Abstract
Deinagkistrodon acutus is one of the unique venomous snakes native to Southeast Asia. Limb injury caused by this species is the main cause of disability in snake bite patients, while the relevant pathogenesis mechanism and intervention strategies need to be further explored. In recent years, studies have established that mesenchymal stem cell-derived exosome (MSC-Exo) exerts a positive therapeutic effect on reducing limb injuries caused by a variety of factors, but this effect in limb injuries caused by snake bite is still unclear. Immunity-and-matrix regulatory cell (IMRC) is a type of mesenchymal stem cell derived from human embryonic stem cells, characterized by its unique capabilities in immune regulation and regulation of extracellular matrix production. In this study, IMRC was selected to investigate the effects and mechanisms of its Exo on limb injury induced by Deinagkistrodon acutus venom. Eighteen healthy male white rabbits were divided into Sham (S) group, Snake venom (SV) group and SV + IMRC-Exo group according to a random number table, with 6 rabbits in each group. Rabbit models of snakebite were established by limb injection of 1.5 mg/kg snake venom, followed by intravenous injection of 80 U/kg antivenom 2 h later. Additionally, subcutaneous injection of 7.5 × 1010 particles of Exo in the SV + IMRC-Exo group was given. After modeling, the limb circumference was measured regularly and the serum levels of muscle injury markers such as Creatine Kinase (CK) and Myoglobin (Mb) were detected. At the end of the experiment, muscle tissue samples of the injured limb were obtained to detect gross pathological damage and cell apoptosis. Ferroptosis-related products including iron deposition, reactive oxygen species(ROS), malondialdehyde (MDA), 4-hydroxynonenal (4-HNE), glutathione (GSH), and superoxide dismutase (SOD)were quantified, and key proteins including acyl-CoA synthetase long chain family member 4 gene (ACSL4), nicotinamide adenine dinucleotide phosphate oxidase 1 (NOX1), cyclooxygenase-2 (COX2), glutathione peroxidase 4 (GPX4), and ferritin heavy chain 1 (FTH1) were measured. Compared with group S, the limb circumference and CK and Mb levels were significantly increased after modeling in the SV group and the SV + IMRC-Exo groups. However, the limb circumference and levels of muscle injury markers were significantly lower in the SV + IMRC-Exo group than in the SV group. Histopathological analysis showed that those animals in the SV and SV + IMRC-Exo groups had obvious muscle tissue damage and apoptosis compared with the S group. However, these pathological changes were significantly milder in the SV + IMRC-Exo group than in the SV group. In addition, compared with group S, the levels of iron deposition, ROS, MDA and 4-HNE, and the mRNA expression of ACSL4, NOX1 and COX2 in muscle tissues in the SV and SV + IMRC-Exo groups were significantly increased while the levels of GSH and SOD, and the mRNA expression of GPX4 and FTH1 were significantly decreased. However, compared with the SV group, the application of IMRC-Exo significantly reversed the changes in ferroptosis-related indices mentioned above. IMRC-Exo has a protective effect on limb injury induced by Deinagkistrodon acutus venom in rabbits, in which the mechanism is potentially related to the inhibition of ferroptosis.
Similar content being viewed by others
Introduction
Snake bites are a major tropical emergency with a disability rate of 25-30%1,2,3. The incidence of snake bites has always been high in corresponding regions in China, with species mainly including Deinagkistrodon acutus (D. acutus), Naja atra, and Trimeresurus stejnegeri, etc., with D. acutus bites having a higher incidence and causing greater harm4. Research has shown that after the bite of Deinagkistrodon acutus, its toxins usually invade the whole body through the limbs, initially causing local muscle tissue edema, ulceration and even necrosis, as well as abnormal coagulation system and multi-organ disfunction5,6,7. Metalloproteinases in the venom destroy muscle tissue by affecting the extracellular matrix, causing tissue destruction, muscle necrosis, etc5. Current treatments include antivenom, preventing venom spread, detoxification, and supportive care8, but traditional methods for managing limb injuries, such as incision and drainage, fail to stop muscle damage progression9,10. Consequently, the disability rate of snake bite patients is still high. Therefore, exploring effective interventions for D. acutus-induced limb muscle injury is essential to improving patient outcomes.
