticoid therapy (log-rank, P = 0.603 and 0.540, respectively) (Fig. 2). The site of relapse did not differ between the two groups.
Clinical profile of the possible group
The clinical profile of the possible group is shown in Table 4. Extra-pancreatic lesions were absent in all cases. Although pathological examinations were conducted in 17 cases (77.3%), none met more than two diagnostic criteria. Glucocorticoid therapy was initiated in all cases for both diagnostic and therapeutic purposes. The relapse rate in the possible group was 18.2% (4/22).
Relapse predictors in patients without elevated serum IgG4 levels
The results of univariate analyses, which were performed to identify the predictors of relapse in patients without elevated serum IgG4 levels (IgG4-negative and possible groups), are shown in Table 5. Since no variables showed a statistically significant association in the univariate analysis, multivariate analysis was not performed.
Discussion
The serum IgG4 level is a useful parameter for diagnosing AIP. However, cases of AIP without elevated serum IgG4 levels are also frequently observed, making diagnosis difficult. Similar to the present study, four other studies have compared cases of AIP with and without elevated serum IgG4 levels10,11,12,13. In this study, jaundice was significantly more prevalent in the IgG4-positive group, which was also reported in a previous study12. Additionally, the IgG4-negative group showed significantly more frequent segmental or focal irregular narrowing of the MPD and a tendency toward more segmental or focal enlargement of the pancreas, as reported in previous studies12. These results suggest a possible correlation between serum IgG4 levels and the extent of pancreatic inflammation. Furthermore, in this study, the IgG4-negative group included a higher proportion of patients who underwent surgical resection, and a similar finding was noted in a previous report11,13. In this study, most of the patients who underwent surgery showed focal pancreatic enlargement or focal narrowing of the MPD, suggesting that surgery was performed due to difficulty distinguishing these cases from pancreatic cancer. In particular, in cases with negative serum IgG4 levels, differentiation from pancreatic cancer may be even more challenging. In the histopathological analysis, cases fulfilling two or more diagnostic criteria were significantly more frequent in the IgG4-negative group, which is likely attributable to the higher proportion of surgical cases in this group. However, characteristic imaging findings of AIP, such as a capsule-like rim and delayed enhancement of the pancreatic parenchyma, may help distinguish it from pancreatic cancer. Nishino et al. reported that characteristic ERCP findings of AIP, compared to pancreatic cancer, include a longer MPD narrowing, the presence of branch ducts within the narrowed segment, and a smaller upstream MPD diameter15. Sekito et al. reported that the apparent diffusion coefficient (ADC) value of localized AIP was significantly lower than that of pancreatic ductal adenocarcinoma16. To avoid unnecessary surgery, the diagnosis should be made cautiously, considering not only these imaging findings but also extra-pancreatic lesions, pathological findings, and the response to glucocorticoid therapy.
Regarding extra-pancreatic lesions, our findings differ from previous reports10,11,12,13, which described a lower frequency in the IgG4-negative group. In contrast, our study demonstrated a higher frequency of extra-pancreatic lesions in the IgG4-negative group, particularly retroperitoneal fibrosis. This difference may be due to the changes in patient selection and diagnostic criteria over time. Kamisawa et al.10 diagnosed AIP based on pancreatic enlargement, irregular narrowing of the MPD, histologically confirmed lymphoplasmacytic sclerosing pancreatitis (LPSP), or steroid responsiveness, and included possible cases in the IgG4-negative group. Matsubayashi et al. used the Asian criteria17, which do not include extra-pancreatic lesions as part of the diagnostic criteria. Paik et al.11 applied the International Consensus Diagnostic Criteria 2011 (ICDC 2011)1, which incorporate extra-pancreatic involvement; however, they adopted their own criteria, focusing on serum IgG4 levels and histopathological findings. Therefore, IgG4-negative cases with extra-pancreatic lesions may have been excluded. Sano et al. also applied the ICDC 20111, but included AIP-NOS (Not Otherwise Specified) cases—corresponding to the possible group in our study—in the IgG4-negative category, which may have contributed to discrepancies in the observed results. In our study, AIP was diagnosed using the JPS 2018 criteria14, which includes extra-pancreatic lesions as part of the diagnostic criteria. These findings imply that extra-pancreatic lesions, especially retroperitoneal fibrosis, may be important in the diagnosis of AIP without elevated serum IgG4 levels.
