Abstract
Metabolic myopathies impair muscle energy metabolism. Exercise is challenging, as patients may suffer from rhabdomyolysis attacks. This study investigated long-term effects of an individualized strength training program. Eighteen patients (12–45 years) with fatty acid oxidation disorders and glycogen storage diseases (types III and V) participated in a supervised progressive strength exercise program over two periods: biweekly assessments for 12 weeks and monthly in the second 12 weeks. Clinical assessments (anthropometry, food intake, laboratory tests), muscle strength/endurance (isokinetic tests), balance (postural stability tests), aerobic capacity (exercise duration, anaerobic threshold rate, maximal oxygen consumption), and quality of life (calculated in eight subscales) were assessed at baseline, 12, and 24 weeks. Biweekly nutritional and medical adjustments were made based on physiological responses, including modifications in caloric intake, macronutrient composition, pre-exercise sucrose and creatine supplementation. Significant improvements in strength (p < 0.001), balance (p < 0.05), and aerobic capacity (p < 0.001) were observed after 12 weeks and persisted for 24 weeks. No patients experienced rhabdomyolysis. Quality of life improved, particularly in the subscales of emotional role limitations, pain, and general health (p < 0.05). These findings suggest that dynamic, multidisciplinary, and individualized exercise program, combined with medical nutrition therapy and pharmacological adjustments, is effective and safe for patients with metabolic myopathies.
Similar content being viewed by others
Introduction
Metabolic myopathies are a heterogeneous group of inherited metabolic disorders caused by impaired energy production, storage, mobilization and/or utilization in muscle tissue. The most important diseases in this group include glycogen storage disorders (GSD) with muscle involvement, fatty acid oxidation defects (FAOD) and mitochondrial diseases. Although there is a wide spectrum of symptoms, the main clinical features of metabolic myopathies include myalgia, weakness, cramps, stiffness, myoglobinuria and recurrent rhabdomyolysis1,2,3.
Exercise increases the energy requirements of the muscle tissue and tends to worsen the symptoms. Exercise intolerance and exercise-induced muscle pain and rhabdomyolysis attacks are a major problem in most patients with metabolic myopathy. Due to their underlying disease, physical activity restrictions and exercise prohibitions significantly limit the quality of life of these patients4. Some patients not only have difficulties with sport, but also with everyday activities5. In healthy individuals, the bioenergetic processes in muscle tissue during exercise are dynamically regulated by various physiological, nutritional and environmental factors such as exercise intensity and duration, body weight and nutritional status6,7. Obviously, impaired carbohydrate or lipid metabolism together with mitochondrial inhibition further complicates the process that occurs during exercise in patients with metabolic myopathy.
Studies have shown that patients with metabolic myopathies exhibit an exaggerated cardiorespiratory response with a marked decrease in maximal oxygen uptake (V̇O2max), indicating increased oxygen consumption during exercise8. Studies on exercise in muscle glycogenosis have mainly focused on glycogen storage disorder type V (GSD V). It has been suggested that regular supervised exercise programs may be effective in GSD V, and several studies have been conducted with different exercise modalities, including aerobic training, mainly in adults9,10. Patients with glycogen storage disease type III (GSD III) have been shown to develop exercise-induced muscle symptoms during dynamic exercise despite the absence of muscle weakness11. However, there are no longitudinal studies on exercise in GSD III patients in the literature. In FAOD patients, studies on exercise are limited to long-chain FAODs (LC-FAODs). These studies are limited to single case reports and no large clinical cohort studies have been conducted12,13,14.
Here we report the long-term outcomes of an individualized strength-training program coordinated under the supervision of the departments of metabolism, nutrition and sports medicine in patients with metabolic myopathy.
Methods
Ethics and informed consent
Written informed consent was obtained from the patients and their families, and the study was approved by the local ethics committee (E-22686390-050.01.04-10987).
Participants
This prospective study was conducted between October 2022 and May 2024 by the Departments of Pediatric Nutrition and Metabolism and Sports Medicine. Eighteen patients diagnosed with metabolic myopathy were enrolled.
Inclusion criteria
-
Confirmed diagnosis of a metabolic myopathy (e.g., muscle glycogenosis, FAOD, mitochondrial disease) based on molecular and/or biochemical analyses.
-
Age between 12 and 45 years (A lower age limit of 12 years was established, as accurate positioning during isokinetic strength assessment with the Humac Norm II isokinetic dynamometer (Cybex, USA) requires the patient to be at least 150 cm tall.)
-
Evidence of a complete metabolic control by clinical and laboratory assessment.
-
The mental and psychological ability to follow the prescribed exercise regimen.
