Abstract
IgA nephropathy (IgAN) is a common glomerular disease in adults with a smoldering course and high risk of progression to end-stage kidney disease (ESKD) in South Asians. We investigated serum IgA/C3 ratio as a potential biomarker for IgAN. We measured serum levels of IgA and C3 in 258 patients with IgAN and 90 controls with non-IgAN primary glomerular disease and examined if serum IgA/C3 ratio differentiates IgAN from other glomerular diseases and if it predicts renal survival in IgAN. The primary outcome was lack of renal survival, defined as irreversible decline in eGFR > 50% from baseline or progression to ESKD. Median serum IgA/C3 ratio was higher in IgAN patients compared to controls (2.4, IQR: 1.9-3.0 vs. 1.8, IQR: 1.3–2.5, p < 0.001). The AUC for the receiver operating curve of IgA/C3 ratio was 0.6760 (95% CI: 0.6074–0.7446). The sensitivity and specificity of IgA/C3 ratio > 2.0 were 70.5% and 62.2% respectively, for differentiating IgAN from other non-IgAN glomerular diseases. With a median duration of follow-up of 35.0(IQR 16-56.8) months, 26.7% patients reached the primary outcome. Compared to patients with a low IgA/C3 ratio(≤ 2.0), those with a high ratio(> 2.0) were significantly older [median age 34 vs. 29 years, p = 0.003], more likely to have hypertension (70.6% vs. 50.5%, p = 0.001), had lower median eGFR [47.7 mL/min/1.73 m² vs. 77.7 mL/min/1.73 m², p < 0.001], lower urine protein creatinine ratio [2.0 g/g vs. 2.5 g/g, p = 0.015] and a significantly higher proportion of segmental glomerulosclerosis (S1 lesions) (80.4% vs. 62.1%, p = 0.005). Renal disease progression was comparable (26.3% vs. 27.0%, p = 0.906) between the low and high IgA/C3 ratio groups respectively. High IgA/C3 ratio (> 2.0) was not a significant predictor of primary outcome (HR = 1.32 95% CI: 0.80–2.2, p = 0.278). Serum IgA/C3 ratio is elevated in IgAN compared to other glomerular diseases but has limited diagnostic and prognostic utility in our patients.
Similar content being viewed by others
Introduction
IgA nephropathy (IgAN) is a common primary glomerular disease in adults and a leading cause of chronic kidney disease(CKD). The pathogenesis is linked to the aberrant glycosylation of O-linked glycans in the hinge region of immunoglobulin A1. This results in elevated levels of galactose-deficient IgA1 molecules (Gd-IgA1), which are recognized by glycan-specific IgA and IgG. The kidney is the primary target organ for damage due to the deposition of Gd-IgA1-IgA complexes1,2. In the Indian population, IgAN is reported to have a more aggressive clinical course with poor renal survival3,4.
Clinical presentation varies from asymptomatic microscopic hematuria, sub-nephrotic proteinuria, nephrotic range proteinuria to rapidly progressive renal dysfunction1. Definite diagnosis requires kidney biopsy, an invasive investigation which patients are often reluctant to undergo, especially when they have mild disease. Prognosis is also variable and the outcome difficult to predict with accuracy in individual patients. Baseline parameters like severity of hypertension, proteinuria, estimated glomerular filtration rate (eGFR) and histological features are conventionally used to predict long term outcomes. The International IgA nephropathy network prediction tool can be used for prognostication but not for deciding treatment5. Unfortunately, it underestimates renal survival in Indian patients4.
Multiple studies have evaluated a range of biomarkers to facilitate non-invasive diagnosis of IgAN and for prognostication and treatment, though none of them are currently recommended for use in routine clinical practice6,7. Some studies have examined the role of the serum IgA/C3 ratio as a non-invasive diagnostic marker of IgAN8,9,10,11. IgA/C3 ratio has also been reported to be useful in predicting outcomes in these patients11,12,13,14,15,16 and may co-relate with disease histology17,18. Both serum IgA and C3 assays are widely accessible in clinical practice. Given the aggressive clinical course of IgAN in the Indian population, and considering most studies investigating the utility of serum IgA and C3 have been conducted in non-Indian populations, we aimed to evaluate the diagnostic and prognostic relevance of these biomarkers in Indian patients.
Method
We compared the baseline serum IgA/C3 ratio measured at time of kidney biopsy, between adult IgAN patients (cases) and non-IgA glomerular disease(controls).
