Abstract
Tonicity-responsive enhancer binding protein (TonEBP) protects kidney tubular cells against hypertonicity. Calcineurin inhibitors (CNI) are known to suppress TonEBP by hampering nuclear translocation. Moreover, sodium inversely activates TonEBP. We investigated whether CNI-induced nephrotoxicity in transplant recipients could be due to impaired TonEBP activity, and whether sodium restriction exacerbates the intoxication. Immunohistochemical analysis using biopsy specimens from 128 patients revealed that TonEBP was mainly located in the cytoplasm in cases of CNI nephrotoxicity, while it showed nuclear-cytoplasmic staining in cases of rejection or interstitial fibrosis and tubular atrophy. This suggests that TonEBP transactivation is limited in CNI nephrotoxicity. A retrospective observational analysis of 308 kidney transplant recipients at our institute between 1984 and 2018 showed a positive correlation between dietary salt intake and eGFR slope. A low-salt diet is linked to a rapid annual decline in eGFR, with adjusted odds ratios of 2.40 and a 95% confidence interval of 1.18–4.90. These findings suggest that the recommended salt intake for kidney transplant recipients may require reassessment.
Similar content being viewed by others
Introduction
The results of renal transplants have improved in the last several decades due to the progress of immunosuppressive agents that prevent graft rejection. Then, preventing grafts from the toxicity of immunosuppressants has emerged as a new problem for maintaining long-term kidney survival. Calcineurin inhibitors (CNI) cause graft injury characterized by tubular atrophy, interstitial fibrosis, arteriolar hyalinosis, and glomerulosclerosis, and the underlying mechanism of CNI nephrotoxicity is explained by vasoconstriction triggered by endothelin imbalance and direct cytotoxicity to tubular cells. High blood CNI concentrations are associated with nephrotoxicity; however, high concentrations are not necessarily required for the pathogenesis1,2.
CNIs are known to inhibit tonicity-responsive enhancer binding protein (TonEBP) transactivation, which is a transcription factor for osmoprotection that protects cells against hypertonicity by adapting the osmolar gap across membranes3. Under hypertonic conditions, TonEBP shifts from the cytoplasm to the nucleus for the transactivation of tonicity-responsive genes such as sodium-myo-inositol cotransporter, sodium-chloride-betaine cotransporter, and aldose reductase, which allows cells to accumulate organic osmolytes to adjust to extracellular hypertonicity4. TonEBP-deficient cells cannot survive under hypertonic conditions and CNIs are known to suppress TonEBP by hampering its nuclear translocation3,5. We hypothesized that CNI-induced renal tubular injury in transplant recipients may be due to impaired TonEBP activity.
Although the precise mechanism of osmosensors in cells has not been elucidated, sodium chloride is the strongest factor that activates TonEBP6. Generally, sodium homeostasis is tightly regulated in most organs, however, the kidneys need to generate an extracellular sodium gradient to produce various degrees of urine concentrations for maintaining adequate body fluid, suggesting that TonEBP plays a crucial role in the kidney7. It is plausible that impaired TonEBP in the kidney fails to maintain water homeostasis or protects tubular cells from osmotic stress.
We hypothesized that TonEBP transactivation could be chronically suppressed by CNIs in the kidneys of transplant recipients, and that an optimal amount of sodium chloride is necessary for appropriate TonEBP activity and maintaining renal functions. In this study, we searched for the association between the amount of salt intake and renal prognosis in transplant recipients to maintain kidney function in terms of preventing CNI nephrotoxicity.
Results
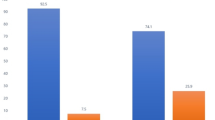
In most cases of 22 native kidney biopsy samples, TonEBP was predominantly located in the nucleus, regardless of underlying diseases, including IgA nephropathy, diabetic nephropathy, nephrosclerosis, membranous nephropathy, and thin basement membrane disease (Fig. 1a,b). Three out of 22 samples (13%) demonstrated cytoplasmic staining, among which two cases took diuretics and one case restricted the dietary salt intake to 3.4 g/day. As TonEBP nuclear translocation is strongly associated with extracellular hypertonicity, it is plausible that interstitial hypotonicity resulting from diuretics or salt restriction leads to cytoplasmic TonEBP distribution8. Among 106 kidney transplant biopsy samples, TonEBP cytoplasmic distributions diagnosed with rejection (n = 56, T cell-mediated and antibody-mediated) was 41%, IF/TA (n = 5) was 40%, and recurrent or de novo glomerulonephritis (n = 7) was 42% (Fig. 1b). In contrast, 75% of CNI nephrotoxicity cases (n = 38) showed significant cytoplasmic patterns (Fig. 1b). These results imply that CNI could be the strong factor that confines TonEBP intracellular shift. A rejection case of medication nonadherence for 10 months demonstrated a clear nuclear distribution of TonEBP, suggesting that rejection itself does not inhibit TonEBP nuclear translocation (Fig. 1c).
