Abstract
This study aimed to establish a rat model of thin endometrium and investigate the effects of super-activated platelet lysate (sPL) and umbilical cord mesenchymal stem cells (UCMSCs) on the thin endometrium in rats. Thin endometrium models were induced by infusing absolute ethyl alcohol into the uteri of female Sprague-Dawley (SD) rats. Rats were randomly assigned to several groups (Control, Model, Extracellular matrix (ECM) + sPL, ECM + cell, Gel + sPL, Gel + cell) and treated for 21 or 42 days. Histopathological structures and endometrial thickness were observed using hematoxylin-eosin (HE) staining. ELISA was used to detect PDGF-BB, TGF-β1, E2 and FSH expression levels in serum. Furthermore, Western blot and immunohistochemical staining were used to assess the expression levels of cyclin D1, CD34, pan-keratin, cytokeratin 18, and vimentin in uterine tissue. HE staining revealed improvements in endometrial thickness, gland number, and blood vessels following treatment with sPL and UCMSCs in the thin endometrium rat model. Compared to the model group, ELISA results demonstrated that the PDGF-BB, E2, TGF-β1 and FSH serum in treatment groups returned to control levels. Immunohistochemical staining and Western blot results indicated decreased keratin, cytokeratin, and vimentin expression levels in the model group, which were significantly increased by sPL perfusion or UCMSCs transplantation. Intrauterine perfusion of sPL and UCMSCs improves endometrium thickness, morphology, function, and repair capacity in rats with thin endometrium.
Similar content being viewed by others
Introduction
Fertility issues have garnered considerable attention within the field of gynecology and obstetrics, with their manifestation suffering adverse influence by both age and various gynecological diseases1. Among the challenges encountered is the presence of a thin endometrium, often accompanied by compromised endometrial receptivity, recognized as a pivotal factor in the success of embryo implantation and prevention of pregnancy failure2. Numerous research has been published in the literature investigating the impact of various therapeutic interventions, particularly biological therapies. Preclinical experiments have demonstrated a significant enhancement in thin endometrium thickness following the infusion of stem cells into the uterus3. Clinical reports further substantiate these findings, revealing a substantial increase in endometrial thickness in patients with thin endometrium following stem cell treatments4.
Consequently, estrogen and stem cell therapy emerge as promising modalities for addressing thin endometrium and, thereby, augmenting the likelihood of successful pregnancies5. Previous research has explored the administration of high doses of estrogen, specifically estradiol (E2), and the utilization of stem cells and amnion to improve thin endometrium thickness6. However, despite these endeavors, clinical outcomes have not significantly improved with the currently available treatments7. Given the pivotal roles that good endometrial receptivity and adequate endometrial thickness play in embryo implantation and subsequent pregnancy8, developing effective treatment protocols for patients with thin endometrium remains a substantial challenge requiring prompt solutions.
In recent years, Mesenchymal Stem Cell (MSC) therapy has emerged as a novel therapeutic modality for fostering tissue regeneration, leveraging its inherent self-renewal, robust proliferation, and differentiation capacities9. Current investigations underscore the widespread utilization of MSCs in preclinical and clinical settings, encompassing distinct types such as bone marrow mesenchymal stem cells, adipose mesenchymal stem cells, and umbilical cord blood mesenchymal stem cells10. Particular attention has been directed towards Umbilical Cord Mesenchymal Stem Cells (UCMSCs) among the various MSC types due to their facile isolation and versatile application11. Extensive research has revealed that MSCs, including UCMSCs, play a pivotal role in tissue regeneration when employed as standalone cell therapy or in conjunction with biomaterials within the framework of tissue engineering techniques12. The principal functions of MSCs are primarily ascribed to their capabilities in migration and chemotaxis, facilitating subsequent differentiation into functional cells at sites of tissue damage13. Notably, UCMSCs have demonstrated efficacy in promoting tissue regeneration and repairing damaged endometrium in various preclinical applications to address thin endometrium-related therapeutic challenges14.