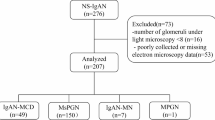
We included 258 patients with biopsy-proven IgAN, diagnosed between 2012 and 2022 at our centre. For the control group, we selected 90 patients with non-IgAN glomerular diseases, including membranous nephropathy-MN(n = 49) and primary focal segmental glomerulosclerosis (FSGS) and minimal change disease (MCD) patients(n = 41, FSGS:21, MCD:20).
Patients with a documented history of immunosuppressive therapy prior to kidney biopsy were excluded from the study. We also excluded patients with secondary causes of IgAN such as chronic liver disease, Henoch Schoenlein Purpura, second coexisting disease on kidney biopsy such as diabetic nephropathy, systemic disease like diabetes or malignancy which can affect kidney function, inadequate or missing clinical records and < 6 months follow-up unless they reached the primary endpoint within 6 months from biopsy.
The serum IgA and C3 levels measured at time of kidney biopsy were recorded. If these reports were not available, serum IgA and C3 levels were measured for both cases and controls in sera collected at the time of kidney biopsy and stored in our biorepository at −80℃.
Complement C3 level was measured by immunoturbidimetric method using Cobas c 702. The measuring range is 0.04 to 5.0 g/L (4‑500 mg/dL). The lower detection limit of the assay corresponds to 0.04 g/L (4 mg/dL). Human C3c forms a precipitate with a specific antiserum which is determined turbidimetrically. Serum and lithium heparin plasma are acceptable sample types for this assay; serum samples were used in this study.
Serum IgA level was measured by immunoturbidimetric method using Cobas c 702. The measuring range is 0.50 to 8.00 g/L (3.13‑50 µmol/L, 50‑800 mg/dL). The lower detection limit of the assay corresponds to 0.05 g/L (0.31 µmol/L, 5 mg/dL). Anti‑IgA antibodies react with antigen in the sample to form an antigen/antibody complex. Following agglutination, this is measured turbidimetrically. Samples used are serum and lithium heparin plasma and K2‑EDTA plasma.
Details of laboratory investigations i.e. serum creatinine, serum albumin, and urinary protein creatinine ratio (UPCR) and treatment prescribed i.e. angiotensin converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARBs) as well as immunosuppression were noted from outpatient records. The estimated glomerular filtration rate(eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD- EPI) equation. The MEST-C score of IgA nephropathy was recorded from biopsy reports.
We defined our primary endpoint (renal disease progression) as > 50% reduction in eGFR from baseline or progression to end stage kidney disease(ESKD) requiring dialysis or transplant.
The study was approved by the Institute Ethics Committee at All India Institute of Medical Sciences, New Delhi(IECPG-464) and conducted according to the declaration of Helsinki. Informed consent was taken for patients recruited prospectively while waiver of consent had been granted for samples tested retrospectively from the biorepository.
Statistical analysis
We conducted our analysis using Stata 14.0 (College Station, Texas, USA). Data were summarized as frequency (%) or median (Inter quartile range). A p-value of < 0.05 was taken as statistically significant. The Chi square test was used to compare the categorical variables between the groups. The Wilcoxon rank sum test or the t test were used to compare continuous variables between two groups depending on the normality of their distribution in the population. The receiver operating characteristic curve for serum IgA/C3 level was constructed to evaluate the diagnostic performance of the serum IgA/C3 ratio. The clinical and laboratory profiles were compared between the low and high IgA/C3 ratio subgroups. Kaplan meier survival analysis was done to compare renal survival (defined as lack of renal disease progression) between the two subgroups. Cox regression analysis was done to identify predictors of renal disease progression. Spearman co-relation test was used to investigate collinearity between baseline proteinuria and IgA/C3 ratio.
Results
The baseline parameters of the cases and controls are provided in Table 1.
Clinical characteristics of patients and controls
The IgAN cohort had a median age of 32(25–40) years, with 73.3% being male. Their baseline median eGFR was 54.8(34.9–98.2) mL/min/1.73m2 and urine protein creatinine ratio(UPCR) was 2.5(1.2–3.4) g/g. In the control group, median age was 33.5(23–46) years, with 60% males. Their baseline median eGFR was 99.1(61.4-124.9) mL/min/1.73m2 and UPCR was 5(2.9-8.0) g/g.