(a) TonEBP immunohistochemistry of kidney tubular cells. TonEBP intracellular localization was scored according to its staining pattern. (b) TonEBP scores in native (non-transplanted, n = 22), rejections (n = 56), CNI nephrotoxicity (n = 38), IF/TA (n = 5) and recurrent or de novo glomerulonephritis (n = 7) specimens. *P < 0.05 was considered statistically significant. (c) TonEBP staining of a 22-year-old case of rejection from medication non-adherence for 10 months. Banff scores were i2, t2, v1, g1, ptc2, ti2, iIFTA2, c4d3, cg3, mm3, ah3, aah1, cv0, ci3 and ct3. TonEBP was predominantly located in the nucleus.
Generally, CNI nephrotoxicity manifests as acute kidney injury, which can be reversed by reduction of medical doses, and as chronic kidney disease caused by irreversible arterial endothelial damage. A Banff aah score representing CNI-related arteriopathy was plotted using 38 cases diagnosed as CNI nephrotoxicity (Fig. 2a), and the results demonstrated a clear correlation between the aah score and time after the surgery, indicating that long-term CNI treatment could be associated with endothelial arteriopathy in the transplanted kidney. Next, we divided the transplant biopsy cases of 56 rejections and 38 CNI nephrotoxicity samples into three groups according to the transplant period: less than one year after the surgery (rejections; n = 15, CNI; n = 13), one-10 years (rejections; n = 19, CNI; n = 9), and > 10 years (rejections; n = 22, CNI; n = 16) (Fig. 2b). As a result, regardless of the time following the transplant, samples diagnosed with CNI nephrotoxicity displayed more cytoplasmic distribution of TonEBP than rejections, suggesting that hampered TonEBP intracellular shift is not associated with chronic CNI-induced arteriopathy, but with ongoing CNI-induced nephrotoxicity.
(a) Correlation between Banff aah score and time after transplant surgery among 38 CNI nephrotoxicity-diagnosed patients. (b) TonEBP scores in rejections (n = 56) and CNI nephrotoxicity (n = 38) specimens. Samples were divided into three groups according to the time after transplant surgery. *P < 0.05 was considered statistically significant.
We then measured the blood tacrolimus trough concentration of kidney transplant recipients in accordance with the time after transplantation. Among transplant recipients receiving kidney biopsy within a year after the transplant, blood tacrolimus trough concentrations were significantly higher in CNI nephrotoxicity than in rejections; however, the differences were dismissed among patients more than one year after transplantation (Fig. 3). The data indicate that a high tacrolimus concentration might not be necessary, but other factors could contribute to the development of chronic CNI nephrotoxicity.
To elucidate the impact of a low-sodium diet on kidney outcomes in transplant recipients, we examined the association between salt intake and the annual eGFR decline. Among 302 participants, we selected 198 patients who had no episodes of rapid eGFR decline, including rejections, acute kidney disease, or recurrence of underlying diseases, for the retrospective observational analysis. Within this group, 32 patients consumed less than 10 g/day of salt throughout the observation period (consistent low-salt consumers), while 55 patients consumed more than 10 g/day of salt (consistent high-salt consumers), and salt intake was variable among the remaining 111 patients. Figure 4 illustrates eGFR decline rates of consistent low-salt consumers and high-salt consumers. The difference was not statistically significant, but the rate of eGFR decline appeared to be faster in low-salt consumers than in high-salt consumers, suggesting that high salt intake may be associated with maintaining eGFR.
Then, to find out the optimal amount of salt intake, Fig. 5a illustrates the distribution of salt intake of 198 transplant recipients over a total of 1200 person-years. In Fig. 5b, 1200 plots of the association between dietary salt intakes and annual eGFR slopes show a weak but positive correlation (y = 0.0013x). Figure 5c depicts the annual eGFR slope in relation to dietary salt intake, with an average slope of 0.985. The data indicates that the slope of the low-salt diet (less than 5 g/day) might be lower than that of the diet with more than 6 g/day, although the difference is not statistically significant. We then measured the odds ratio for annual eGFR decline with adjustments for age, sex, eGFR, body mass index, diabetes status, and type of calcineurin inhibitors. Annual eGFR declines of 2, 3, and 4 ml/min/1.73m2 were presented in Fig. 5d. These findings suggest that a dietary salt intake of less than 6 g/day was related to a rapid decline in eGFR among transplant recipients. The adjusted odds ratios (with 95% confidence intervals) of salt intake less than 6 g/day for eGFR decline of 3/year and 4/year were 2.40 (1.18–4.90) and 2.26 (1.00–5.12) respectively.