In recent years, Exosome (Exo) derived from mesenchymal stem cells (MSCs) have been shown to protect against muscle damage caused by ischemia and diabetes11,12. Other studies have shown that, immune and matrix regulatory cells (IMRCs) derived from human embryonic stem cells demonstrate superior cell quality, immunomodulatory capabilities, and injury repair capacities compared to adult-tissue-derived MSCs13,14. Consequently, IMRCs are preferred for Exo production13. However, no studies have yet reported the therapeutic effects of MSC-Exo on snakebite-induced limb muscle injuries. In this study, we examined the effects of IMRC-Exo on such injuries. In addition, ferroptosis, as a new form of cell death related to iron-mediated lipid peroxidation, has been found to be involved in the pathogenesis of muscle damage caused by ischemia, heat stroke and other causes15,16, but its involvement in snakebite-induced limb muscle injury remains unconfirmed. We hypothesized that ferroptosis is involved in the process of limb muscle injury caused by venomous snake bite and that IMRC-Exo can reduce the degree of limb muscle injury caused by Deinagkistrodon by inhibiting ferroptosis. These results may provide a novel effective treatment strategy for limb muscle injury caused by D. acutus.
Results
A total of 18 rabbits were included in this study. Prior to the experiment, the basic vital signs, such as body weight, heart rate, pulse oxygen saturation, and body temperature, were normal in all groups, and there was no significant difference between the groups (Fig. 1). After modeling, all animals survived for 24 h: the 24-hour survival rate of all three groups was 100% (6/6). There was no significant difference in any parameters among the groups.
Baseline characteristics of each group. (A) Body weight. (B) Heart rate. (C) Oxygen saturation. (D) Body temperature. S, sham; SV, snake venom; IMRC-Exo, immunity and matrix regulatory cells derived exosome. The data were shown as mean ± standard deviation, and then analyzed by one-way analysis of variance followed by Bonferroni test. In (A-D), all P > 0.05 versus S group or SV group.
Before modeling, there were no significant differences in limb circumference and muscle injury markers among the three groups. After modeling, the limb circumference and serum muscle injury markers in the SV group and SV + IMRC-Exo group gradually increased. Compared with group S, the limb circumference and serum muscle injury markers in the SV group and SV + IMRC-Exo group increased significantly at 6 h after snake venom injection, and the differences between the groups were statistically significant, suggesting that limb muscle injury occurred after snake venom injection. However, the limb circumference and serum muscle injury marker levels of animals in the SV + IMRC-Exo group were consistently lower than those in the SV group, with significant between-group differences in CK at all time points after modeling, and limb circumference and Mb at 12 h and 24 h after modeling (Fig. 2).
Changes in limb circumference and muscle injury biomarkers in each group. Limb circumference. (B) Creatine kinase(CK). (C) Creatine kinase isoenzyme (Mb). BL, baseline; S, sham; SV, snake venom; IMRC-Exo, immunity and matrix regulatory cells derived exosome. The data were shown as mean ± standard deviation, and then analyzed by one-way analysis of variance followed by Bonferroni test. In (A-C), **P < 0.01, ***P < 0.001 versus S group; #P < 0.05, ##P < 0.01, ###P < 0.01 versus SV group.
Histopathological analysis showed that muscle fiber necrosis and inflammatory infiltration occurred in the muscle tissues of animals in the SV group and SV + IMRC-Exo group after modeling, whereas the pathological injury of the SV + IMRC-Exo group was significantly milder than that of animals in the SV group (Fig. 3). In addition, TUNEL assay showed that cell apoptosis occurred in the SV group and SV + IMRC-Exo group after modeling, and the apoptosis rate was significantly higher than that in the S group. However, compared with the SV group, animals in the SV + IMRC + Exo group exhibited a significantly lower apoptosis rate (Fig. 4).
Pathological changes in muscle tissue of each group. Representative micrograph of muscle tissue stained with hematoxylin eosin (200x magnification). The arrow points to “inflammatory cell infiltration and necrosis of muscle cells”. S, sham; SV, snake venom; IMRC-Exo, immunity and matrix regulatory cells derived exosome.
Analysis of cell apoptosis in limb muscle tissue in each group. (A) Representative micrograph at 200x magnification; (B) apoptosis ratio S, sham; SV, snake venom; IMRC-Exo, immunity and matrix regulatory cells derived exosome. The data were shown as mean ± standard deviation, and then analyzed by one-way analysis of variance followed by Bonferroni test. In (B), ***P < 0.001 versus S group; ###P < 0.01 versus SV group.