Nevertheless, emerging studies have progressively indicated that transplanted MSCs exhibit limited survival durations, impeding their capacity to fully differentiate into functional cells and contribute significantly to endometrial regeneration. During the reproductive phase in females, the endometrium undergoes a cyclical process of shedding, proliferation, differentiation, and repair, collectively known as menstruation15. The regulatory actions of the steroid hormone estrogen within the endometrial tissue primarily orchestrate this intricate cycle16. Estrogen play pivotal roles in the complex regulation of female reproductive functions, with a significant focus on the uterus and ovaries17. The management of uterine growth, contraction, endometrial physiology, menstruation, and the maintenance of a successful pregnancy has traditionally centered around controlling estrogen and progesterone (Pg) levels through hormonal therapies18. Super-active platelet lysate (sPL) is a rapid release of high-concentration bioactive factors in platelets based on the patented platelet-efficient induction–activation culture technique. An emerging approach involves using sPL derived from platelet-rich plasma (PRP) through ultra-low temperature freeze-thawing. sPL exhibits elevated levels of growth factors, including platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), and epidermal growth factor (EGF), etc19. These growth factors promote tissue regeneration and inhibit local inflammatory responses20. sPL has higher safety than PRP and can be stored for a longer period of time. During preparation of sPL, residual solid cellular components and white cells are removed, while immunogenicity is reduced. sPL can be directly injected into the damage position without activating platelets. Compared to traditional PRP, sPL can enhance hormone secretion, directly activate MSCs and expedite tissue regeneration21. Furthermore, there is a need to develop more specific protocols for applying sPL in gynecological diseases. Additionally, further exploration warrants the treatment of hormone-dependent and immune-related disorders using sPL.
We established a thin endometrium model to assess the potential pharmacological application of sPL in a clinical context. Subsequently, we performed comprehensive histological, biological, and functional analyses to explore the potential of sPL or UCMSCs administration in reinstating endometrial functionality and enhancing pregnancy outcomes.
Results
Effect of sPL and UCMSCs on the morphology of endometrium
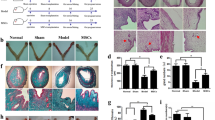
H&E staining showed that the uterine tissues observed visible blood vessels and glands, and the cells were tightly arranged in the endometrium of rats. The uterus in the model group was significantly thin and had extensive necrosis of the endometrium. Rats in the intervention groups (sPL and UCMSCs) had significantly thicker endometrium and increased endometrial glands compared with the model group after 21 days of treatment (Fig. 1). The structure of epithelium was more integrity in treatment groups. The endometrial thicknesses of the control, Model 21, extracellular matrix (ECM) + sPL 21, ECM + cell 21, Gel + sPL 21, and Gel + cell 21 was showed as follows: 917.5 ± 83.6 μm, 181.1 ± 41.2 μm, 538.5 ± 51.6 μm, 360.6 ± 63.7 μm, 546.8 ± 73.2 μm and 339.8 ± 74.9 μm, respectively (Table 1). The intervention groups showed relatively intact endometrium structures, with increased capillaries and glands and thicker endometrium at 42 days. There was no significant difference between the intervention groups after 42 days of treatment (Fig. 1B and C). In 21 days and 42 days intervention groups (sPL and UCMSCs), the number of endometrial glands was increased significantly compared with the model group (Fig. 1D and E). The effects of 21 Days groups and 42 Days groups remodeling uterine structure, endometrial glandsand epithelial repair remained stable.
HE staining shows the morphology of the endometrium. (A) Results of HE analysis of each group (scale bar = 1,000 μm); (B) Results of endometrial thicknesses after 21 days treatment, *P < 0.05, **P < 0.01; (C) Results of endometrial thicknesses after 42 days treatment, *P < 0.05. **P < 0.01; (D) Results of Glands number after 21 days treatment, ***P < 0.001; (E) Results of Glands number after 42 days treatment, ***P < 0.001, compared with model group. n = 6. Gel: chitosan. ECM: extracellular matrix. sPL: super-activated platelet lysate.
Protein contents in endometrium after sPL and UCMSCs treatment
The expression of cyclin D1, CD34, and cytokeratin was examined using IHC to assess the effect of sPL and UCMSCs transplantation on a thin endometrium. According to IHC staining, cyclin D1 and cytokeratin were predominantly expressed in the cytoplasm of endometrial epithelial cells, and CD34 and vimentin in the cytoplasm of endometrial stroma cells (Fig. 2). Based on the results, we found that the fluorescence intensities of cyclin D1, CD34, vimentin, and cytokeratin in the intervention groups (sPL and UCMSCs) were significantly higher than in the model group (P < 0.01). Western blot results showed that sPL and UCMSCs increased CK18 and stromal cell marker vimentin expression levels in a thin-endometrium rat model. Cyclin protein changes are a sign of cell proliferation.
In comparison with the model group, cyclin D1 and CD34 expression levels were higher in the intervention group (sPL/Gel treatment), but no significant differences were found between sPL and UCMSC treatments (Figs. 2 and 3). A higher level of cytokeratin and vimentin expression was observed in the intervention group (sPL/Gel treatment) compared to the control group (P < 0.01). There were no statistically significant differences between the control and sPL groups. A significant difference was observed in the expression of cytokeratin and vimentin between the subcutaneous and model groups (Figs. 2 and 3). Based on Western blot analysis, the experimental group showed significantly higher cytokeratin and vimentin expression than the control group (P < 0.05). In contrast, no significant difference was observed between the control and experimental groups (Fig. 3).