Histological characteristics
Based on the Oxford classification of IgAN, 220(85.3%) of patients had mesangial hypercellularity(M1), 18(6.2%)had endocapillary hypercellularity(E1), 190(73.6%) had segmental glomerular sclerosis (S1), 109(42.2%) had tubular atrophy/interstitial fibrosis (T1/T2) and 40(16.1%) had crescents (C1/C2).
IgA/C3 Ratio
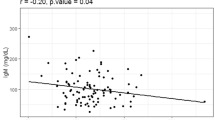
The median serum IgA level of the IgAN cases was 316.6 (249.0-392.1) mg/dL and serum C3 level was 136.00 (115.2-154.6) mg/dL. For the control group, the median serum IgA was 248.2 (180.3-347.3) mg/dL and serum C3 level was 145.3 (121.7–169.0) mg/dL. Median IgA/C3 ratio was higher in IgAN patients (2.4, IQR:1.9-3.0) compared to controls (1.80, IQR: 1.3–2.5), p < 0.001, Fig. 1. The median IgA/C3 ratio of patients with IgAN, MCD/FDGS and MN are shown in supplementary Fig. 1.
On plotting a Receiver Operating Characteristic (ROC) curve of the baseline IgA/C3 ratio for the cases and controls we got a curve with AUC of 0.6760 (95% CI: 0.6074–0.7446), Fig. 2. The sensitivity and specificity of IgA/C3 ratio > 2.0 for differentiating IgAN from other non-IgAN glomerular diseases were 70.5% and 62.2% respectively. The cut-off of > 2.0 was used as it gave the optimal sensitivity and specificity which is essential for an effective diagnostic biomarker. If we used a higher threshold of > 2.4 based on its median value in the IgAN cohort(Table 1) the sensitivity decreased to 50.39% with specificity of 72.22%.
When examining the clinical characteristics stratified by IgA/C3 ratio, several notable differences emerge between the low (≤ 2.0) and high (> 2.0) subgroups(Table 2). Compared to patients with a low IgA/C3 ratio, those with a high ratio were significantly older [median age 34 years (26–42) vs. 29 years (23–36), p = 0.003], more likely to have hypertension (70.6% vs. 50.5%, p = 0.001), had lower eGFR [47.7 mL/min/1.73 m² (31.4–73.1) vs. 77.7 mL/min/1.73 m² (40.1–124.2), p < 0.001], and lower UPCR [2.0 g/g (1.0–3.1) vs. 2.5 g/g (1.5–4.0), p = 0.015]. In addition, a significantly higher proportion of patients in the high IgA/C3 ratio group had segmental glomerulosclerosis (S1 lesions) (80.9% vs. 62.8%, p = 0.005). No significant differences were observed in the distribution of other MEST-C lesions between the two groups. When patients were categorized based on the degree of proteinuria, those with lower proteinuria had significantly higher IgA/C3 ratios(supplementary Fig. 2 A and 2B). The median IgA/C3 ratio was 2.7 (IQR: 2.1–3.1) in patients with proteinuria < 1 g/g, compared to 2.3 (IQR: 1.8–2.9) in those with proteinuria ≥ 1 g/g (p = 0.02). Similarly, the median IgA/C3 ratio was 2.4 (IQR: 1.9–3.0) in patients with proteinuria < 3.5 g/g, versus 2.2 (IQR: 1.6–2.7) in those with proteinuria ≥ 3.5 g/g (p = 0.014).
With a median duration of follow-up of 35.0(16-56.8) months, 69 (26.7%) IgAN patients reached the primary endpoint of renal disease progression. The proportion of patients who received immunosuppressive therapy following biopsy did not differ significantly (55.8% vs. 47.9%, p = 0.219) and renal disease progression was comparable (26.3% vs. 27.0%, p = 0.906) between the low and high IgA/C3 ratio groups respectively.
There was no statistically significant difference when comparing the renal disease progression(lack of renal survival) between low (≤2.0) and high (> 2.0) IgA/C3 ratio groups, using Kaplan Meir analysis, (log rank p = 0.275), Fig. 3. Kaplan Meir analysis after stratifying patients using higher cut-off levels of IgA/C3 ratio of 2.4(median value in present IgAN cohort), and 2.8 and 3.0 based on other studies9,10,14,16,19 also did not reveal any significant difference in renal survival.
There was no association between the histological severity of IgAN as defined by the Oxford MEST-C classification and serum IgA/C3 ratio (, supplementary Table 1).
