Abstract
This in vivo animal study aimed to evaluate the diagnostic reliability of a damping capacity assessment by correlating dental implant stability with peri-implant bone loss in a beagle dog model. Thirty-two bone-level implants were immediately placed in four beagle dogs without bone grafting. Implants were divided into platform switching and platform matching groups. Each implant was loaded with a superstructure after a 4-week healing period, followed by 8 weeks of functional loading. Damping capacity assessment and resonance frequency analysis were performed every 4 weeks. Peri-implant bone loss, supporting bone volume, and bone-to-implant contact were assessed using micro-CT and histological analysis. Two implants in the platform switching group failed during the study. Among the remaining 30 implants, final implant stability indices by damping capacity assessment ranged from 44 to 80. Peri-implant bone loss varied from 0.25 mm to 6.96 mm. A strong negative correlation was found between final stability indices and bone loss (r = − 0.745; p ≤ 0.0001). Supporting bone volume and bone-to-implant contact ratio showed significant positive correlations with implant stability. Damping capacity assessment demonstrated diagnostic relevance by reflecting peri-implant bone loss and the degree of osseointegration. However, its ability to detect early bone resorption is uncertain.
Similar content being viewed by others
Introduction
Dental implant therapy has become a widely accepted and predictable option for tooth replacement, relying heavily on the achievement and maintenance of osseointegration. Historically, this process involved an unloaded healing period of 3 to 6 months to ensure stable bone-implant integration1,2,3,4. Recent advances in implant macro- and micro-design, surface modifications, and surgical protocols have dramatically shortened healing periods and enabled immediate or early loading in cases with sufficient primary stability5. In line with these advancements, immediate implant placement has become feasible for implants with high primary stability, making it an increasingly attractive solution for both patients and clinicians6,7. This treatment alternative offers several advantages, including reduced treatment time and fewer surgical interventions, which in turn enhances patient satisfaction. However, immediate implant placement may lead to a higher failure rate due to unpredictable future changes in soft and hard tissue levels. Thus, careful case selection is needed to ensure a successful outcome8,9,10,11,12,13.
Implant stability is typically divided into primary (mechanical) and secondary (biological) stability. Primary implant stability is known to be one of the key factors for success associated with placement and loading protocols14,15,16,17,18,19. Accurate assessment of stability is critical, particularly when applying immediate or early loading protocols. Various methods have been proposed and investigated clinically for evaluating implant stability20. Two widely accepted non-invasive methods for assessing implant stability are resonance frequency analysis (RFA) and damping capacity assessment (DCA). RFA is a technique that measures the stiffness function of the bone/implant complex to assess implant stability21,22. Measured resonance frequency of a transducer attached to the implant is converted into a value called Implant Stability Quotient (ISQ). Typically, implant stability is measured using an ISQ value in the range of 45 to 85. Implants with ISQ values above 60 may be suitable for early loading23. DCA measures deflection/deceleration of a tooth or implant that has been struck by a metal rod from inside the instrument’s handpiece. The contact time of the accelerated metal rod to the implant, which moves according to the strike, is calculated as a value called Periotest™ value (PTV), which ranges from − 8 to + 50 with decreasing stability of the tooth or implant24. PTVs above 10 are associated with failure of osseointegration25,26. Several studies have investigated the ability and usefulness of RFA and DCA to measure implant stability. These studies suggested that these methods are correlated and significantly reflected the peri-implant bone level. However, the RFA method in which the metal peg is directly connected to the implant is limited in its use once the prosthesis is installed. On the other hand, the DCA method using repeated impacts should be used carefully when the initial stability is low right after implant placement20,27,28.
Recently, a modified DCA device (Anycheck, Korea) designed to display implant stability on a scale of 1 to 100 has proven to be clinically useful due to its simplicity and versatility in both clinical and research settings29. Clinical validation studies have demonstrated the effectiveness of modified DCA devices, showing comparable reliability to both conventional DCA systems such as Periotest M and RFA-based systems such as Osstell during healing periods30,31. Moreover, recent investigations have emphasized the diagnostic reliability of non-invasive tools, including DCA, across diverse bone resorption patterns and implant loading conditions32. Comparative analyses suggest that DCA exhibits moderate to strong agreement with RFA, although its sensitivity may vary depending on device configuration33. In addition, site-specific variability in DCA measurements, influenced by the direction of measurement and surrounding bone morphology, continues to present a challenge in clinical practice34. Therefore, more studies are needed to further explore the accuracy of implant stability measured by DCA and its potential clinical utility25,35.
Therefore, this in vivo animal study aimed to evaluate the diagnostic reliability and clinical applicability of DCA by analyzing its correlation with peri-implant bone loss, supporting bone volume, and histological bone-to-implant contact.