Influence of sPL and UCMSCs transplantation of the endometrium. (A) The expression of the cyclin D1, CD34, keratin, cytokeratin 18 and vimentin protein in each group was detected by western blotting. The original images are shown in the appendix. (B) A histogram shows the relative expression level of the target proteins to that of an internal standard. *P < 0.05, **P < 0.01, ***P < 0.001, compared with Model group. n = 3 uteri.
sPL and UCMSCs promote the expression levels of PDGF-BB, E2, TGF-β1 and FSH
Compared with the control group, serum PDGF-BB and TGF-β1 levels in the model groups were increased (P < 0.05), E2 and FSH levels were decreased (P < 0.05). After 42 days of treatment, intervention group compared with the model group, the levels of the PDGF-BB (ECM + sPL), TGF-β1 (ECM/Gel + sPL), E2 (ECM + cell) and FSH (ECM + cell) were decreased (P < 0.05). Based on (Fig. 4), there was no statistical difference between the two groups.
Discussion
It has been estimated that the endometrium undergoes more than 400 cycles of development, growth, and shed during a woman’s reproductive years. Endometrial thinness occurs as a result of inflammation, endometrial dysfunction, and uterine surgery. To gain a better understanding of the mechanisms underlying endometrial injury and repair, this study investigated the effects of sPL and UCMSCs on thin endometrium. Using histomorphology, Western blotting, and immunocytochemistry to diagnose and repair the damaged microenvironment. It is well-established that absolute ethyl alcohol perfusion in rats induces endometrial injury, offering advantages such as easy modeling and high success rates. Several in vivo experiments demonstrated that the morphology and thickness of the endometrium were both significantly improved by sPL and UCMSCs. In previous studies, UCMSCs have been shown to thicken the endometrium, increase glands, and increase sub-endometrial angiogenesis, all of which improve pregnancy rates22. The therapeutic effectiveness of sPL significantly exceeded that of UCMSC transplantation in rats with thin endometrium by enhancing endometrial thickness, morphology, function, and repair capacity. Our study suggests that sPL combined with UCMSC therapy may be a promising approach for treating thin endometrium.
This study suggests that intrauterine administration of autologous sPL has proliferative and anti-fibrotic effects on damaged endometrium. It has been well documented that sPL and UCMSCs initiates tissue repair via the regulate on the level of biologically active molecules and growth factor. sPL contains growth factors and cytokines contributing to rapid healing and tissue regeneration by accelerating cell proliferation, angiogenesis, and migration23. PDGF has been demonstrated to play several pivotal roles in endometrial cell proliferation, survival, and migration, primarily effect on cells of mesenchymal origin24. Diverse growth factors, such as TGF-β, insulin-like growth factors, and colony-stimulating factors have been recognized for their participation in autocrine and paracrine functions. These growth factors influencing endometrial cell growth, differentiation and implantation25,26,27. E2 plays a decisive role in maintaining ovarian function and mitigating ovarian aging. Additionally, FSH participates during the late follicular stage, to promote the development and optimal functioning of follicles. The ECM + cell group has not only a low E2 level, but also significantly decreased FSH level, this may E2 deficiency in a low gonadotropin state in rats. These findings highlight the therapeutic potential of UCMSC in the modulation of angiogenesis signaling pathway within the thin endometrium and ovary. TGF-β1 can induce the collagen formation and the change from the epithelial to mesenchymal phenotype, resulting in fibrosis. TGF-β1 is a regulator of the ECM and pro-fibrosis, playing a critical role in the development of fibrogenesis and organ dysfunction in a number of diseases. In our study, we demonstrated that sPL and UCMSCs increased endometrial thickness and promoted ECM deposition probably by down-regulating the expression of PDGF-BB, E2 and TGF-β1 in the endometrium of rat model.
Moreover, to the extent of our knowledge, this study is the first to reveal that autologous sPL can enhance endometrial proliferation, decrease fibrosis, and mitigate intrauterine adhesions by using a thin endometrial model. In a recent study, sPL has been demonstrated to have an anti-fibrotic effect on skeletal muscle healing after injury28. However, the result of this study showed that sPL inhibited excessive collagen deposition, which may decrease fibrosis progression in thin endometriums. Compared with the model group, the thickness of the endometrium and the number of glands in sPL or UCMSC treatment groups were significantly higher, and the interstitial blood vessels were denser.