(a) Histogram demonstrating the dietary salt intake from 24-h urine collections of 198 participants over 1200 person-years. (b) Correlation between the annual eGFR slope and dietary salt intake of 198 participants over 1200 person-years. (c) Annual eGFR slope (eGFR/eGFR of a year ago) in relation to dietary salt intakes. (d) Spline curves showing adjusted odds ratio and 95% CIs for annual eGFR decline > 2 ml/min/1.73 m2, annual eGFR decline > 3 ml/min/1.73 m2 and annual eGFR decline > 4 ml/min/1.73 m2 associated with dietary salt intake. Models were adjusted for age, sex, eGFR, BMI, with or without diabetes, Tacrolimus or Cyclosporine users, and living or deceased donors.
Discussion
TonEBP nuclear translocation in renal tubular cells is restricted in most transplant recipients. Since those of native kidneys and the solo non-adherence transplant kidney showed predominantly nuclear staining, CNI is associated with a certain degree of impaired TonEBP activation. A restricted TonEBP may anticipate two unphysiological renal responses: reduced urine-concentrating ability and tubular damage from osmotic stress. In animal models, long-term cyclosporine-administered rats showed TonEBP downregulation in renal tubular cells3. TonEBP haplo-deficient mice demonstrated reduced urine osmolality with low blood pressure, and elevated plasma renin activity, demonstrating chronic dehydration from an impaired urine concentrating system3,9. From this point of view, kidney transplant recipients need proper hydration as they may easily lose fluid from disrupted urine-concentrating ability by CNI-induced TonEBP inhibition.
Another anticipation from TonEBP downregulation is an attenuated adaptive response of renal tubular cells to hypertonicity. Decreased TonEBP-driven osmoprotective gene expressions lead to diminished accumulation of organic osmolytes in renal tubular cells, which could incessantly damage tubular cells from osmotic stress5. This mechanism may be crucial to comprehend the present results. Generally, CNI nephrotoxicity includes tubular injury and arteriolar hyalinosis. In our results, arteriolar injury; Banff aah score, correlated with time after the surgery, suggesting that accumulated CNI toxicities may link to vascular damage. However, tubular TonEBP score was associated with ongoing CNI intoxications regardless of transplant vintage indicating that CNI-induced tubular injury develops by a different mechanism than arteriolar injury such as hyalinosis. It may be possible that TonEBP-mediated CNI nephrotoxicity directly affects the interstitium and tubules and reflects the progression of interstitial fibrosis and tubular atrophy. Although the pathogenesis of CNI nephrotoxicity is multifactorial, sodium supplementation may effectively prevent CNI-induced tubular injury by upregulating TonEBP.
TonEBP is also known as the nuclear factor of activated T cell 5 (NFAT5). NFATs have been discovered as transcriptional factors controlling IL-2 activation in T cells and are therapeutic targets of CNIs, because calcineurin regulates NFAT nuclear translocation by interacting with a calcineurin binding domain conserved in the NFAT family10,11. However, unlike NFAT1-4, this domain is absent in TonEBP, suggesting that CNIs do not directly inhibit TonEBP. Lim et al. argued on this issue and found that kidney interstitial hypotonicity resulting from the reduction of sodium transporters downregulates TonEBP activity in cyclosporine-induced nephrotoxicity because TonEBP activity is reversed by upregulation of sodium transporters3. From this point of view, interstitial hypotonicity could be key to developing CNI nephrotoxicity. Interestingly, a low-salt diet is necessary to generate cyclosporine-induced nephropathy rat models, and salt-depleted CNI rats demonstrate worsened striped fibrosis and impaired renal function12,13,14,15,16. Together, interstitial hypotonicity is essential for developing CNI nephrotoxicity, and preventing interstitial hypotonicity by optimal salt intake could be effective against CNI nephrotoxicity in transplant recipients.
We then investigated that a low dietary salt intake was associated with significant rapid eGFR decline. Thus, our results do not support the idea that lower salt intake is associated with better renal outcomes in transplant recipients. There are some differences between non-transplant chronic kidney disease (CKD) and transplanted CKD: (i) transplant recipients have already been exposed to uremia of end-stage kidney diseases, (ii) they had a single kidney instead of two kidneys, (iii) they were always exposed to non-self-antigens, and (iv) they were required to continuously take immunosuppressive agents. These differences make CKD management difficult for transplant recipients. In this study, we clarified the differences in the management of non-transplanted and transplanted CKD with salt restriction. Under immunosuppressive conditions with CNIs, a low-salt diet less than 6 g/day could be a risk factor for accelerated renal damage.