Results
Data analysis
Two platform-switched implants with internal connection types in the test group failed at 8 and 12 weeks after placement, respectively. Sample size was adjusted except for the two failed implants (n = 30). Final implant stability indices measured at 12 weeks were mostly in the range of 60 to 80, except for two samples that were 44; the mean ± standard deviation was 69.81 ± 8.13. The amount of peri-implant bone loss measured in four directions varied from 0.25 mm to 6.96 mm, and the mean value was 3.06 mm. The mean and standard deviation of supporting bone volume was 21.95 ± 2.83 mm3. The maximum value was 27.93 mm3 and the minimum value was 15.44 mm3. Bone-to-implant contact was analyzed based on combined buccal and lingual values, ranging from a maximum of 74% to a minimum of 22.1%, with a mean value of 46.94%. The raw data for the variables in this study are available in Supplementary Table S1 online.
Normality test results showed that most stability indices did not satisfy normality except for the RFA index at 8 weeks, whereas peri-implant bone loss of the parameters excluding the lingual side met the criteria for normality. Normality test results showed that both bone to implant contact and supporting bone volume satisfied the normality assumption. Since several factors failed to satisfy the normality test, non-parametric statistical analysis was selected (Table 1).
Correlation with stability indices for each variable
In all samples, the peak insertion torque values of the implants were not significantly correlated with any variables. As a result of categorizing and grouping the data on the final implant stability indices and peri-implant bone loss, box plot graphs showing a negative correlation trend were observed in both criteria (Fig. 1). When the samples were grouped according to final stability index and compared based on mean peri-implant bone loss, the median value for the three samples with a stability index less than 65 was 5.37 mm, and the median value for the six samples with a stability index between 65 and less than 70 was 3.68 mm. The median value was observed to be 3.52 mm in 14 samples with a stability index between 70 and 75, and the median value was 1.39 mm in 7 samples with a stability index between 75 and 80. On the other hand, as a result of classifying the final stability index of all samples based on the mean value of peri-implant bone loss, the median value of the stability index for 10 samples with bone loss less than 2 mm was 77, and for 12 samples with bone loss from 2 mm to 4 mm, the median value was 70.7. The median stability index for eight samples with bone loss > 4 mm was 65.8.
Box plot graphs visualized using data grouping to determine distribution and trends between Anycheck value (ACV) and peri-implant bone loss (pIBL). Distribution of ACV data divided into three groups based on pIBL 2 mm and 4 mm as the boundary (a). Distribution of pIBL data divided into four groups based on the final stability indices referred as ACV (b).
The peri-implant bone loss pattern measured in four directions was found to have a statistically significant correlation with the final stability index in all directions: buccal (r = − 0.736; p ≤ 0.0001), lingual (r = − 0.712; p ≤ 0.0001), mesial (r = − 0.688; p ≤ 0.0001), and distal (r = − 0.678; p ≤ 0.0001). Mean, maximum, and minimum values of peri-implant bone loss calculated for each implant also showed statistically significant negative correlations with final implant stability indices (Fig. 2), with the minimum value showing the strongest negative correlation: mean (r = − 0.745; p ≤ 0.0001), maximum (r = -0.721; p ≤ 0.0001), and minimum (r = − 0.800; p ≤ 0.0001).
A statistically significant positive correlation (r = 0.593; p = 0.0004) was observed between supporting bone volume and final implant stability index. The bone to implant contact ratio also showed a statistically significant positive correlation with the final stability index for both buccal (r = 0.563; p ≤ 0.0001) and lingual (r = 0.728; p ≤ 0.0001) sides. A relatively low correlation was confirmed on the buccal side than on the lingual side (Fig. 3).
Analysis related to implant stability indices and measurement methods
A statistically significant correlation was observed between implant stability indices measured at 4 and 8 weeks using the RFA and DCA methods. A moderate positive correlation (r = 0.750; p ≤ 0.0001) was observed at 4 weeks. A high positive correlation (r = 0.887; p ≤ 0.0001) was observed at 8 weeks. However, at 12 weeks, the stability index using the RFA method could not be measured due to the implant’s superstructure. Therefore, correlation could not be assessed.
Changes in implant stability over time were evaluated by comparing differences in stability index measured with the same method. Correlations with other variables were also analyzed. As a result, differences in stability index between individual time points (4, 8, and 12 weeks) did not show a significant correlation with peri-implant bone loss or bone to implant contact ratio. However, they showed a correlation with the three-dimensional volume of the supporting bone. Except for the difference in stability index between 4 and 8 weeks, all differences including the 12-week stability index showed statistically significant correlation with the supporting bone volume (Table 2).