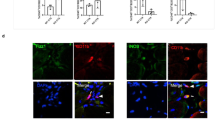
Prussian blue staining revealed blue grain-like precipitates in the muscle tissue of SV group and SV + IMRC-Exo group after modeling and the positive staining area was significantly higher than that of S group. However, the area of positive staining in the SV + IMRC-Exo group was significantly smaller than that in the SV group, suggesting that IMRC-Exo could reduce the level of iron deposition in muscle tissue after modeling. Immunofluorescence staining revealed differences in fluorescence intensity across muscle tissues after modeling. SV group (control group with stimulus) and SV + IMRC-Exo group (intervention group) exhibited significantly stronger fluorescence intensity compared to the S group (baseline control). However, the fluorescence intensity in the SV + IMRC-Exo group was significantly weaker than in the SV group (despite both being stronger than the S group). Since fluorescence intensity correlates with reactive oxygen species (ROS) levels, this indicates: IMRC-Exo reduces ROS production in muscle tissue post-modeling. In addition, the contents of MDA and 4-HNE in muscle tissues in the SV group and SV + IMRC-Exo group were significantly higher than those in the S group, while GSH content and SOD activity were significantly lower than those in the S group. However, the SV + IMRC-Exo group could significantly reverse the changes of the above indicators, suggesting that IMRC-Exo could reduce the peroxide content and promote the increase of antioxidants in muscle tissue after model establishment. These results suggest that snake venom can cause ferroptosis in muscle tissue, and that IMRC-Exo can reduce the degree of ferroptosis in muscle tissue (Fig. 5).
Changes in ferroptosis-related productions in limb muscle tissue in each group. (A,B) Representative image of Prussian blue staining (200x magnification) and its positive area analysis; (C,D) Representative images of reactive oxygen species (ROS) immunofluorescence staining (200x magnification) and their corresponding intensity analysis; (E-H) Malondialdehyde (MDA), 4-hydroxynonenal(4-HNE), glutathione(GSH) contents and superoxide dismutase (SOD) activity. S, sham; SV, snake venom; IMRC-Exo, immunity and matrix regulatory cells derived exosome. The data were shown as mean ± standard deviation, and then analyzed by one-way analysis of variance followed by Bonferroni test. In (B, D, E-H), *P < 0.05, **P < 0.01, ***P < 0.001 versus S group; ##P < 0.01, ###P < 0.01 versus SV.
Compared with the S group, the mRNA levels of ACSL4, NOX1 and COX2 were significantly increased, while the mRNA levels of GPX4 and FTH1 were significantly decreased in the SV group and SV + IMRC-Exo group after model establishment (all P < 0.05). However, compared with the SV group, IMRC-Exo could significantly downregulate the expression levels of ACSL4, NOX1 and COX2 and upregulate the expression levels of GPX4 and FTH1, suggesting that IMRC-Exo could reverse the expression levels of key genes of ferroptosis in muscle tissue after model establishment (Fig. 6).
Acyl CoA synthetase long-chain family member 4(ACSL4), NADPH Oxidase 1 (NOX1), Cyclooxygenase-2 (COX2), Glutathione Peroxidase 4 (GPX4), and Ferritin heavy chain 1 (FTH1) mRNA expression in limb muscle tissue in each group. S, sham; SV, snake venom; IMRC-Exo, immunity and matrix regulatory cells derived exosome. The data were shown as mean ± standard deviation, and then analyzed by one-way analysis of variance followed by Bonferroni test. In (A-E), *P < 0.05, **P < 0.01, ***P < 0.001 versus S group; #P < 0.05, ##P < 0.01, ###P < 0.01 versus SV group.
Discussion
In this study, rabbits were selected as experimental subjects to establish a limb injury model of poisoned rabbits bitten by D. acutus by limb injection of snake venom. The venom concentration of 1.5 mg/kg was selected based on a comprehensive literature review and preliminary experimental findings17. Next, the effect and mechanism of local injection of IMRC-Exo on the limb injury of rabbits bitten by D. acutus were investigated. The results showed that the rabbits’ limbs showed typical injury manifestations after snake venom injection, including obvious swelling of the limbs and significantly increased levels of muscle damage markers. Histopathological examination revealed that muscle tissue was seriously damaged and cell apoptosis was significantly increased. However, IMRC-Exo can significantly reduce the limb injury and histopathological damage, suggesting that IMRC-Exo has a protective effect on limb injury caused by snake venom in rabbits. Furthermore, we found that ferroptosis-related products and key genes were significantly abnormal in limb muscle tissue after snake venom injection, suggesting the occurrence of ferroptosis. Meanwhile, the application of IMRC-Exo could significantly inhibit the degree of ferroptosis, suggesting that the protective effect of IMRC-Exo may be related to the inhibition of ferroptosis in muscle tissue.