In the univariable cox regression analysis, IgA/C3 ratio > 2.0 was not a significant predictor of renal disease progression (HR = 1.32 95% CI: 0.80–2.2, p = 0.278. It was also not a significant predictor when examined as a continuous variable (HR = 1.10 95% CI: 0.84–1.44, p = 0.501) and using a higher threshold of > 2.4(HR = 1.11, 95% CI: 0.69–1.80, p = 0.659). IgA/C3 ratio also did not predict renal disease progression in 237 IgAN patients after excluding those with nephrotic range(> 3.5 g/day) proteinuria, (HR = 1.42, 95% CI: 0.79–2.53, p = 0.237).
We examined two models for multivariable cox regression analysis(supplementary Table 2). Model 1 included all variables from the univariable analysis with p<0.1, along with age and gender and IgA/C3 ratio. Proteinuria was excluded here due to collinearity with IgA/C3 ratio(co-relation coefficient of −0.267, p < 0.001). E1 lesion (aHR = 2.41, 95% CI: 1.00-5.78, p = 0.049), T1/T2 lesions (aHR = 2.99, 95% CI: 1.66–5.40, p < 0.001) and baseline eGFR < 45 ml/min/1.73m2 (aHR = 3.43, 95% CI: 1.81–6.51, p < 0.001) were independently associated with increased risk of renal disease progression. Model 2 which included all variables in model 1 except IgA/C3 ratio, and proteinuria showed similar findings. Baseline proteinuria was not significantly associated with renal disease progression on multivariable analysis.
Discussion
Initial information regarding serum IgA/C3 ratio as a surrogate marker originated from Japan8,9 where studies suggested that high IgA/C3 ratio could distinguish IgAN from other primary renal diseases9. In a large cohort of 1095 primary glomerular disease patients from China10, IgA/C3 ratio was a good diagnostic marker of IgAN especially in those with mild disease(≤1 g/day). The IgA/C3 ratio was significantly higher in our IgAN patients compared to non-IgAN glomerular disease controls, consistent with previous studies8,9,10,11. However, the median IgA/C3 ratio in our IgAN cohort (2.4) was lower than that reported in other IgAN populations9,10,11,12,13,14,16,17. The area under the ROC curve of IgA/C3 ratio, was 0.6760 (95% CI: 0.6074–0.7446). The optimal sensitivity and specificity of IgA/C3 ratio cut-off of > 2.0 for differentiating IgAN from other non-IgAN glomerular diseases were only 70.5% and 62.2% respectively These findings suggest IgA/C3 ratio may not be an effective diagnostic biomarker in Indian patients. The baseline characteristics of IgAN especially proteinuria differed from controls in our study as they are known to have disparate clinical manifestation. Non-IgA diseases i.e. MCD, FSGS and membranous nephropathy primarily present as nephrotic syndrome while IgAN may have varied presentation from minimal to nephrotic range proteinuria. Previous studies8,9,11 have also included patients of MN and FSGS though they have not specified the difference in clinical and laboratory profile of cases and controls. Gong et al.10 examined IgA/C3 ratio in only patients with mild disease(< 1 g/day proteinuria). However, when we are considering a potential diagnostic biomarker, to be clinically relevant, it should be effective irrespective of degree of proteinuria especially since a sizable number of IgAN patients do have significant proteinuria in South Asian population3,4.
When stratified into low(≤2.0) and high(> 2.0) subgroups, the combination of older age, higher prevalence of hypertension, lower eGFR, lower proteinuria, and more frequent glomerulosclerosis in the high IgA/C3 ratio subgroup(Table 2) suggests a more long-standing disease process with features of chronic kidney injury rather than acute inflammation. This pattern may account for the lower C3 levels and higher IgA concentrations observed in this cohort, ultimately leading to a higher IgA/C3 ratio. A previous study also observed that a higher IgA/C3 ratio was associated with more advanced kidney disease and lower levels of proteinuria, suggesting a smoldering disease course19. Unlike other studies17,18, there was no significant difference in the median IgA/C3 ratio across MEST-C scores. This paucity of histological co-relation was also observed in a pediatric cohort from China15.