Discussion
DCA method is considered a valuable clinical tool, along with RFA method, for the prevention, diagnosis, and prediction of implant failures, which can aid in the maintenance and management of implants21,36,37. The validity of these technologies has been determined through methods such as mechanical testing, radiographic examinations, and histological evaluations38,39,40,41,42. This study aimed to evaluate the reliability of the final stability index through the DCA method for implants with various bone levels by evaluating the amount of bone loss around the implant, bone-implant contact ratio, and implant supporting bone volume. A previous in vitro study demonstrated significantly different indices of implant stability measurements by the DCA method at a peri-implant bone level of approximately 2 mm29. However, this suggests that measuring implant stability in a dynamic environment before osseointegration as opposed to after stable osseointegration can show significant differences. In this in vivo study, despite applying an immediate placement protocol to implants, it was intentional to perform early loading and exclude bone grafting to simulate a range of bone resorption states. Most samples in each group showed a normal distribution. The final stability index was confirmed to follow a normal distribution except for two values below 60. However, other stability index groups did not follow a normal distribution due to an insufficient number of samples. Therefore, non-parametric statistical analysis was applied. Ultimately, all implants involved in this experiment showed adequate results for implant stability measurements intended in this study. This animal study with immediate placement and early loading protocol could potentially be recognized as a single stability experimental model with a standard normal distribution for a variety of stability evaluations.
In this study, effects of immediate implantation and early loading protocols were observed using two different types of implants. Although all implants showed stable insertion torque values (10 to 40 N·cm) and were initially submerged without loading for 4 weeks, the fact that loading began early, after 4 weeks, may have contributed to implant failure in some cases. Depending on the implant design, early loading protocols without bone grafting might still pose risks despite adequate insertion torque. In particular, in the case of conical internal connection type implants developed to evenly distribute the load throughout the entire surface of implant body, excessive early loading force could be a detrimental condition for osseointegration. However, external connection implants without a platform switching design might have provided relative stability even in the early stages of osseointegration due to axial load transmission through the abutment screw.
The mean values of peri-implant bone loss for the 30 implants in this study ranged from 0.52 mm to 6.23 mm for an 8.5 mm long implant. As shown in Fig. 1, when divided into three groups based on the 2 mm interval of peri-implant bone loss, a significant median difference (> 4 ACV units) was observed between groups. This indicates that the same DCA measuring device demonstrated in our previous in vitro study is also effective in distinguishing 2 mm of peri-implant bone loss, even in vivo. Results of this study indicated a significant correlation between the amount of alveolar bone loss and the final ACV. The final ACV measured from all four aspects (buccal, lingual, mesial, distal) exhibited a negative correlation with the amount of alveolar bone loss. Particularly, in the table depicting the relationship between minimum values of alveolar bone loss and the final ACV, it could be observed that minimum values of alveolar bone loss were clustered near the upper limit of the linear regression line. It was shown that if osseointegration was achieved by contact with the alveolar bone in only one direction around the implant, implant stability could be sustained via localized bone support in that direction.
In this study, the 3-dimensional supporting bone volume around the implant was quantitatively measured using micro-CT. The results of measuring the bone volume outside the implant in a cylindrical area bordered with a diameter of 4.25 mm, which is representing a 25% increase over the core diameter (3.0 mm, threads excluded), were expected to be related to the density or elasticity of the supporting bone around the implant. BIC was determined by histomorphometry as the area of the implant surface in contact with bone. The previous inconsistency in the correlation between implant stability measurements and BIC might be attributed to the use of 2-dimensional histomorphometric evaluations43,44,45,46. This is because one or a few tissue cross-sections cannot provide a comprehensive view of the implant-bone interface. In addition, unlike in other studies limited to marginal bone loss, the range of peri-implant bone loss in this study varied from 5.9 to 73.3%. Therefore, significant differences in BIC might have resulted from the heterogeneous patterns of peri-implant bone loss. As a result, both supporting bone volume and BIC showed significant correlations with the final implant stability indices.
Previous in vivo studies have reported that RFA and DCA device values show moderate (between 0.3 and 0.7) or weak (less than 0.3) correlations47. In this study, implant stability measured by RFA and DCA at 4 and 8 weeks demonstrated a strong correlation. A strong relationship (r = 0.856) was observed between O8 and A8 measured at 8 weeks, indicating that DCA devices and RFA devices have similar reliability in implant measurements. This is consistent with results reported in other studies29,48,49. However, the use of metal peg with RFA devices prevented the measurement of implant stability at 12 weeks when the superstructure was connected. Therefore, final stability comparisons between DCA and RFA could not be performed due to measurement constraints at 12 weeks. Furthermore, the change in implant stability over time showed a significant correlation with the supporting bone volume in outcomes such as A12-A4 and A12-A8, which included the final implant stability index measured at 12 weeks. A12-A4 showed a low positive correlation, while A12-A8 showed a moderate positive correlation. This suggests that as time progresses and osseointegration is completed, a higher correlation between supporting bone volume and implant stability becomes evident.