Several studies have reported an association between IgG4 and retroperitoneal fibrosis. Hamano et al.18 reported no difference in serum IgG4 levels between patients with AIP with retroperitoneal fibrosis and those without. In contrast, Wallace et al.19 reported that in IgG4-related diseases, the group with retroperitoneal fibrosis had significantly lower serum IgG4 levels, noting that among 23 patients with retroperitoneal fibrosis, 85% were diagnosed via biopsy but had normal serum IgG4 levels. In addition, Liao et al.20 reported that among 16 cases of retroperitoneal fibrosis due to IgG4-related diseases, only five (31%) showed elevated serum IgG4 levels. They highlighted a strong correlation between IgG4 and IgE levels, suggesting that when serum IgG4 levels are normal, IgE could be useful for diagnosis and assessment of activity and relapse20. The reason serum IgG4 levels are rarely elevated in cases of AIP with retroperitoneal fibrosis is currently unknown, and further investigation is needed as more data are accumulated.
In our study, sclerosing dacryoadenitis and sialadenitis were only observed in patients with elevated serum IgG4 levels. Hamano et al.18 and Kuruma et al.21 reported that patients with AIP, sclerosing dacryoadenitis, and sialadenitis had significantly higher serum IgG4 levels than those without these conditions. Additionally, Kuruma et al.21 reported a significantly higher relapse rate in AIP complicated by sclerosing dacryoadenitis and sialadenitis compared to AIP without these complications. Similarly, Ishii et al.22 reported that sclerosing dacryoadenitis and sialadenitis were predictive factors for AIP relapse. Considering these previous reports and the results of the present study, serum IgG4 levels appear to be closely associated with disease activity and are often elevated in patients with sclerosing dacryoadenitis and sialadenitis.
In this study, the prevalence of extra-pancreatic lesions in the IgG4-positive group—or in the overall cohort—was lower than that reported by Sano et al.13 and Masamune et al.23. However, in these previous studies, sclerosing cholangitis involving the intra-pancreatic bile duct was also classified as an extra-pancreatic lesion, accounting for 33.4% and 40.9%, respectively. In contrast, the JPS 2018 criteria14 adopted in our study do not consider intra-pancreatic sclerosing cholangitis as an extra-pancreatic lesion. Therefore, the apparent discrepancy in the frequency of extra-pancreatic lesions may be attributed to differences in classification rather than a true difference in prevalence. Furthermore, in cases of asymptomatic sclerosing dacryoadenitis and sialadenitis or pulmonary lesions, the absence of head, neck, or chest imaging may have resulted in an underestimation of the frequency of extra-pancreatic lesions.
Although the usefulness of EUS-FNA in the histological diagnosis of AIP has been reported24, no established consensus exists regarding the histopathological findings that reflect the relationship between serum IgG4 levels and the number of IgG4-positive plasma cells within pancreatic lesions. Our study also could not draw definitive conclusions regarding this relationship. Previous studies have reported that the number of IgG4-positive plasma cells per high-power field tends to be higher in patients with serum IgG4-positive AIP11,13. However, other reports have indicated that among 160 patients with suspected LPSP, 60 (37%) showed no elevation in serum IgG4 levels25. In addition, several case reports have demonstrated the presence of IgG4-positive plasma cells in the pancreas, salivary glands, and liver tissue, even without elevated serum IgG4 levels26,27. Therefore, when AIP is suspected based on imaging findings, obtaining tissue for pathological examination using EUS-FNA remains crucial, regardless of serum IgG4 levels.