Despite that limb injury has long been the main cause of the high disability rate of snake envenomation, there is a lack of effective treatment methods in this field. In recent years, studies have shown that MSC-Exo contains a variety of bioactive components and has positive therapeutic effects on muscle injuries caused by a variety of causes. Yan et al. established an ischemic muscle injury model in rats and found that human umbilical cord-derived MSC-Exo prevented ischemic injury by releasing circHIPK3 and also inhibited cell pyroptosis and the release of IL-1β and IL-18, thereby reducing muscle damage and repairing ischemic muscle damage18. Moreover, Wang et al. established a muscle-specific Rb1 gene knockout mouse model of ischemic limb injury and found that human umbilical cord-derived MSC-Exo reduced the release of inflammatory factors by inhibiting the NLRP3 inflammasome pathway mediated by tumor suppressor Rb1, thus alleviating limb injury11. Song et al. used the mouse muscle atrophy model caused by diabetes to demonstrate that human umbilical cord-derived MSC-Exo significantly enhance the muscle strength and mass of mice, confirming that human umbilical cord-derived MSC-Exo can reduce muscle atrophy caused by diabetes and obesity by enhancing AMPK/ULK1-mediated autophagy12. Cho et al. established a mouse specific dermatitis model and found that adipose-derived MSC-Exo reduced inflammatory response by regulating immune cells and remodeling inflammatory microenvironment, thus exerting a therapeutic effect on tissue damage19. Yang et al. established a mouse second-degree burn model and indicated that human umbilical cord-derived MSC-Exo could effectively accelerate wound healing and inhibit scar formation through multiple functions mediated by miR-21-5p20. However, the therapeutic effect of MSC-Exo on limb muscle injury caused by snake venom remained to be clarified.
In this study, after combining the actual clinical situation, the relevant literature7 and preliminary experimental results, we selected the method of using 1.5 mg/kg snake venom injection for 2 h followed by 80 U/kg anti-agitatus venom serum treatment to establish an animal model. This method can not only cause serious damage to the limb muscle tissue but also ensures that there are enough viable experimental animals at the end of the experiment to complete the detection process. In addition, we combined with the literature to show that IMRC is a mesenchymal stem cell differentiated from human embryonic stem cells. Compared with mesenchymal stem cells derived from traditional adult tissues such as umbilical cord, bone marrow, and adipose tissue, IMRC has better cell quality and stronger damage prevention and repair ability, and can penetrate biological barriers, regulate immune cells, and remediate inflammatory microenvironment, hence it plays a role in repairing muscle damage14,18,21. Therefore, we preferentially selected Exo from IMRC for this study and injected it locally into the injured limb to obtain a better therapeutic effect. The results showed that compared with the SV group, animals in the IMRC-Exo group had significantly reduced limb swelling and muscle injury markers, the pathological damage of muscle tissue was significantly milder, and the degree of apoptosis was significantly reduced, suggesting that the topical administration of IMRC-Exo had a positive therapeutic effect on reducing limb muscle injury caused by D. acutus.
Research has shown that ferroptosis is a newly discovered programmed cell death pathway driven by iron overload and lipid peroxidation. Its main mechanism is characterized by an increase in ferrous ions in damaged tissue cells due to various stimuli, which catalyze the production of ROS through the Fenton reaction. The latter undergoes lipid peroxidation with polyunsaturated fatty acids, leading to oxidative damage to biological membranes, proteins and nucleic acids, resulting in the ferroptosis of cells22. Studies have increasingly confirmed that ferroptosis is involved in the pathogenesis of muscle injuries caused by various reasons and has become one of the effective therapeutic targets23,24. Ju et al. established a muscle atrophy rat model and demonstrated the involvement of ferroptosis in the process of muscle atrophy. The application of renal failure nutrition capsules to activate the HIF-1 α/SLC7A11 pathway can inhibit ferroptosis and improve muscle function25. He et al. established a mouse model of exercise-induced rhabdomyolysis. The results showed that ferroptosis is involved in the process of muscle necrosis, and its main mechanism is related to ACSL4 overactivation mediated ferroptosis of muscle cells16. Wang et al. constructed a mouse model of lower limb ischemia/reperfusion injury and used syringic acid to block the HMGB1 pathway, inhibiting ferroptosis of skeletal muscle cells and reducing lower limb injury15. In addition, MSC Exo has been found to exert therapeutic effects in alleviating various diseases by regulating the ferroptosis pathway. Zhang et al. established iron-mediated cell death models and radiation-induced lung injury mouse models, and confirmed by in vitro and in vivo experiments that miR-486-5p engineered MSC-Exo can alleviate radiation-induced acute lung injury and late stage pulmonary fibrosis by inhibiting ferroptosis26. Chen et al. used a BV2 cell iron-mediated cell death model and a spinal cord injury rat model to find that bone marrow-derived MSC-Exo can inhibit iron-mediated cell death and alleviate the severity of acute spinal cord injury by regulating the Nrf2/GCH1/BH4 signaling axis27. Lin et al. established a mouse model of acute liver injury and found that MSC-Exo can inhibit ferroptosis by stabilizing SLC7A11 activity, thereby reducing the severity of acute liver injury28. Based on the above literature, we opted to observe the phenomenon of muscle tissue ferroptosis caused by snake venom and explore the potential regulatory role of MSC-Exo. The results showed that after injection of D. acutus venom, the levels of peroxides MDA and 4-HNE in limb muscle tissue significantly increased, while the expression of iron-mediated cell death-promoting genes ACSL4, NOX1 and COX2 was significantly upregulated. At the same time, the levels of antioxidants GSH and SOD were significantly reduced and the expression of anti-iron-mediated cell death genes GPX4 and FTH1 was significantly downregulated, indicating that snake venom caused ferroptosis in muscle tissue. However, compared with the SV group, the application of IMRC-Exo local injection therapy could significantly reduce the above-mentioned peroxide content, inhibit the expression of ferroptosis promoting genes, and significantly increase the level of antioxidants and promote the expression of anti-ferroptosis genes. Therefore, it is asserted that IMRC Exo can play a protective role in reducing limb muscle damage caused by snake venom by inhibiting the process of ferroptosis.