26.7% IgAN patients had renal disease progression during the study period consistent with the outcomes observed in other South Asian cohorts who tend to have a poorer and more aggressive prognosis3,4. There was no difference in renal survival between low and high IgA/C3 ratio groups. The use of immunosuppression after diagnosis, which could influence disease trajectory did not differ significantly between the two subgroups. Serum IgA/C3 ratio was effective in predicting renal outcome of IgAN patients in Japanese11,12,13 and Chinese14,15 populations with higher baseline IgA/C3 ratio being associated with poor renal survival. Serum IgA/C3 ratio ≥3.32 was associated with increased risk of disease progression in Chinese patients14. In a European cohort of 95 patients16,patients with IgA/C3 ratio < 2.9 had a tendency to better renal survival, though it had poor sensitivity (68%) and specificity (55%) and was not statistically significant(p > 0.5). Based on previous studies9,10,14,16,19 we also investigated higher IgA/C3 ratio cut-offs for prognostication [2.4(median value in IgAN cohort),2.8 and 3.0] but they did not significantly predict renal survival. Our IgAN patients had lower eGFR and higher proteinuria compared to other cohorts10,12,13,14,17,18. However, IgA/C3 ratio did not predict renal survival in our cohort even after excluding patients with nephrotic range proteinuria. The European cohort which failed to show a statistically significant association between IgA/C3 ratio and renal survival also had more severe disease with lower eGFR and higher proteinuria like our patients16. Our patients also had more histologically chronic disease with higher proportion of T1/T2 and S1 and less E1 lesions compared to other studies14,17,18.
The lower median IgA/C3 ratio in our cohort (2.4) compared to other IgAN populations9,10,11,12,13,14,16,17 and lack of co-relation with the diagnosis and renal outcomes can be attributed to some extent to chronic disease with less activity due to delayed diagnosis (attributed to late presentation). It may also reflect ethnic differences in the disease phenotype which needs to be explored further.
Though not examined in our study, on serial monitoring,15% reduction in serum IgA/C3 ratio was associated with higher likelihood of complete remission in Japanese patients13 and further studies are needed to explore the role of serial monitoring of IgA/C3 ratio in these patients. Since production of galactose deficient IgA1(Gd-IgA1) is considered to be the initial trigger for pathogenesis of IgAN, Gd-IgA1/C3 ratio has also been examined and found to be associated with CKD progression in IgAN, independent of clinical and histological disease profile20. Plasma Gd-IgA1 assay is still a research tool and not approved for clinical use.
This was a retrospective single center study and lacked a control group of healthy volunteers. We examined the association between single, baseline serum IgA/C3 ratio and renal outcome and did not measure serial values for dynamic changes in the disease activity. Serum C3 levels were measured using the immunoturbidimetric assay which may affect the precision of results compared to the nephalometric assay. We recognize Gd-IgA1 levels may be superior in this context considering mechanistic pathway of IgAN, but it is still a research tool, expensive and not widely accessible like the serum IgA assay.
Despite these limitations, this is the first study from India, in a reasonably large cohort of patients testing an easily available investigation as a putative biomarker in IgAN. Although serum IgA/C3 ratio is higher in our IgAN patients compared to other primary glomerular diseases, it lacks enough specificity and sensitivity to be used as a non-invasive diagnostic biomarker for IgAN and did not predict renal survival in our patients. Prospective studies with serial monitoring of IgA/C3 ratio in larger cohorts with preferably more active disease are needed to delineate its role in Indian patients.
Data availability
All relevant data are available within the manuscript and supplementary file. The corresponding author may be contacted for additional data if required.
References
Rodrigues, J. C., Haas, M. & Reich, H. N. IgA nephropathy. Clin. J. Am. Soc. Nephrol. 12 (4), 677–686. https://doi.org/10.2215/CJN.07420716 (2017). Epub 2017 Feb 3.
Suzuki, H., Novak, J., IgA & Nephropathy Significance of IgA1-Containing immune complexes in clinical settings. J. Clin. Med. 13 (15), 4495. https://doi.org/10.3390/jcm13154495 (2024).
Alexander, S. et al. Three-Year clinical outcomes of the first South Asian prospective longitudinal observational IgA nephropathy cohort. Kidney Int. Rep. 7 (2), 305–318 (2021).
Bagchi, S. et al. The international IgA nephropathy network prediction tool underestimates disease progression in Indian patients. Kidney Int. Rep. 7 (6), 1210–1218 (2022).
Kidney Disease. Improving global outcomes (KDIGO) glomerular diseases work group. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 100 (4S), S1–S276 (2021).
Schena, F. P. & Cox, S. N. Biomarkers and precision medicine in IgA nephropathy. Semin Nephrol. 38 (5), 521–530 (2018).
Suzuki, H. Biomarkers for IgA nephropathy on the basis of multi-hit pathogenesis. Clin. Exp. Nephrol. 23 (1), 26–31. https://doi.org/10.1007/s10157-018-1582-2 (2019). Epub 2018 May 8.