In this study, no significant difference in relapse rates was observed between the sIgG4-positive and sIgG4-negative groups. This finding contrasts with previous studies12,13, and several factors may explain the discrepancy. First, differences in patient composition may be relevant. In the study by Sano et al., the frequency of extra-pancreatic lesions was lower in the sIgG4-negative group. In contrast, in our study, extra-pancreatic involvement—including retroperitoneal fibrosis—was more common in the sIgG4-negative group, suggesting the presence of cases with higher disease activity. The inclusion of AIP-NOS cases in the sIgG4-negative group in the study by Sano et al.13 may also have influenced their results. Second, differences in follow-up duration may have influenced the relapse rates. In addition, variations in tapering speed and discontinuation criteria for glucocorticoid therapy among institutions may have also contributed to differences in relapse outcomes. Relapse may have occurred during long-term observation, even in the sIgG4-negative group. Even in the possible group, some patients experienced relapse or showed increased serum IgG4 levels during the disease course. Fluctuations in serum IgG4 levels have been reported to be associated with disease activity28, suggesting that diagnosis may have occurred during a phase of relatively low disease activity. These findings suggest that the serum IgG4 level at the time of diagnosis alone may not accurately reflect disease activity or relapse risk. A comprehensive assessment that considers the long-term clinical course is essential regardless of IgG4 status.
This study had some limitations. First, this was a retrospective study, and there was a substantial difference in the number of cases between the sIgG4-positive and sIgG4-negative groups. While this imbalance is to some extent unavoidable, as sIgG4-negative AIP represents a minority among all AIP cases, the small absolute number of patients in the IgG4-negative group may have affected the generalizability and interpretation of the results. Second, the data used in this study were restricted to individuals of Japanese descent; therefore, conclusions regarding other racial or ethnic groups should be drawn with caution.
Conclusions
Type 1 AIP without elevated serum IgG4 levels is characterized by a high incidence of segmental or focal irregular narrowing of the MPD, pancreatic enlargement, and retroperitoneal fibrosis. To avoid misdiagnosis of pancreatic tumors and unnecessary surgery, these findings should be taken into consideration, and pathological examinations should be performed to obtain an appropriate diagnosis. Additionally, patients diagnosed as “possible” may require careful follow-up, similar to other AIP patients.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Shimosegawa, T. et al. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the international association of pancreatology. Pancreas 40, 352–358 (2011).
Hamano, H. et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl. J. Med. 344, 732–738 (2001).
Yoshida, K. et al. Chronic pancreatitis caused by an autoimmune abnormality: proposal of the concept of autoimmune pancreatitis. Dig. Dis. Sci. 40, 1561–1568 (1995).
Okazaki, K. & Chiba, T. Autoimmune related pancreatitis. Gut 51, 1–4 (2002).
Umehara, H. et al. A novel clinical entity, IgG4-related disease (IgG4RD): general concept and details. Mod. Rheumatol. 22, 1–14 (2012).
Lee, S. C., Yang, C. H., Chang, C. T. & Yu, K. H. Diagnostic utility of serum IgG4 in autoimmune pancreatitis: an updated comprehensive systematic review and meta-analysis. J. Clin. Gastroenterol. 56, 810–817 (2022).
Ghazale, A. et al. Value of serum IgG4 in the diagnosis of autoimmune pancreatitis and in distinguishing it from pancreatic cancer. Am. J. Gastroenterol. 102, 1646–1653 (2007).
Choi, E. K. et al. The sensitivity and specificity of serum Immunoglobulin G and Immunoglobulin G4 levels in the diagnosis of autoimmune chronic pancreatitis: Korean experience. Pancreas 35, 156–161 (2007).
Kamisawa, T., Okamoto, A. & Funata, N. Clinicopathological features of autoimmune pancreatitis in relation to elevation of serum IgG4. Pancreas 31, 28–31 (2005).
Kamisawa, T. et al. Serum IgG4-negative autoimmune pancreatitis. J. Gastroenterol. 46, 108–116 (2011).
Paik, W. H. et al. Clinical and pathological differences between serum Immunoglobulin G4-positive and -negative type 1 autoimmune pancreatitis. World J. Gastroenterol. 19, 4031–4038 (2013).
Matsubayashi, H. et al. Characteristics of autoimmune pancreatitis based on serum IgG4 level. Dig. Liver Dis. 43, 731–735 (2011).
Sano, T. et al. Serum IgG4-negative and IgG4-positive type 1 autoimmune pancreatitis present with different clinicopathological features: an analysis of a nationwide survey in Japan. Pancreatology 25, 82–88 (2025).
Kawa, S. et al. Japanese clinical diagnostic criteria for autoimmune pancreatitis, 2018: revision of Japanese clinical diagnostic criteria for autoimmune pancreatitis, 2011. Pancreas 49, e13–e14 (2020).