Based on the results of this in vivo study, the following conclusions could be drawn. Various patterns of peri-implant bone loss have been observed when immediate implant placement and early loading protocols excluding bone grafting were used. Peri-implant bone loss evaluated by radiographic or histomorphometric analysis was significantly correlated with the implant stability index measured using the DCA method. However, the diagnostic ability of early bone resorption of implants is controversial because the clinical efficacy of the DCA method is determined by significant progression of peri-implant bone loss.
Materials and methods
Dental implants and abutment
A total of 32 commercial dental implants with a diameter of 3.5 mm, a length of 8.5 mm, and a sandblasted and acid-etched surface (TSIII SA & USII SA Fixture, Osstem Implant, Seoul, Korea) were used. Half of these bone level implants were of the platform switching type with an internal connection (TSIII SA) as a test group. The other half were of the platform matching type with an external connection (USII SA) as a control group. One-piece titanium abutments (5 mm height) were used during the healing period, while two-piece customized titanium abutments (6 mm height, 7 mm diameter) were fabricated for the functional loading period.
Experimental design
This study was performed with the approval of the Ethics Committee of Animal Experimentation of the Institutional Animal Care and Use Committee (CRONEX-IACUC approval no. 201906003; Cronex, Hwasung, Korea). In addition, all the study procedures, including animal selection, management, preparation, and surgical protocols, were conducted in accordance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines. Therefore, all methods were carried out in accordance with relevant guidelines and regulations.
The experimental design was referenced from a previous beagle dog study by Blanco et al. in 2012, which involved an immediate implantation and immediate loading protocol after tooth extraction50. Nevertheless, because the purpose of this study was different from previous studies, some methods such as implant type, placement position, loading time, and superstructures were modified. This experimental design compared implant stability and bone resorption patterns of two types of commercially available implants with clinically proven success rates, a sample size of four animals was judged to be sufficient for verification.
Four male beagle dogs (Orientbio, Seongnam, Korea) aged approximately one year were used in this study. Anesthesia was induced with subcutaneous atropine (0.05 mg/kg), followed by intravenous xylazine (2 mg/kg) and ketamine hydrochloride (10 mg/kg). Maintenance was achieved via endotracheal enflurane inhalation (2%). Conventional dental infiltration anesthesia (lidocaine 40 mg, 0.2% epinephrine) was administered at the surgical sites. Bilateral extractions of mandibular premolars and molars (P2–M1) were performed. Crestal incisions were performed in the premolar–molar region of the mandible. Full-thickness mucoperiosteal flaps were elevated.
Two consecutive implants with identical connection types were randomly assigned in 16 different regions, symmetrically divided into left and right, anterior and posterior regions, using a randomized block design to minimize differences between individuals and between implantation sites. As a result, four tapered implants were immediately placed on each side of mandible by a single clinician under sterile conditions. Peak insertion torque value was measured manually by a torque wrench. Liquid diets were provided postoperatively to minimize masticatory trauma during healing. All implants were submerged for the first four weeks. After four weeks of implant placement, a healing abutment was connected to the implant with a constant force of up to 10 N·cm using a digital torque meter. After soft tissue healing, a final titanium prosthesis was loaded to the implant during the final four weeks. Titanium abutment screws were torqued to 30 N·cm and the screw hole was filled with polytetrafluoroethylene and resin materials. At 12 weeks, experimental animals were sacrificed by administering concentrated sodium pentobarbital IV (Euthasol, Delmarva Laboratories, Inc., Midlothian, VA, USA).
Implant stability measurement
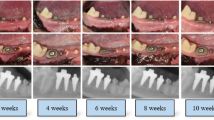
Implant stability (three repeated sets) was measured using a damping capacity assessment device (Anycheck, Neobiotech, Seoul, Korea). This device measured the contact time between the metal tapping rod and the implant superstructure using six consecutive impacts in one set, displaying results as a stability index called the Anycheck value (ACV). DCA measurements were performed consistently on the buccal side. During both healing and loading phases, measurements were conducted approximately 5 mm above the implant platform, corresponding to the top of the healing abutment (5 mm) or near the top of the final abutment (6 mm), ensuring consistency across time points.