This study was limited by the fact that histological assessment was not performed using protocol biopsies. We hypothesized that impaired TonEBP activation in the kidney could lead to renal injury; however, due to selection bias, the correlation between cytoplasmic TonEBP distribution and renal prognosis was not evaluated. Additionally, as the study was conducted within a Japanese population, known for higher dietary salt intake compared to regions like the US, UK, and China, the relatively small low-salt group may have obscured the detrimental effects of salt restriction on kidney transplant recipient17. Further studies with other ethnic populations might clarify the association between salt restriction and renal outcomes in patients with transplanted CKD.
In summary, restricted TonEBP nuclear translocation was observed in CNI nephrotoxicity. Low dietary salt intake may exacerbate kidney dysfunction by reducing TonEBP activation. From this point of view, appropriate sodium intake may need to be reevaluated for kidney transplant recipients.
Methods
Study population
A non-interventional retrospective cohort study was conducted at the outpatient department of JCHO Sendai Hospital. A Total of 302 kidney transplant recipients who underwent transplant surgery at the hospital from 1984 to 2018 and six kidney transplant recipients who were referred to our hospital after the surgery were enrolled in this study.
Immunohistochemistry of kidney biopsy samples
Kidney biopsy was performed in 106 transplant recipients when decreased graft function and rejection episodes were suspected. Among them, 56 were diagnosed with rejections (T cell-mediated and antibody-mediated), 38 with CNI nephrotoxicity, 5 with interstitial fibrosis and tubular atrophy (IF/TA), and 7 with recurrent or de novo glomerulonephritis. The diagnosis of rejection was based on the Banff criteria 2022. The diagnosis of CNI nephrotoxicity required both histological and clinical features: 1) pathological characteristics such as isometric vacuolization in the renal tubular cells, stripe pattern of interstitial fibrosis, arteriolar hyalinosis, and glomerular segmental sclerosis, 2) clinically no other recognizable cause of decreased graft function and the function could be recovered by CNI reduction. TonEBP immunohistochemistry was performed on 22 non-transplanted kidney disease biopsy samples as well. TonEBP intracellular localization in the cortical distal tubules was scored using three grades: (1) predominant nuclear staining, (2) equivalent density in the nucleus and cytoplasm, and (3) predominant cytoplasmic staining. Two pathological specialists conducted histological assessments.
Retrospective observational study
The correlation between eGFR slope and dietary salt intake was evaluated using medical records. Individual dietary salt intake was calculated from 24-h urine collected at patients’ visits using Tanaka’s equation: daily salt intake (g/day) = urine sodium concentration (mEq/L) × urine volume (L)/17. Dietary sodium intake was calculated annually. Patients without pooled urine data were excluded. To measure long-term kidney transplant survival, patients with any history of rejection, acute kidney disease or recurrence of underlying diseases were excluded. After the exclusion process, 198 patients were followed up for up to 23 years and the total person-years was 1200. Annual eGFR slope and odds ratios of eGFR decline were calculated in relation to dietary salt intake. Patients with a dietary salt intake consistently below 10 g/day during the observation period were classified as low-salt consumers, while those with a consistent intake above 10 g/day were classified as high-salt consumers. The blood concentrations of tacrolimus were measured. Due to the retrospective nature of the study, JCHO Sendai Hospital Institutional Review Board waived the need of obtaining informed consent. The study protocol was approved by the Ethics Committee of JCHO Sendai Hospital. The study adhered to the principles of the Declaration of Helsinki.
Statistical analyses
Continuous variables were compared using Student’s t-test or Mann–Whitney U-test. The correlation between TonEBP intracellular localization and pathological diagnosis was assessed using an ordinal logistic regression analysis. The annual eGFR slope was calculated as eGFR divided by eGFR in the past year. The odds ratio of annual eGFR decline by 2, 3, and 4 ml/min/1.73 m2 were calculated in relation to dietary salt intake after adjusting for age, sex, eGFR, BMI, presence of diabetes, Tacrolimus or Cyclosporine use, and whether the donor was living or deceased. All statistical analyses were performed using IBM SPSS Statistics for Windows, Version 19.0. (IBM Corp., Armonk, NY, USA) and P < 0.05 was considered statistically significant.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Nankivell, B. J. et al. Calcineurin inhibitor nephrotoxicity: Longitudinal assessmment by protocol histology. Transplantation 78, 557–565 (2004).