Limitations
This study has several limitations. Firstly, in this study, we focused exclusively on the effects of apoptosis and ferroptosis, without investigating other factors such as inflammation, mitochondrial damage, or pyroptosis. Additionally, our current research is limited to evaluating the efficacy of exosomes, and it remains unclear whether this approach represents the most optimal treatment strategy compared to alternative options. Third, this study had a small sample size and used only a single dose of IMRC-Exo. Future studies with a larger sample size are needed to confirm the effectiveness of IMRC-Exo in reducing limb injury caused by snake bites and its optimal use.
Conclusions
This study provides the first evidence that IMRC Exo has a therapeutic effect in reducing limb damage caused by D. acutus bites in rabbits, and the relevant protective mechanism may be related to inhibiting the process of ferroptosis.
Materials and methods
Animals
A total of 18 healthy New Zealand white rabbits (male, 5–7 months old, 2.5–3 kg) were purchased from Hongfeng Rabbit Farm, Fuyang, Hangzhou City. All experimental animals were fed under standard conditions, such as a room temperature of 20 ~ 25℃, humidity of 60 ~ 80%, alternating day and night cycle (12 h/12 h), regular cleaning, drinking ad libitum, and routine feeding. After the animals were stable for one week, then they subjected to the experiment. The study was approved by the Animal Ethics Committee of Lishui University School of Medicine (No. 2025YD0001) and conducted in accordance with Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes. All methods were performed in accordance with the relevant guidelines and regulations. Additionally, all sections of this report comply with the ARRIVE Guidelines for reporting animal research and comply with the 3R Principles for animal research.
Anesthesia and surgical preparation
Before the experiment, rabbits were fasted for 12 h with free access to water. During the experiment, the rabbits were weighed, placed in the experimental animal fixator, and set in the prone position to fix the limbs. The left lower limb and right ear of the animals were routinely skin-prepared with a professional razor and the left lower limb was fully exposed. The outer middle part of the left thigh was marked as the injection point of snake venom, and the thigh circumference of the marked point was measured and recorded. The heart rate and oxygen saturation were measured and recorded, along with the body temperature through the right ear of the experimental rabbits using an ear thermometer. Anesthesia was performed by intravenous injection of 3% pentobarbital solution (1 mL/kg) through the ear edge vein.
Experimental procedures
Animal grouping and model establishment
The experimental animals were randomly divided into sham operation group (group S), snake venom group (SV) and SV + IMRC-Exo group (n = 6 in each group). In the SV and SV + IMRC-Exo groups, rabbit snakebite models were established by limb injection of 1.5 mg/kg snake venom followed by intravenous injection of 80 U/kg antivenom 2 h later. The Agkistrodon acutus antivenin was procured from Shanghai Seron Biotechnology Co., LTD. (Batch No.: 20230905; Manufacturing Date: Sep 26, 2023; Expiry Period: 36 months). For each administration, the lyophilized powder was reconstituted aseptically by adding sterile 0.9% (w/v) sodium chloride injection. The resulting mixture was gently agitated until complete dissolution was achieved. The solution was then further diluted with sterile saline to a final volume of 20 mL, yielding a final concentration of 4 U/mL. Lyophilized powder of Deinagkistrodon venom was purchased from Shanghai Seron Biotechnology Co., LTD. (Batch No. 20200410). The lyophilized powder was dissolved in normal saline and prepared at a concentration of 10 mg/mL before use. The specific method of modeling was as follows: the position of the middle part of the left thigh was selected as the injection point, the vertical needle was inserted, the depth was 5 mm, and 1.5 mg/kg snake venom was injected. After completion of the injection, a cotton swab was used to press the injection site for 1 min to prevent fluid leakage. In group S, the same volume of normal saline was injected into the limb and vein.