A significant increase in endometrial thickness and gland number was observed in the sPL-treated or UCMSC-treated groups, and interstitial blood vessels were decreased significantly. Vimentin and cytokeratin are often considered specific epithelial cell markers29. In endometrial cells, keratin and vimentin are important components of the intermediate filament protein cytoskeleton30. Their role is crucial in promoting cell proliferation and differentiation, and they are often considered dynamic structures29,31. In rat endometrial epithelial cells and luminal epithelial cells, cytokeratin 18 is predominantly found in the cytoplasm32. Within the mesenchyme cytoplasm of rats, vimentin is predominantly expressed in stromal cells33. Rat endometrium was found to have high levels of CK18 and vimentin after intrauterine perfusion with sPL and UCMSC. Microscopic examination reveals an intact and tight arrangement of the glandular epithelium and stroma of the endometrium due to changes in endometrial thickness and cyclin D1, CD34, CK18, and vimentin expression levels. Our research showed that sPL and UCMSC perfusion could restore endometrial thickness after intrauterine perfusion and facilitate the rejuvenation of glandular epithelial cells and stromal cells in a thin endometrial structure.
According to previous studies, estrogen and progesterone regulate VEGF expression in the endometrium, transforming it from thin and dense to thick and transparent34. VEGF could influence the endometrium’s proliferative, secretory, and shedding in combination with female hormones35. Low levels of VEGF in the menstrual phase are associated with endometrial shedding36. In contrast, high levels of VEGF in the secretory phase promote neovascularization and vascular permeability, both of which are beneficial to endometrium repair and embryo implantation during pregnancy37. In our study, VEGF was expressed throughout the implantation window in rat endometrium, suggesting that it could contribute to endometrial receptivity. Furthermore, sPL has also been applied to medical and surgical fields, including skin rejuvenation and hair regrowth, lung dysfunction, neural repair, and orthopedics due to its no-side effects properties.
In conclusion, sPL and MSCs can significantly promote endometrium growth, offers significant benefits for thin endometrium treatments, providing a therapeutic option for thin endometrium. Despite ongoing challenges and difficulties, sPL shows significant promise for future clinical trials and could provide an alternative to PRP-based treatments. Furthermore, these promising results may contribute to the development of innovative therapeutic strategies for conditions associated with endometrial dysfunction. The combination of sPL and UCMSCs emerges as a potential candidate for clinical interventions aimed at enhancing endometrial regeneration and improving reproductive outcomes. sPL and MSCs hold great translational potential for regenerative medicine. We believe that further studies are necessary to determine the composite sPL + MSCs was generally superior to its corresponding monotherapy.
Materials and methods
Animal study
Animals’ experiments
Specific pathogen-free Sprague-Dawley females’ rats (aged 8–10 weeks, weighing 220 ± 20 g) were purchased from Heilongjiang University of Chinese Medicine. All the Rats were housed in a specific virus-free laboratory at an ambient temperature of 23 °C, and a 12-hour light/dark cycle and ad libitum access to food and water were provided after one week of acclimatization. The experiment was conducted on rats with complete sexual cycles.
sPL preparations
An average of 8 mL of blood was extracted from 20 anaesthetized rats by cardiac puncture and collected in 15 mL centrifuge tube containing 20 U sodium heparin. The red blood cells were removed, and the platelet was collected by centrifuging fresh blood at 1,500 g for 15 min. Platelet activation was achieved by incubating PRP with 20 mM CaCl2 and 2 U/mL sodium heparin for 2 h at 37 °C. Then, the mixture was frozen at −80 °C for 2 h, followed by thawing at 37 °C and cooling at 4 °C to allow fibrin coalescence. sPL is collected from the platelet through ultra-low temperature freeze-thawing. After centrifugation at 3,000 g for 8 min, the supernatant was filtered through a 0.22 μm filter (Millipore, Darmstadt, Germany) to obtain the sPL38. A total of 16 mL sPL was collected from 160 mL blood, the prepared and actived sPL was frozen and stored at −20 °C.
Culture and amplification of UCMSCs
The UCMSCs were from the mothers who just gave birth, and informed consent was obtained from hospitalized mothers at The First Affiliated Hospital of Harbin Medical University, and rapidly processed. The Ethics Committee of Heilongjiang University of Chinese Medicine approved this study. All experiments were performed in accordance with relevant guidelines and regulations. The cells were harvested and cultured by centrifugation at 1,200 g for 10 min. The cells were resuspended and cultured in DMEM/F12 media with 10% fetal calf serum and 1% P/S at 37 °C in a humidified incubator with 5% CO2. Three times a week, the culture medium was changed. 0.05% trypsin-EDTA was used to harvest adherent UCMSCs at 90% confluence. After obtaining the third passage of culture, the UCMSCs were used for transplantation. Trypsin-EDTA was used to harvest the cells, followed by two washes with DMEM/F12 before resuspending them at 62,500 cells/µL in DMEM/F1239,40,41.