Nishino, T., Oyama, H., Toki, F. & Shiratori, K. Differentiation between autoimmune pancreatitis and pancreatic carcinoma based on endoscopic retrograde cholangiopancreatography findings. J. Gastroenterol. 45, 988–996 (2010).
Sekito, T. et al. The role of apparent diffusion coefficient value in the diagnosis of localized type 1 autoimmune pancreatitis: differentiation from pancreatic ductal adenocarcinoma and evaluation of response to steroids. Abdom. Radiol. (NY). 46, 2014–2024 (2021).
Otsuki, M. et al. Asian diagnostic criteria for autoimmune pancreatitis: consensus of the Japan-Korea symposium on autoimmune pancreatitis. J. Gastroenterol. 43, 403–408 (2008).
Hamano, H. et al. Prevalence and distribution of extra-pancreatic lesions complicating autoimmune pancreatitis. J. Gastroenterol. 41, 1197–1205 (2006).
Wallace, Z. S. et al. IgG4-related disease: clinical and laboratory features in one hundred twenty-five patients. Arthritis Rheumatol. 67, 2466–2475 (2015).
Liao, S. et al. Comparison of two subsets of Chinese patients with retroperitoneal fibrosis in terms of IgG4 immunohistochemical staining. Rheumatol. (Oxf Engl). 58, 455–462 (2019).
Kuruma, S. et al. Clinical characteristics of patients with autoimmune pancreatitis with or without mikulicz’s disease and mikulicz’s disease alone. Gut Liver. 7, 96–99 (2013).
Ishii, Y. et al. Impact of sclerosing dacryoadenitis/sialadenitis on relapse during steroid therapy in patients with type 1 autoimmune pancreatitis. Scand. J. Gastroenterol. 54, 259–264 (2019).
Masamune, A. et al. Nationwide epidemiological survey of autoimmune pancreatitis in Japan in 2016. J. Gastroenterol. 55, 462–470 (2020).
Kanno, A. et al. Diagnosis of autoimmune pancreatitis by EUS-guided FNA using a 22-gauge needle: A prospective multicenter study. Gastrointest. Endosc. 84, 797–804e1 (2016).
Kamisawa, T. et al. Clinical profile of autoimmune pancreatitis and its histological subtypes: an international multicenter survey. Pancreas 40, 809–814 (2011).
Zhang, M. M. et al. Contrast enhanced ultrasonography in the diagnosis of IgG4-negative autoimmune pancreatitis: A case report. J. Interv Gastroenterol. 1, 182–184 (2011).
Hideshima, K. et al. IgG4-related hepatic inflammatory pseudotumor in a patient with serum IgG4-negative type 1 autoimmune pancreatitis. Clin. J. Gastroenterol. 16, 895–900 (2023).
Tabata, T. et al. Serial changes of elevated serum IgG4 levels in IgG4-related systemic disease. Intern. Med. 50, 69–75 (2011).
Acknowledgements
We would like to thank all the participants in this study and the medical staff at the participating institutions for their invaluable contributions to this research. Ethical approval for this study was granted by the ethics committee of Hiroshima University (approval number: E-246). Finally, we would like to acknowledge Professor Shiro Oka for his helpful feedback and suggestions during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Yumiko Yamashita conducted data analysis and authored the manuscript, while Yasutaka Ishii designed the study, contributed to data analysis and interpretation, and assisted in manuscript preparation. Keiji Hanada, Tamito Sasaki, Yoshifumi Fujimoto, Atsushi Yamaguchi, Ken Hirao, Masahiro Serikawa, Bunjiro Noma, Tomoyuki Minami, Akihito Okazaki, Masanobu Yukutake, Teruo Mouri, Yumiko Tatsukawa, Shinya Nakamura and Juri Ikemoto contributed to data collection. Shiro Oka critically reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All procedures were performed in accordance with the ethical standards outlined in the 1964 Declaration of Helsinki and its amendments, and the study was approved by the ethics committee of Hiroshima University (approval number: E-246).
Informed consent
The requirement for informed consent was waived due to the retrospective nature of this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yamashita, Y., Ishii, Y., Hanada, K. et al. Clinicopathological features of type 1 autoimmune pancreatitis without elevated serum IgG4 level. Sci Rep 15, 24518 (2025). https://doi.org/10.1038/s41598-025-10478-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-10478-0