Implant stability quotient (ISQ) was also acquired three times each as a reference using a resonance frequency analysis device (Osstell ISQ, Integration Diagnostics, Gothenburg, Sweden). Both implant stability measurements were performed every four weeks (Fig. 4). However, ISQ values were not obtained at 12 weeks when the superstructure was fixed to the implant. Using RFA, the difference between indices measured at 8 weeks (O8) and 4 weeks (O4) was calculated. Using DCA, differences between indices were measured at 4 weeks (A4), 8 weeks (A8), and 12 weeks (A12).
Evaluation of peri-implant supporting bone
All samples including implants, hard tissues, and soft tissues were scanned using microcomputed tomography (micro-CT) to evaluate the morphology of the peri-implant supporting bone. Samples were reoriented along the implant axis and scanned using a micro-CT device (SkyScan1173; Bruker-CT, Kartuizersweg 3B 2550 Kontich, Belgium) with the region of interest in the center. SkyScan1173 control software version 1.6 (Bruker-CT) was used for measurement. Imaging conditions of 130 kVp, 60 µA, 1 mm aluminum filter, 500 ms exposure time, 2240 × 2240 pixels were used to obtain a total of 800 high-resolution images. After radiographic assessment, samples were fixed with 4% formalin, decalcified with 10% ethylene-diamine-tetra-acetic acid (pH 7.0), dehydrated through a graded series of ethanol solutions and 100% acetone, and embedded in methyl methacrylate. Tissue slides (25 μm) were then prepared in the buccolingual direction parallel to the axis of the implant with an EXAKT 400CS grinding machine (Leica, Wetzlar, Germany). Slides were then stained with basic fuchsin, toluidine blue, and Goldner’s trichrome.
Radiographic analysis
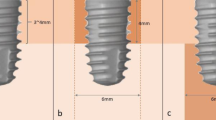
To analyze the pattern of peri-implant bone loss, four types of dimensions were measured from implant shoulder to the most coronal bone-to-implant contact in micro-CT sections. Distance calibration was performed using ImageJ version 1.53 for image processing and analysis for every radiograph to compensate for linear distortion. The following linear measurements were performed parallel to the long axis of each implant at four points: buccal, lingual, mesial, and distal sections (Fig. 5).
Peri-implant bone loss was evaluated by measuring linear dimensions in four directions from the implant shoulder to the most coronal point of surrounding bone attached implant threads on micro-CT cross sections: Buccolingual section (a) and mesiodistal section (b). (L: lingual, B: buccal, M: mesial, D: distal)
To analyze supporting bone volume, projection images obtained from micro-CT were reconstructed into cross-sectional slices using NRecon software version 1.7.0.4 (Bruker-CT) and analyzed with CTAn software version 1.17.7.2 (Bruker-CT). The region of interest (ROI), encompassing the trabecular bone surrounding the implant, was defined as a cylinder from the implant axis and a length of 8.5 mm from the top of the implant. Limit of ROI was defined as an outer cylindrical border of 4.25 mm in diameter with the same axis as the implant body, which had a diameter of approximately 3.0 mm excluding threads. A width of 375 μm was defined for the circular ROI margin because it was 25% of the radius of the implant body, a commonly used distance. All images were individually evaluated. ROI mask was applied to each image. Bone tissue was segmented using a threshold of 50–80 Hounsfield units within the ROI (Fig. 6).
Representative 3D micro-CT image showing the process of peri-implant supporting bone volume calculation. From left to right: The implant boundary was digitally segmented from the raw scan data. A cylindrical region of interest (ROI) with a diameter of 4.25 mm and a height of 8.5 mm, aligned with the implant axis, was defined starting from the implant platform. The volume of the implant was subtracted from this ROI to isolate the supporting bone volume. The ROI included a 375 μm margin beyond the implant surface to encompass the surrounding trabecular bone.
Histomorphometric analysis
Tissue slide images were captured and analyzed with a light microscope (Olympus BH2 with S Plan FL2 lens, Tokyo, Japan) and a computer-digitized image analysis system (Leica Imaging System, Cambridge, England). Bone-to-implant contact (BIC) was calculated as linear percentage of the interface with direct contact between bone and the implant to the total interface of the implant in the cancellous bone. Considering the damping effect on buccal impact when evaluating implant stability, the measurement of BIC was divided into buccal and lingual parts based on the long axis of the implant (Fig. 7).
Representative histological section showing bone-to-implant contact (BIC) on buccal (B) and lingual (L) aspects. The specimen was stained using Masson’s trichrome, where mineralized bone appears blue. Buccal and lingual regions were separately evaluated along the implant’s long axis to reflect directional variations.