Preparation of injection mixtures
To obtain a proper mixture, an efficient dispersion of the composition of the mixture must be provided to avoid precipitation. The chitosan powders were dissolved in 0.01 mol/L of acetic acid solution. The mixture was heated to 60 °C under continuous magnetic stirring (200 g) for 180 min for better dissolution and cooling to 4 °C. This can be accomplished through a three-way stopcock device, connecting a syringe with 160 µL sPL or UCMSCs on one side and another syringe with 40 µL 5% chitosan (Sigma-Aldrich, St. Louis, USA) or ECM (354263, BD Biosciences, Bedford MA, USA) on the opposite side. The device ensures a homogeneous mixture, consolidating components within a single syringe for injection.
Model Preparation and treatment
The estrus status of rats was monitored daily through vaginal smears. Nembutal sodium was used for general anesthesia during the operation on rats in stable estrus. Rats were placed in the supine position on the operating table, and the lower abdomen was scraped and disinfected with ethanol. The abdomen skin and muscles were deeply opened, starting with a longitudinal incision of 1.5 to 2 cm, followed by layer-by-layer openings. Near the vagina and ovary, the uterus was ligated. Then, 200–300 µL PBS was kept in the uterus for 5 min in the control groups and 200–300 µL 95% ethanol was injected in the model and treatment groups for 5 min, respectively. In addition to extracting the remaining ethanol, the uterine cavities were washed twice with physiological saline. Three rounds of iodophor were applied to the incision site and sutured to return the uterus to its normal anatomical position after the incision was made42,43.
The control group was maintained normally without intervention. Model rats were divided into five groups: Model group (40 µL 5% chitosan, 40 µL ECM and 120 µL PBS/rat, n = 20), ECM + sPL group (40 µL ECM and 160 µL sPL/rat, n = 20), ECM + cell group (40 µL ECM and 1 × 107 UCMSC/160 µL/rat, n = 20), Gel + sPL group (40 µL 5% chitosan and 160 µL sPL/rat, n = 20), and Gel + cell group (40 µL 5% chitosan and 1 × 107 UCMSC/160 µL/rat, n = 20). After modeling for 6 to 8 h, we perfused the therapeutic mixture into the uterus cavity with a medical syringe. The 21 days groups were treated at day 0, and the 42 days groups were treated at day 0 and day 21. To evaluate the endometrium status, rats were anesthetized for sampling at 21 days and 42 days after treatment. To further investigate, the uterus of rats was sectioned and preserved at −80 °C or 4% paraformaldehyde.
Histopathological examinations
The rat uterus was removed in each group and placed in paraffin. The uterus was sliced at a thickness of 5 μm and dried at 45 °C. The sections were covered in xylene and ethanol, varying from high to low concentrations. We stained the sections with HE stain kit (G1120, Solarbio), and used CaseViewer image processing software (3DHISTECH, Hungary) to analyze the vertical distance between the uterine cavity and the myometrium. A comparison of group differences in endometrial morphology and thickness was made by obtaining data from five random views of the endometrium.
Immunohistochemistry
The uterine was stored in 4% paraformaldehyde and placed in wax blocks. To block endogenous peroxidase activity and non-specific antigens, the sections were deparaffinized in xylene, rehydrated with ethanol, then incubated with 3% H2O2 for 15 min and 10% goat serum for 20 min. The sections were incubated with the primary antibodies CD34 (1: 2,500, ab81289, Abcam), cyclin D1 (1: 100, ab134175, Abcam), cytokeratin 18 (1: 500, ab133263, Abcam) and vimentin (1: 200, sc-6260, Santa Cruz) at a dilution of 1: 100 overnight at 4 °C. Slides were incubated with 50 µL secondary antibodies and followed by 3,3′-diaminobenzidine tetrahydrochloride (DAB) solution for 1 h. A hematoxylin solution was then applied to the slides for 3 min, and the slides were analyzed with CaseViewer image processing software (3DHISTECH, Hungary).
Western blot analysis
Uterine tissues were lysed by RIPA lysis buffer to extract proteins. After lysing, the Uterine tissues in 400 µL lysis buffer (P0013C, Beyotime) and protein (30 µg/20 µL) were stained with loading dye, separated with 8 − 12% SDS-PAGE, transferred to polyvinylidene difluoride (PVDF) membranes and incubated with 5% skim milk. The membranes were exposed to cyclin D1 antibody (1: 2,000, ab134175, Abcam), CD34 antibody (1: 5,000, ab81289, Abcam), Pan-keratin antibody (1: 1,000, #4545, cell signaling technology), cytokeratin 18 antibody (1: 1,000, ab133263, Abcam) and vimentin antibody (1: 200, sc-6260, Santa Cruz) overnight. The membranes were washed with TBST and incubated with secondary anti-rabbit (1: 5,000, ab6721, Abcam) or anti-mouse IgG (1: 5,000, ab6789, Abcam) for 1 h at room temperature. The chemiluminescent signal was developed using the ECL Kit (Invitrogen, Waltham, MA, USA) and the protein contents was detected using ImageJ 1.52a (National Institutes of Health, USA).