Lusco, M. A., Fogo, A. B., Najafian, B. & Alpers, C. E. AJKD atlas of renal pathology: Calcineurin inhibitor nephrotoxicity. Am. J. Kidney Dis. 69, e21-22 (2017).
Sun, W. L. et al. Downregulation of renal sodium transporters and tonicity-responsive enhancer binding protein by long-term treatment with cyclosporin A. J. Am. Soc. Nephrol. 18, 421–429 (2007).
Sang, D. L. et al. TonEBP stimulates multiple cellular pathways for adaptation to hypertonic stress: Organic osmolyte-dependent and -independent pathways. Am. J. Physiol. Renal Physiol. 300, 707–715 (2011).
Go, W. Y., Liu, X., Roti, M. A., Liu, F. & Ho, S. N. NFATS/TonEBP mutant mice define osmotic stress as a critical feature of the lymphoid microenvironment. Proc. Natl. Acad. Sci. USA 101, 10673–10678 (2004).
Kumar, R. et al. NFAT5, which protects against hypertonicity, is activated by that stress via structuring of its intrinsically disordered domain. Proc. Natl. Acad. Sci. USA 117, 20292–20297 (2020).
Grist, J. T. et al. Visualization of sodium dynamics in the kidney by magnetic resonance imaging in a multi-site study. Kidney Int. 98, 1174–1178 (2020).
Sheen, M. R. et al. Interstitial tonicity controls TonEBP expression in the renal medulla. Kidney Int. 75, 518–525 (2009).
Choi, S. Y. et al. Tonicity-responsive enhancer-binding protein mediates hyperglycemia-induced inflammation and vascular and renal injury. J. Am. Soc. Nephrol. 29, 492–504 (2018).
McCaffrey, P. G. et al. Isolation of the cyclosporin-sensitive T cell transcription factor NFATp. Science 262, 750–754 (1993).
Rao, A., Luo, C. & Hogan, P. G. Transcription factors of the NFAT family: Regulation and function. Annu. Rev. Immunol. 15, 707–747 (1997).
Young, B. A. et al. Cellular proliferation and macrophage influx precede interstitial fibrosis in cyclosporine nephrotoxicity. Kidney Int. 48, 439–448 (1995).
Li, C. et al. Pravastatin treatment attenuates interstitial inflammation and fibrosis in a rat model of chronic cyclosporine-induced nephropathy. Am. J. Physiol. Renal Physiol. 286, 46–57 (2004).
Lim, S. W. et al. Long-term treatment with cyclosporine decreases aquaporins and urea transporters in the rat kidney. Am. J. Physiol. Renal Physiol. 287, 139–151 (2004).
Elzinga, L. W., Rosen, S. & Bennett, W. M. Dissociation of glomerular filtration rate from tubulointerstitial fibrosis in experimental chronic cyclosporine nephropathy: Role of sodium intake. J. Am. Soc. Nephrol. 4, 214–221 (1993).
Klawitter, J. et al. Low-salt diet and cyclosporine nephrotoxicity: Changes in kidney cell metabolism. J. Proteome Res. 11, 5135–5144 (2012).
Anderson, C. A. M. et al. Dietary sources of sodium in China, Japan, the United Kingdom, and the United States, Women and Men Aged 40 to 59 Years: The INTERMAP Study. J. Am. Diet Assoc. 110, 736–745 (2010).
Acknowledgements
We thank Professor Hyug Moo Kwon (Ulsan National Institute of Science and Technology, Korea) for the helpful comments and the kind gift of TonEBP antibody. We would especially like to thank Dr. Yoshiko Moriya, Miki Miyazawa, Mie Uematsu, and the other Division of Nutrition Management members at JCHO Sendai Hospital for their enthusiastic support for the patients and long-term monitoring of dietary salt data. We also thank Dr. Hiroshi Kitamura for his excellent immunohistochemical technique.
Author information
Authors and Affiliations
Contributions
Research design: Satoru Sanada, Satoshi Sekiguchi and Mitsuhiro Sato. Writing of the paper: Satoru Sanada, Kengo Asami and Hiroyuki Kumata. Performance of the research: Satoru Sanada and Saki Katano. Data analysis: Satoru Sanada and Saki Katano
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sanada, S., Katano, S., Asami, K. et al. Correlation between dietary salt intake and renal outcomes related to calcineurin inhibitor nephrotoxicity in kidney transplant recipients. Sci Rep 15, 26466 (2025). https://doi.org/10.1038/s41598-025-11292-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-11292-4