Statistical analysis
The Shapiro–Wilk test was performed to assess the normality of each variable. Spearman’s rank correlation analysis was also performed to analyze the correlation between results of implant stability and peri-implant bone level results analyzed by various methods. In addition, univariate linear regression analysis was performed to determine the effect of each outcome on implant stability. The significance level set in the analysis was 5% (p = 0.05). All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA). The sample size (n = 32 implants) was determined via power analysis (α = 0.05, power = 0.80, effect size = 0.5) to detect significant correlations between implant stability and bone-related parameters.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Brånemark, P. I. et al. Intra-osseous anchorage of dental prostheses. I. Experimental studies. Scand. J. Plast. Reconstr. Surg. 3, 81–100. https://doi.org/10.3109/02844316909036699 (1969).
Schroeder, A., Pohler, O. & Sutter, F. Tissue reaction to an implant of a titanium Hollow cylinder with a titanium surface spray layer. SSO Schweiz. Monatsschr Zahnheilkd. 86, 713–727 (1976).
Brånemark, P. I. et al. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand. J. Plast. Reconstr. Surg. Suppl. 16, 1–132 (1977).
Schroeder, A., van der Zypen, E., Stich, H. & Sutter, F. The reactions of bone, connective tissue, and epithelium to endosteal implants with titanium-sprayed surfaces. J. Maxillofac. Surg. 9, 15–25. https://doi.org/10.1016/s0301-0503(81)80007-0 (1981).
Belser, U. C., Schmid, B., Higginbottom, F. & Buser, D. Outcome analysis of implant restorations located in the anterior maxilla: A review of the recent literature. Int. J. Oral Maxillofac. Implants. 19 (Suppl), 30–42 (2004).
Attard, N. J. & Zarb, G. A. Immediate and early implant loading protocols: A literature review of clinical studies. J. Prosthet. Dent. 94, 242–258. https://doi.org/10.1016/j.prosdent.2005.04.015 (2005).
Ostman, P. O. Immediate/early loading of dental implants. Clinical documentation and presentation of a treatment concept. Periodontol 2000. 47, 90–112. https://doi.org/10.1111/j.1600-0757.2007.00244.x (2008).
Chen, S. T., Wilson, T. G. Jr. & Hämmerle, C. H. Immediate or early placement of implants following tooth extraction: Review of biologic basis, clinical procedures, and outcomes. Int. J. Oral Maxillofac. Implants. 19 (Suppl), 12–25 (2004).
Araújo, M. G., Sukekava, F., Wennström, J. L. & Lindhe, J. Ridge alterations following implant placement in fresh extraction sockets: An experimental study in the dog. J. Clin. Periodontol. 32, 645–652. https://doi.org/10.1111/j.1600-051X.2005.00726.x (2005).
Barone, A., Ricci, M., Tonelli, P., Santini, S. & Covani, U. Tissue changes of extraction sockets in humans: A comparison of spontaneous healing vs. ridge preservation with secondary soft tissue healing. Clin. Oral Implants Res. 24, 1231–1237. https://doi.org/10.1111/j.1600-0501.2012.02535.x (2013).
Avila-Ortiz, G., Elangovan, S., Kramer, K. W., Blanchette, D. & Dawson, D. V. Effect of alveolar ridge preservation after tooth extraction: A systematic review and meta-analysis. J. Dent. Res. 93, 950–958. https://doi.org/10.1177/0022034514541127 (2014).
Araújo, M. G., Silva, C. O., Misawa, M. & Sukekava, F. Alveolar socket healing: What can we learn? Periodontol 2000. 68, 122–134. https://doi.org/10.1111/prd.12082 (2015).
Buser, D., Chappuis, V., Belser, U. C. & Chen, S. Implant placement post extraction in esthetic single tooth sites: When immediate, when early, when late? Periodontol 2000. 73, 84–102. https://doi.org/10.1111/prd.12170 (2017).
Jaffin, R. A. & Berman, C. L. The excessive loss of Branemark fixtures in type IV bone: A 5-year analysis. J. Periodontol. 62, 2–4. https://doi.org/10.1902/jop.1991.62.1.2 (1991).
Chiapasco, M., Gatti, C., Rossi, E., Haefliger, W. & Markwalder, T. H. Implant-retained mandibular overdentures with immediate loading. A retrospective multicenter study on 226 consecutive cases. Clin. Oral Implants Res. 8, 48–57. https://doi.org/10.1111/j.1600-0501.1997.tb00007.x (1997).
Lazzara, R. J., Porter, S. S., Testori, T., Galante, J. & Zetterqvist, L. A prospective multicenter study evaluating loading of osseotite implants two months after placement: One-year results. J. Esthet Dent. 10, 280–289. https://doi.org/10.1111/j.1708-8240.1998.tb00505.x (1998).