Biochemical and histopathological analysis
All blood samples collected by abdominal aortic blood were stood at room temperature for 1 h, then the samples were centrifuged at 6,000 g for 15 min to collect the serums. The serums extracted from the blood samples of the experimental rats were separated and stored at −20 °C. The concentrations of PDGF-BB, E2, TGF-β1 and FSH were performed using ELISA kits (R&D Systems, Inc., Minneapolis, MN, USA) according to the instructions provided by the manufacturer.
Statistical analysis
Analysis was performed using the statistical software SPSS 22.0. The data in our study are presented as mean ± standard deviation (SD). Students’ T-tests, or a one-way analysis of variance (ANOVA) were used for comparisons between the two groups, followed by Tukey post-hoc comparison. Statistical significance was indicated by P < 0.05.
Data availability
Data underlying the results presented in this paper are not publicly available at this time but may be obtained from the corresponding authors (Ling-Qi Meng: 523336674@qq.com and Yi Zhang: neo_yi_zhang@163.com) upon reasonable request.
References
AAyhan, A. et al. Oncologic and obstetric outcomes of early-stage epithelial ovarian cancer patients who underwent fertility-sparing surgery: A retrospective study. Int. J. Gynaecol. Obstet. 162, 711–717. https://doi.org/10.1002/ijgo.14732 (2023).
Kieu, V. et al. A survey study of endometrial receptivity tests and immunological treatments in in vitro fertilisation (IVF). Aust N Z. J. Obstet. Gynaecol. 62, 306–311. https://doi.org/10.1111/ajo.13466 (2022).
Gharibeh, N. et al. Cell-based therapy in thin endometrium and Asherman syndrome. Stem Cell. Res. Ther. 13, 33. https://doi.org/10.1186/s13287-021-02698-8 (2022).
Cakiroglu, Y. et al. Treatment options for endometrial hypoproliferation. Curr. Opin. Obstet. Gynecol. 35, 254–262. https://doi.org/10.1097/GCO.0000000000000863 (2023).
Sapozhak, I. M. et al. Application of autologous endometrial mesenchymal stromal/stem cells increases thin endometrium receptivity: a case report. J. Med. Case Rep. 14, 190. https://doi.org/10.1186/s13256-020-02515-5 (2020).
Ebrahim, N. et al. Human mesenchymal stem cell-derived extracellular vesicles/estrogen combined therapy safely ameliorates experimentally induced intrauterine adhesions in a female rat model. Stem Cell Res. Ther. 9, 175. https://doi.org/10.1186/s13287-018-0924-z (2018).
Oh, J. Y. et al. 17β-Estradiol protects mesenchymal stem cells against high glucose-induced mitochondrial oxidants production via Nrf2/Sirt3/MnSOD signaling. Free Radic. Biol. Med. 130, 328–342. https://doi.org/10.1016/j.freeradbiomed.2018.11.003 (2019).
Jacobs, E. A. et al. Endometrial thickness: how thin is too thin? Fertil. Steril. 118, 249–259. https://doi.org/10.1016/j.fertnstert.2022.05.033 (2022).
Zheng, J. et al. Epitranscriptomic modifications in mesenchymal stem cell differentiation: advances, mechanistic insights, and beyond. Cell. Death Differ. 31, 9–27. https://doi.org/10.1038/s41418-023-01238-6 (2024).
Squillaro, T., Peluso, G. & Galderisi, U. Clinical trials with mesenchymal stem cells: an update. Cell Transplant. 25, 829–848. https://doi.org/10.3727/096368915X689622 (2016).
Zhuang, M. et al. Human umbilical cord mesenchymal stromal cells promote the regeneration of severe endometrial damage in a rat model: hUCMSCs promote the recovery of severe endometrial damage. Acta Biochim. Biophys. Sin (Shanghai). 54, 148–151. https://doi.org/10.3724/abbs.2021015 (2022).
Yulia Suzdaltseva, S., Zhidkih, S. L. & Kiselev, V. Stupin. Locally delivered umbilical cord mesenchymal stromal cells reduce chronic inflammation in long-term nonhealing wounds: a randomized study. Stem Cells International 5308609. (2020). https://doi.org/10.1155/2020/5308609 (2020).
Xie, Z. et al. TNF-alpha-mediated m(6)A modification of ELMO1 triggers directional migration of mesenchymal stem cell in ankylosing spondylitis. Nat. Commun. 12, 5373. https://doi.org/10.1038/s41467-021-25710-4 (2020).