IMRC-Exo was purchased from Hangzhou Luyuan Biotechnology Co., LTD. At the time of serum injection during the modeling period, the SV + IMRC-Exo group was subcutaneously injected with 7.5 × 1010 particles of IMRC-Exo diluted in normal saline, and the other two groups were injected with the same amount of normal saline. IMRC-Exo was purchased from Hangzhou Luyuan Biotechnology Co., LTD. and prepared into 5 × 1010 particles/mL solution, with a total volume of 1.5 mL with normal saline for reserve. The specific application method was as follows: with the injection point of snake venom as the center, 4 positions with equal spacing in the circumference of 0.5 cm and 6 positions with equal spacing in the circumference of 1 cm were selected to inject IMRC-Exo in a total of 10 places, and each place was injected with 0.15 mL of Exo.
All experimental animals were observed for 6 h after the administration of snake venom and then returned to the rabbit cage for 18 h. Subsequently, the animals were euthanized by intravenous injection of sodium pentobarbital 150 mg/kg, and the main organs of the animals were routinely dissected to observe the general condition of limb muscles.
Measurements
Physiological monitoring and gross morphological assessment
Heart rate, pulse oxygen saturation, body temperature, and other general vital signs were dynamically observed, and survival was assessed after venom injection. The circumference of the thigh at the injection site was measured at baseline and subsequently at 6 h, 12 h and 24 h after venom injection.
Serum biomarker analysis
Venous blood samples (2 mL) were collected at baseline and at 6 h, 12 h, and 24 h after venom injection. Samples were centrifuged to obtain plasma, which was then stored. Serum levels of creatine kinase (CK), myoglobin (Mb), and other muscle injury markers were measured by enzyme-linked immunosorbent assay (ELISA) kits (purchased from Shanghai Meixuan Biotechnology Co., LTD.) according to the manufacturer’s instructions.
HE staining, TUNEL staining, and Prussian blue staining
At the end of the experiment, the animals were euthanized and the muscle tissue samples around the injection points of snake venom were quickly obtained. Some samples were fixed with 4% paraformaldehyde for 24 h, and then pathological samples were prepared by paraffin, dehydration, section and other steps. HE staining was performed to observe the gross pathological changes of muscle tissue.
Pathological samples were prepared as above and stained with an in situ Nick end labeling (TUNEL) assay kit (purchased from Wuhan Baodu Company) according to the manufacturer’s instructions. Three randomly selected fields were photographed under a light microscope (C31 Biological microscope, Olympus, Japan) at 200 × magnification. The number of TUNEL-positive cells and total cells were counted, and the percentage of TUNEL-positive cells in total cells was used as the apoptotic rate.
Similarly, pathological samples were made, and the sections were rehydrated, incubated with Prussian blue staining solution, rinsed in distilled water, then stained with nuclear fast red. Finally, the Prussian blue-stained specimens were photographed at ×200 magnification under an optical microscope, and then the percentage of positive staining area was analyzed.
(ROS) measurement
Reactive oxygen species (ROS) in the muscle was measured with the dedicated assay kit (Solarbio, Beijing, China) according to the manufacturer’s instructions, in which tissue samples were rapidly frozen in liquid nitrogen, sliced into 5-µm sections, incubated with 2’,7’- dichloroflurorescein diacetate (1:500) at 37℃ for 30 min, and finally observed with a fluorescence microscope (Olympus, Tokyo, Japan). Three random fields were photographed at 200×magnification, and the intensity of fluorescence was analyzed with Image J image analysis software (NIH, Bethesda, MD).