Szmukler-Moncler, S., Salama, H., Reingewirtz, Y. & Dubruille, J. H. Timing of loading and effect of micromotion on bone-dental implant interface: Review of experimental literature. J. Biomed. Mater. Res. 43, 192–203. (1998).
Testori, T. et al. A multicenter prospective evaluation of 2-months loaded osseotite implants placed in the posterior jaws: 3-year follow-up results. Clin. Oral Implants Res. 13, 154–161. https://doi.org/10.1034/j.1600-0501.2002.130205.x (2002).
Schrott, A., Riggi-Heiniger, M., Maruo, K. & Gallucci, G. O. Implant loading protocols for partially edentulous patients with extended edentulous sites–a systematic review and meta-analysis. Int. J. Oral Maxillofac. Implants. 29 Suppl, 239–255. https://doi.org/10.11607/jomi.2014suppl.g4.2 (2014).
Bischof, M., Nedir, R., Szmukler-Moncler, S., Bernard, J. P. & Samson, J. Implant stability measurement of delayed and immediately loaded implants during healing. Clin. Oral Implants Res. 15, 529–539. https://doi.org/10.1111/j.1600-0501.2004.01042.x (2004).
Meredith, N., Alleyne, D. & Cawley, P. Quantitative determination of the stability of the implant-tissue interface using resonance frequency analysis. Clin. Oral Implants Res. 7, 261–267. https://doi.org/10.1034/j.1600-0501.1996.070308.x (1996).
Meredith, N. A review of nondestructive test methods and their application to measure the stability and osseointegration of bone anchored endosseous implants. Crit. Rev. Biomed. Eng. 26, 275–291. https://doi.org/10.1615/critrevbiomedeng.v26.i4.20 (1998).
Meredith, N. Assessment of implant stability as a prognostic determinant. Int. J. Prosthodont. 11, 491–501 (1998).
Schulte, W. et al. [Periotest–a new measurement process for periodontal function]. Zahnarztl Mitt. 73, 1229–1230 (1983). 1233 – 1226.
Olivé, J. & Aparicio, C. Periotest method as a measure of osseointegrated oral implant stability. Int. J. Oral Maxillofac. Implants. 5, 390–400 (1990).
Schulte, W. & Lukas, D. Periotest to monitor osseointegration and to check the occlusion in oral implantology. J. Oral Implantol. 19, 23–32 (1993).
Friberg, B., Jemt, T. & Lekholm, U. Early failures in 4,641 consecutively placed Brånemark dental implants: A study from stage 1 surgery to the connection of completed prostheses. Int. J. Oral Maxillofac. Implants. 6, 142–146 (1991).
Cochran, D. L., Morton, D. & Weber, H. P. Consensus statements and recommended clinical procedures regarding loading protocols for endosseous dental implants. Int. J. Oral Maxillofac. Implants. 19 (Suppl), 109–113 (2004).
Pyo, S. W., Kim, H. G., Kwon, O., Otgonbold, J. & Lee, K. W. Reliability verification of damping capacity assessment through in vitro analysis of implant micromotion in Peri-implant bone loss model. Int. J. Oral Maxillofac. Implants. 36, 106–114. https://doi.org/10.11607/jomi.8168 (2021).
Lim, H. J. et al. Comparison of the reliability of implant stability measurements between Periotest M and anycheck devices: An in vivo study. J. Periodontal Implant Sci. 52, 266–276 (2022).
Kwon, H. J., Kim, H. J., Lee, D. W. & Lee, J. H. Validation of an implant stability measurement device using the Osstell® and AnyCheck® devices: An in vivo comparison study. J. Oral Med. Oral Surg. 30, 20 (2024).
Park, Y. et al. Influence of peri-implant bone loss on implant stability parameters: An in vivo study. Sci. Rep. 13, 11091 (2023).
Kim, D. H. et al. Comparative evaluation of implant stability measured using periotest, osstell, and anycheck: A clinical study. Medicina 58, 1570 (2022).
Kim, Y. D. et al. Effect of implant location and bone characteristics on stability parameters in peri-implantitis: An experimental model. PLOS ONE. 17, e0290595 (2022).
Nedir, R., Bischof, M., Szmukler-Moncler, S., Bernard, J. P. & Samson, J. Predicting osseointegration by means of implant primary stability. Clin. Oral Implants Res. 15, 520–528. https://doi.org/10.1111/j.1600-0501.2004.01059.x (2004).
Meredith, N., Friberg, B., Sennerby, L. & Aparicio, C. Relationship between contact time measurements and PTV values when using the Periotest to measure implant stability. Int. J. Prosthodont. 11, 269–275 (1998).