Wang, Y. et al. Umbilical cord mesenchymal stem cell-derived apoptotic extracellular vesicles ameliorate cutaneous wound healing in type 2 diabetic mice via macrophage pyroptosis Inhibition. Stem Cell. Res. Ther. 14, 257. https://doi.org/10.1186/s13287-023-03490-6 (2023).
Hilary, O. D., Critchley, J. A., Maybin, G. M., Armstrong, Alistair, R. W. & Williams Physiology of the endometrium and regulation of menstruation. Physiol. Rev. 100, 1149–1179. https://doi.org/10.1152/physrev.00031.2019 (2020).
Di, X. et al. Activation of SGK1/ENaC signaling pathway improves the level of decidualization in unexplained recurrent spontaneous abortion. Reprod. Sci. 30, 3273–3284. https://doi.org/10.1007/s43032-023-01273-1 (2023).
PSong, T. et al. Zearalenone promotes uterine development of weaned gilts by interfering with serum hormones and Up-Regulating expression of Estrogen and progesterone receptors. Toxins (Basel). 14, 732. https://doi.org/10.3390/toxins14110732 (2022).
Ryan, M., Marquardt, T. H., Kim, J. H., Shin, J. W. & Jeong Progesterone and Estrogen signaling in the endometrium: what Goes wrong in endometriosis?? Int. J. Mol. Sci. 20, 3822. https://doi.org/10.3390/ijms20153822 (2019).
Zhang, Y. et al. Super activated platelet lysate, a novel autologous platelet lysate, regulates the expression of inflammasome and cytokine in the experimental periodontitis in rats. Drug Des. Dev. Therapy. 14, 5535–5543. https://doi.org/10.2147/DDDT.S289753 (2020).
Yilmaz, B. D. & Bulun, S. E. Endometriosis and nuclear receptors. Hum. Reprod. Update. 25, 473–485. https://doi.org/10.1093/humupd/dmz005 (2019).
Chansaenroj, A., Yodmuang, S. & Ferreira, J. N. Trends in salivary gland tissue engineering: from stem cells to secretome and organoid Bioprinting. Tissue Eng. Part. B Rev. 27, 155–165. https://doi.org/10.1089/ten.TEB.2020.0149 (2021).
Zhang, S. et al. Intrauterine injection of umbilical cord mesenchymal stem cell exosome gel significantly improves the pregnancy rate in thin endometrium rats. Cell Transplant. 31, 9636897221133345. https://doi.org/10.1177/09636897221133345 (2022).
Ranjan Verma, S., Kumar, P., Garg, Y. K. & Verma Platelet-rich plasma: a comparative and economical therapy for wound healing and tissue regeneration. Cell. Tissue Bank. 24, 285–306. https://doi.org/10.1007/s10561-022-10039-z (2023).
Zhang, X. et al. PDGFBB improved the biological function of menstrual blood-derived stromal cells and the anti-fibrotic properties of exosomes. Stem Cell. Res. Ther. 14, 113. https://doi.org/10.1186/s13287-023-03339-y (2023).
Apte, R. S., Chen, D. S. & Ferrara, N. VEGF in signaling and disease: beyond discovery and development. Cell 176, 1248–1264. https://doi.org/10.1016/j.cell.2019.01.021 (2019).
Hajipour, H. et al. An update on platelet-rich plasma (PRP) therapy in endometrium and ovary related infertilities: clinical and molecular aspects. Syst. Biol. Reprod. Med. 67, 177–188. https://doi.org/10.1080/19396368.2020.1862357 (2021).
Ma, J. et al. Recent trends in therapeutic strategies for repairing endometrial tissue in intrauterine adhesion. Biomater. Res. 25, 40. https://doi.org/10.1186/s40824-021-00242-6 (2021).
Guo, X. et al. Effect of super activated platelet lysate on cell proliferation, repair and osteogenesis. Biomed. Mater. Eng. 34, 95–109. https://doi.org/10.3233/BME-221426 (2023).
Wang, T. & Tan, J. Therapeutic effect of menstrual blood stem cells in rats with thin endometrium. Zhejiang Da Xue Xue Bao Yi Xue Ban. 52, 13–23. https://doi.org/10.3724/zdxbyxb-2022-0509 (2023).
Chenglin Miao, S., Zhao, S. E. M. & Yaming Jiu. The diverse actions of cytoskeletal vimentin in bacterial infection and host defense. J. Cell. Sci. 136, jcs260509. https://doi.org/10.1242/jcs.260509 (2023).
Nick, A., Kuburich, P., Hollander, J. T., Pietz, Sendurai, A. & Mani Vimentin and cytokeratin: good alone, bad together. Semin Cancer Biol. 86, 816–826. https://doi.org/10.1016/j.semcancer.2021.12.006 (2022).