MDA, GSH, SOD, and 4-HNE measurements
Some fresh muscle tissue samples were obtained and homogenized by rapid freezing in liquid nitrogen and long-term freezing in a −80℃ refrigerator. According to the manufacturer’s protocol, the products related to ferroprosis were detected, including the contents of malondialdehyde (MDA) and glutathione (GSH), the activity of superoxide dismutase (SOD) by biochemical methods, and the content of 4-hydroxynonenal (4-HNE) by ELISA. The corresponding kits were purchased from Nanjing Jiancheng BioEngineering Institute.
qRT-PCR analysis of ferroptosis markers
The mRNA expression levels of ferroptosis-related proteins ACSL4, NOX1, COX2, GPX4, and FTH1 in fresh muscle tissues were detected by qRT-PCR. The protocol details are as follows: Total RNA from muscle tissue samples was extracted using TRIzol reagent (Invitrogen, Carlsbad, USA). RNA quantification was performed using a Nanodrop instrument (TMO, Massachusetts, USA). Subsequently, cDNA synthesis was carried out using a RT kit (Yeasen, Shanghai, China). PCR amplification was conducted using a qPCR Mix (Yeasen, Shanghai, China). GAPDH was used as the internal reference. The relative expression levels were calculated using the 2 − ΔΔCt method. The sequences of the primers are shown in Table 1.
Statistical analysis
SPSS 20.0 software (IBM, USA) was used for statistical analysis. The normality of the distribution of data was confirmed by the Kolmogorov-Smirnov test and the data were presented as the means ± standard deviations. One-way analysis of variance was used for comparisons among the three groups, and the Bonferroni post hoc correction was used for further pairwise comparisons. P < 0.05 was considered as indicative of statistical significance.
Data availability
The raw data in this study are included in the article and supplementary materials. For further inquiries, please contact the corresponding author.
References
Chippaux, J. P. Snakebite envenomation turns again into a neglected tropical disease! J. Venom. Anim. Toxins Incl. Trop. Dis. 23, 38. https://doi.org/10.1186/s40409-017-0127-6 (2017).
Warrell, D. A. & Williams, D. J. Clinical aspects of snakebite envenoming and its treatment in low-resource settings. Lancet 401 (10385), 1382–1398. https://doi.org/10.1016/S0140-6736(23)00002-8 (2023).
Arnold, C. Vipers, Mambas and taipans: the escalating health crisis over snakebites. Nature 537 (7618), 26–28. https://doi.org/10.1038/537026a (2016).
Qin Gongping. Toxicology of Snakes in China [M] 2nd edn p. 46–772 (Guangxi Science and Technology, 1998). [in Chinese].
Seifert, S. A., Armitage, J. O. & Sanchez, E. E. Snake envenomation. Reply. N Engl. J. Med. 386 (11), 1100. https://doi.org/10.1056/NEJMc2201552 (2022).
Ha, S. J. et al. Qualitative analysis of proteins in two snake venoms, Gloydius Blomhoffii and Agkistrodon Acutus. J. Pharmacopunct. 25 (1), 52–62. https://doi.org/10.3831/KPI.2022.25.1.52 (2022).
Lai, L. et al. The establishment and evaluation of a swine model of deinagkistrodon acutus snakebite envenomation. Toxicon 241, 107683. https://doi.org/10.1016/j.toxicon.2024.107683 (2024).
Bhaumik, S. et al. Interventions for the management of snakebite envenoming: an overview of systematic reviews. PLoS Negl. Trop. Dis. 14 (10), e0008727. https://doi.org/10.1371/journal.pntd.0008727 (2020). Published 2020 Oct 13.
Luo, Y. et al. Wei Zhong Bing Ji Jiu Yi Xue ;32(10):1241–1246. doi:https://doi.org/10.3760/cma.j.cn121430-20200316-00217. (2020).
Zheng, Z. et al. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue ;29(11):1026–1029. doi:https://doi.org/10.3760/cma.j.issn.2095-4352.2017.11.013. (2017).
Wang, Y. et al. Stem cell-derived exosomes repair ischemic muscle injury by inhibiting the tumor suppressor Rb1-mediated NLRP3 inflammasome pathway. Signal. Transduct. Target. Ther. 6 (1), 121. https://doi.org/10.1038/s41392-021-00520-8 (2021).
Song, J. et al. Mesenchymal stromal cells ameliorate diabetes-induced muscle atrophy through exosomes by enhancing AMPK/ULK1-mediated autophagy. J. Cachexia Sarcopenia Muscle. 14 (2), 915–929. https://doi.org/10.1002/jcsm.13177 (2023).
Zhao, Y. et al. Human ESC-derived immunity- and matrix- regulatory cells ameliorated white matter damage and vascular cognitive impairment in rats subjected to chronic cerebral hypoperfusion. Cell. Prolif. 55 (5), e13223. https://doi.org/10.1111/cpr.13223 (2022).
Wu, J. et al. Immunity-and-matrix-regulatory cells derived from human embryonic stem cells safely and effectively treat mouse lung injury and fibrosis. Cell. Res. 30 (9), 794–809. https://doi.org/10.1038/s41422-020-0354-1 (2020).