Winkler, S., Morris, H. F. & Spray, J. R. Stability of implants and natural teeth as determined by the Periotest over 60 months of function. J. Oral Implantol. 27, 198–203. (2001)
Martinez, H., Davarpanah, M., Missika, P., Celletti, R. & Lazzara, R. Optimal implant stabilization in low density bone. Clin. Oral Implants Res. 12, 423–432. https://doi.org/10.1034/j.1600-0501.2001.120501.x (2001).
O’Sullivan, D., Sennerby, L. & Meredith, N. Influence of implant taper on the primary and secondary stability of osseointegrated titanium implants. Clin. Oral Implants Res. 15, 474–480. https://doi.org/10.1111/j.1600-0501.2004.01041.x (2004).
Gedrange, T. et al. An evaluation of resonance frequency analysis for the determination of the primary stability of orthodontic palatal implants. A study in human cadavers. Clin. Oral Implants Res. 16, 425–431. https://doi.org/10.1111/j.1600-0501.2005.01134.x (2005).
Ito, Y. et al. Relevance of resonance frequency analysis to evaluate dental implant stability: Simulation and histomorphometrical animal experiments. Clin. Oral Implants Res. 19, 9–14. https://doi.org/10.1111/j.1600-0501.2007.01419.x (2008).
Degidi, M., Perrotti, V., Strocchi, R., Piattelli, A. & Iezzi, G. Is insertion torque correlated to bone-implant contact percentage in the early healing period? A histological and histomorphometrical evaluation of 17 human-retrieved dental implants. Clin. Oral Implants Res. 20, 778–781. https://doi.org/10.1111/j.1600-0501.2008.01599.x (2009).
Nkenke, E. et al. Implant stability and histomorphometry: A correlation study in human cadavers using stepped cylinder implants. Clin. Oral Implants Res. 14, 601–609. https://doi.org/10.1034/j.1600-0501.2003.00937.x (2003).
Fanuscu, M. I., Chang, T. L. & Akça, K. Effect of surgical techniques on primary implant stability and peri-implant bone. J. Oral Maxillofac. Surg. 65, 2487–2491. https://doi.org/10.1016/j.joms.2007.04.017 (2007).
Cha, J. Y. et al. Influence of the length of the loading period after placement of orthodontic mini-implants on changes in bone histomorphology: Microcomputed tomographic and histologic analysis. Int. J. Oral Maxillofac. Implants. 24, 842–849 (2009).
Jun, S. H., Chang, B. M., Weber, H. P. & Kwon, J. J. Comparison of initial stability parameters and histomorphometric analysis of implants inserted into extraction sockets: Human fresh cadaver study. Int. J. Oral Maxillofac. Implants. 25, 985–990 (2010).
Krafft, T., Winter, W., Wichmann, M. & Karl, M. In vitro validation of a novel diagnostic device for intraoperative determination of alveolar bone quality. Int. J. Oral Maxillofac. Implants. 27, 318–328 (2012).
Andreotti, A. M. et al. Relationship between implant stability measurements obtained by two different devices: A systematic review. J. Periodontol. 88, 281–288. https://doi.org/10.1902/jop.2016.160436 (2017).
MA, A. A. et al. Correlation of implant stability between two noninvasive methods using submerged and nonsubmerged healing protocols: A randomized clinical trial. J. Oral Implantol. 46, 571–579. https://doi.org/10.1563/aaid-joi-D-19-00130 (2020).
Blanco, J., Carral, C., Liñares, A., Pérez, J. & Muñoz, F. Soft tissue dimensions in flapless immediate implants with and without immediate loading: An experimental study in the beagle dog. Clin. Oral Implants Res. 23, 70–75. https://doi.org/10.1111/j.1600-0501.2011.02183.x (2012).
Acknowledgements
The authors thank Genoss Co., Ltd. for providing technical support and data analysis services in micro-CT and histomorphometric measurements.
Funding
This research was supported by the Yonsei University College of Dentistry Fund (grantnumber: 6-2021-0018).
Author information
Authors and Affiliations
Contributions
Conceptualization, S.-W. Pyo; methodology, S.-W. Pyo and S. Kim; software, G.Y. Kim; validation, J.-S. Chang; formal analysis, S. Kim; investigation, G.Y. Kim; resources, J.-S. Chang; data curation S. Kim; writing—original draft preparation, S.-W. Pyo ; writing—review and editing, S. Kim; visualization, G.Y. Kim; supervision, S. Kim; project administration, S.-W. Pyo; funding acquisition, S.-W. Pyo.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personalrelationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Pyo, SW., Kim, G.Y., Chang, JS. et al. In vivo validation of damping capacity assessment as a diagnostic tool for peri-implant bone loss. Sci Rep 15, 27984 (2025). https://doi.org/10.1038/s41598-025-11144-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-11144-1