Tomino, Y. et al. Measurement of serum IgA and C3 May predict the diagnosis of patients with IgA nephropathy prior to renal biopsy. J. Clin. Lab. Anal. 14 (5), 220–223 (2000).
Maeda, A. et al. Significance of serum IgA levels and serum IgA/C3 ratio in diagnostic analysis of patients with IgA nephropathy. J. Clin. Lab. Anal. 17 (3), 73–76 (2003).
Gong, W. Y. et al. High serum IgA/C3 ratio better predicts a diagnosis of IgA nephropathy among primary glomerular nephropathy patients with proteinuria ≤ 1 g/d: an observational cross-sectional study. BMC Nephrol. 20 (1), 150. https://doi.org/10.1186/s12882-019-1331-0 (2019).
Ishiguro, C. et al. Serum IgA/C3 ratio May predict diagnosis and prognostic grading in patients with IgA nephropathy. Nephron 91 (4), 755–758 (2002).
Komatsu, H. et al. Relationship between serum IgA/C3 ratio and progression of IgA nephropathy. Intern. Med. 43 (11), 1023–1028 (2004).
Torikoshi, K. et al. Serum IgA/C3 ratio: a useful marker of disease activity in patients with IgA nephropathy. Int. Urol. Nephrol. 3 https://doi.org/10.1007/s11255-024-04104-7 (2024 Jun).
Zhang, J. et al. Serum Immunoglobulin A/C3 ratio predicts progression of Immunoglobulin A nephropathy. Nephrol. (Carlton). 18 (2), 125–131 (2013).
Lang, Y. et al. Serum IgA/C3 ratio and glomerular C3 staining predict progression of IgA nephropathy in children. Transl Pediatr. 10 (3), 666–672 (2021).
Stefan, G. et al. Is there a role for IgA/C3 ratio in IgA nephropathy prognosis?? an outcome analysis on an European population. Iran. J. Kidney Dis. 14 (6), 470–477 (2020).
Karahisar Şirali, S. & Büberci, R. Correlation between IgAC3 ratio and Oxford score in IgA nephropathy. Clin. Exp. Nephrol. 26 (10), 982–987 (2022).
Mizerska-Wasiak, M. et al. Relationship between serum IgA/C3 ratio and severity of histological lesions using the Oxford classification in children with IgA nephropathy. Pediatr. Nephrol. 30 (7), 1113–1120 (2015).
Yang, D. et al. Combined effects of the serum IgA/C3 ratio and glomerular C3 staining on the renal outcome in adult Immunoglobulin A nephropathy. Kidney Blood Press. Res. 49 (1), 246–257 (2024). Epub 2024 Feb 21. PMID: 38382485.
Chen, P. et al. Plasma Galactose-Deficient IgA1 and C3 and CKD progression in IgA nephropathy. Clin. J. Am. Soc. Nephrol. 14 (10), 1458–1465 (2019).
Funding
The study did not receive any funding.
Author information
Authors and Affiliations
Contributions
AS and AA: Collected and analyzed the data and wrote the initial draft of the protocol and manuscript. SKD: Supervised the study investigations and contributed to the manuscript MK: Analysis and interpretation GS and AB: Reviewed kidney biopsies, AKS, RKY, SM and DB: Helped in data collection, SKA: Contributed to study design and supervision, SB: Conceptualized the study protocol, collected and analyzed data and wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
Soumita Bagchi, Arunkumar Subbiah and Dipankar Bhowmik are site investigators/co-investigators for clinical trials in kidney diseases (all funding is received by All Institute of Medical Sciences, New Delhi with no personal financial involvement). The rest of the authors have no competing interests to declare. Remaining authors do not have any competing interests to disclose.
Ethics and informed consent approval
The study was approved by the Institute Ethics Committee at All India Institute of Medical Sciences, New Delhi(IECPG-464/27.09.2018) and conducted according to the declaration of Helsinki. Informed consent was taken for patients recruited prospectively while waiver of consent had been granted for samples tested retrospectively from the biorepository.
Informed consent
Informed consent was taken from patients recruited prospectively while waiver of consent was provided by the Institute Ethics Committee for patients included retrospectively.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Swamy, A., Antony, A., Datta, S.K. et al. Serum IgA/C3 ratio as a diagnostic and prognostic biomarker for IgA nephropathy. Sci Rep 15, 29007 (2025). https://doi.org/10.1038/s41598-025-10578-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-10578-x