Wang, F. et al. Syringic acid suppresses ferroptosis of skeletal muscle cells to alleviate lower limb ischemia/reperfusion injury in mice via the HMGB1 pathway. Chem. Biol. Drug Des. 102 (6), 1387–1398. https://doi.org/10.1111/cbdd.14326 (2023).
He, S. et al. ACSL4 contributes to ferroptosis-mediated rhabdomyolysis in exertional heat stroke. J. Cachexia Sarcopenia Muscle. 13 (3), 1717–1730. https://doi.org/10.1002/jcsm.12953 (2022).
Youya, L. & Xue, X. Effect of extracting venom in ughtening the toxicity of Agkistrodon Acutus injury in rabbits. Hainan Med. J. 24 (19), 2813–2815 (2013).
Yan, B. et al. Stem cell-derived exosomes prevent pyroptosis and repair ischemic muscle injury through a novel Exosome/circHIPK3/ FOXO3a pathway. Theranostics 10 (15), 6728–6742. https://doi.org/10.7150/thno.42259 (2020).
Cho, B. S., Kim, J. O., Ha, D. H. & Yi, Y. W. Exosomes derived from human adipose tissue-derived mesenchymal stem cells alleviate atopic dermatitis. Stem Cell. Res. Ther. 9 (1), 187. https://doi.org/10.1186/s13287-018-0939-5 (2018).
Yang, Y. et al. Exosome/antimicrobial peptide laden hydrogel wound dressings promote scarless wound healing through miR-21-5p-mediated multiple functions. Biomaterials 308, 122558. https://doi.org/10.1016/j.biomaterials.2024.122558 (2024).
Gomzikova, M. O., James, V. & Rizvanov, A. A. Therapeutic application of mesenchymal stem cells derived extracellular vesicles for Immunomodulation. Front. Immunol. 10, 2663. https://doi.org/10.3389/fimmu.2019.02663 (2019).
Stockwell, B. R. et al. Ferroptosis: A regulated cell death Nexus linking metabolism, redox biology, and disease. Cell 171 (2), 273–285. https://doi.org/10.1016/j.cell.2017.09.021 (2017).
Lv, J. et al. The relationship between ferroptosis and diseases. J. Multidiscip Healthc. 15, 2261–2275. https://doi.org/10.2147/JMDH.S382643 (2022). Published 2022 Oct 6.
Riegman, M. et al. Ferroptosis occurs through an osmotic mechanism and propagates independently of cell rupture. Nat. Cell Biol. 22 (9), 1042–1048. https://doi.org/10.1038/s41556-020-0565-1 (2020).
Ju, L. et al. Shenshuai Yingyang Jiaonang ameliorates chronic kidney disease-associated muscle atrophy in rats by inhibiting ferroptosis mediated by the HIF-1α/SLC7A11 pathway. Heliyon 10 (8), e29093. https://doi.org/10.1016/j.heliyon.2024.e29093 (2024). Published 2024 Apr 15.
Zhang, W. Y. et al. S-RBD-modified and miR-486-5p-engineered exosomes derived from mesenchymal stem cells suppress ferroptosis and alleviate radiation-induced lung injury and long-term pulmonary fibrosis. J. Nanobiotechnol. 22 (1), 662. https://doi.org/10.1186/s12951-024-02830-9 (2024).
Chen, Y. et al. Inhibition of ferroptosis by mesenchymal stem Cell-Derived exosomes in acute spinal cord injury: role of Nrf2/GCH1/BH4 Axis. Neurospine 21 (2), 642–655. https://doi.org/10.14245/ns.2448038.019 (2024).
Lin, F. et al. Mesenchymal stem cells protect against ferroptosis via exosome-mediated stabilization of SLC7A11 in acute liver injury. Cell. Death Dis. 13 (3), 271. https://doi.org/10.1038/s41419-022-04708-w (2022). Published 2022 Mar 26.
Author information
Authors and Affiliations
Contributions
L. L. were responsible for drafting the initial manuscript and subsequent revisions. W. D. and H. W. contributed to the animal experiments. W.L. and K.L. contributed to the data collection and analysis. P.S. and L.S. performed immunohistochemistry and other related experiments.J.X. and P. L. provided overall supervision and guidance for the study. All authors discussed results and commented onthe manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lai, L., Du, W., Wu, H. et al. IMRC-exo alleviates limb injury by inhibiting ferroptosis in a rabbit model of deinagkistrodon acutus snakebite envenomation. Sci Rep 15, 24586 (2025). https://doi.org/10.1038/s41598-025-10746-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-10746-z