Tantengco, O. A. G. et al. Progesterone alters human cervical epithelial and stromal cell transition and migration: implications in cervical remodeling during pregnancy and parturition. Mol. Cell. Endocrinol. 529, 111276. https://doi.org/10.1016/j.mce.2021.111276 (2021).
Chen, K. et al. A novel method to repair thin endometrium and restore fertility based on Menstruation-Derived stem cell. Reprod. Sci. 31, 1662–1673. https://doi.org/10.1007/s43032-024-01458-2 (2024).
Jianghong et al. Progress on the role of Estrogen and progesterone signaling in mouse embryo implantation and decidualization. Reproductive Sci. 30, 1746–1757. https://doi.org/10.1007/s43032-023-01169-0 (2023).
Hu, X. et al. Cyclical endometrial repair and regeneration: molecular mechanisms, diseases, and therapeutic interventions. MedComm 4, e425. https://doi.org/10.1002/mco2.425 (2020). (2023).
Naseri, S. et al. A cross-sectional study comparing the inflammatory profile of menstrual effluent vs. peripheral blood. Health Sci. Rep. 6, e1038. https://doi.org/10.1002/hsr2.1038 (2023).
Pérez-Gutiérrez, L. & Ferrara, N. Biology and therapeutic targeting of vascular endothelial growth factor A. Nat. Rev. Mol. Cell Biol. 24, 816–834. https://doi.org/10.1038/s41580-023-00631-w (2023).
Wu, H. et al. The combination of Super-Active platelet lysate and acellular amniotic membrane enhances endometrial receptivity, while simultaneously facilitating endometrial repair in rats. J. Inflamm. Res. 17, 11097–11109. https://doi.org/10.2147/JIR.S483446 (2024).
Juan Wang, S. et al. Manufacture and quality control of human umbilical Cord-Derived mesenchymal stem cell sheets for clinical use. Cells 11, 2732. https://doi.org/10.3390/cells11172732 (2022).
Wang, L. et al. The UCMSC-bFGF/Scaffold system accelerates the healing of the uterine Full-Thickness injury. Tissue Eng. Part. A. 29, 112–125. https://doi.org/10.1089/ten.TEA.2022.0153 (2023).
Zhang, L. et al. Transplantation of umbilical cord-derived mesenchymal stem cells promotes the recovery of thin endometrium in rats. Sci. Rep. 12, 412. https://doi.org/10.1038/s41598-021-04454-7 (2022).
Xi, J. et al. Electroacupuncture Improves Pregnancy Outcomes in Rats with Thin Endometrium by Promoting the Expression of Pinopode-Related Molecules. Biomed Res Int. 6658321. (2021). https://doi.org/10.1155/2021/6658321 (2021).
Zhao, J., Zhang, Q., Wang, Y. & Li, Y. Uterine infusion with bone marrow mesenchymal stem cells improves endometrium thickness in a rat model of thin endometrium. Reprod. Sci. 22, 181–188. https://doi.org/10.1177/1933719114537715 (2015).
Acknowledgements
The authors thank to Ethical Committee for Animal Care and Use of the Heilongjiang University of Chinese Medicine for animal experiment support of this article (Ethics approval number: 2024011615).
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
L.Q. M.†: Conceptualization, Methodology, Software, Validation, Investigation, Data Curation, Writing – Original Draft, Visualization; Y. Z.†: Conceptualization, Methodology, Writing – Original Draft; C.X. L.: Methodology, Validation, Data Curation; I.U.: Writing – Review & Editing; T.Q. Z.: Data Curation; Y. Z.*: Conceptualization, Validation, Investigation, Supervision, writing – review and editing. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Consent for publication
All authors gave consent for publication.
Competing interests
The authors declare no competing interests.
Ethics approval
The animal study was reviewed and approved by the Ethical Committee for Animal Care and Use of the Heilongjiang University of Chinese Medicine (Ethics approval number: 2024011615). All authors confirm that all animal procedures and care were performed in accordance with relevant guidelines and regulations. All animal experiments were performed in accordance with the ARRIVE guidelines.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Meng, LQ., Zhang, Y., Liu, CX. et al. Therapeutic potential of super activated platelet lysate (sPL) and umbilical cord mesenchymal stem cells (UCMSCs) in enhancing endometrial regeneration in rats with thin endometrium. Sci Rep 15, 24698 (2025). https://doi.org/10.1038/s41598-025-10587-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-10587-w
Keywords
This article is cited by
-
Effects of miR-210-3p/SDF2 and miR-31-5p/FGF7 from hypoxic endometrial exosomes on UCB-MSC proliferation, migration, and differentiation
Stem Cell Research & Therapy (2025)