Exclusion criteria
Patients who did not attend regular follow-up visits, had poor adherence to therapy or suffered from movement disorders, dystonia or other conditions that would negatively affect exercise participation were excluded from the study.
Study design
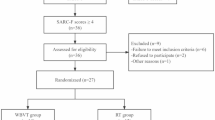
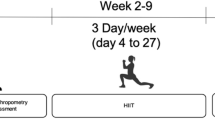
Patients who met the inclusion criteria were first examined in the Department of Nutrition and Metabolism for baseline metabolic status. Clinical visits included anthropometric measurements, analysis of dietary intake records and laboratory tests. Based on the evaluation of all clinical, laboratory and nutritional data, individualized nutritional and pharmacological treatments were designed according to the type of metabolic myopathy. During the first visit to the sports medicine department, initial exercise tests were carried out. On the first day, postural stability tests and measurements of isokinetic muscle strength and endurance were performed, while on the second day a cardiopulmonary exercise test (CPET) was made. Based on these results, patients received tailored exercise prescriptions that progressed every two weeks for the first 12 weeks. These prescriptions started with aerobic exercise and progressively included isometric, isotonic and dynamic balance exercises, with adjustments made according to individual tolerance. At the end of week 12, patients were invited to participate in the second period of the study (12–24 weeks). Patients who agreed to participate in the second period were examined monthly. At the end of both study periods (weeks 12 and 24), all exercise tests were repeated. The primary outcomes were improvement in muscle strength and endurance, balance and stability, aerobic capacity and maintenance of metabolic control. The secondary outcome was improvement in quality of life. The overall design and schedule of the study, consisting of two periods and a total duration of 24 weeks, is shown in Fig. 1.
Procedures
Initial metabolic assessment
Anthropometric measurements including height, body weight, body mass index (BMI), mid-upper arm circumference, measurement of skinfold thickness of triceps, biceps, suprailiac and subscapular, and determination of body fat mass and lean body mass by bioelectrical impedance analysis were assessed. A retrospective five-day food intake record was obtained and analyzed in terms of daily energy, carbohydrate, fat and protein intake. The contents were calculated based on the daily calorie intake (DCI). Detailed laboratory testing including complete blood count, liver and renal functions, plasma ammonia, lactate and creatine kinase (CK) levels, plasma acylcarnitine profile, plasma quantitative amino acid analysis, urinary organic acid analysis was performed. After evaluating all clinical, laboratory and nutritional data, individualized medical nutritional and pharmacological treatments tailored to the underlying type of metabolic myopathy were implemented. The patient was then referred to sports medicine for an initial visit.
Initial visit to the sports medicine department
The baseline exercise tests were carried out on seven consecutive days. A seven-day interval was inserted between the two tests in order to prevent the exertion shown in the first test from leading to inaccurate assessments in the tests on the second day. On the first test day, a postural stability test and measurements of isokinetic muscle strength and endurance were performed. A CPET was carried out on the second test day. Based on the test results and evaluations, patients were issued an initial exercise prescription.
1. Postural stability testing
The “Biodex balance stability system” (Biodex, Inc., Shirley, New York), which is known for its high validity and reliability, was used to measure postural stability. The system, which can tilt up to 20º anteroposterior/right-left, had a 360º platform with a multi-axis structure. The screen of the system was divided into four quadrants and four zones during the test. The system consisted of 12 difficulty levels, with level 12 being the easiest and level 1 being the most difficult. The Biodex balance stability system was calibrated regularly.
To measure postural stability, participants were tested with their eyes open and without shoes or socks. After the participant found the center in a comfortable position, the coordinates of the foot were taken and recorded, the trial measurement was explained to the participants, and the test measurement was started after the participants learned the test. The test consisted of 3 repetitions between levels 12 − 4 with 10-second rest intervals between repetitions. The test was terminated if participants made hand contact during the test. At the end of the test, the participants’ overall, anteroposterior and right-left stability values were recorded and analyzed. As a result of the test, the right-left, anteroposterior and overall stability indices were determined. A decrease in all values was interpreted as good balance15.
Isokinetic testing
The isokinetic strength assessment of the patients was performed using a “Humac Norm II model (Cybex, USA)”, isokinetic dynamometer which is calibrated at regular intervals16. The entire test procedure was carried out by the same researcher. The muscle strength measurements for knee flexion and extension were performed in a seated position. A belt was used for pelvic safety and to prevent the use of the hip flexors. The belt of the device prevented the upper region from supporting the force generated.
The leg muscle strength measurement was performed with three repeated trials and four repeated test measurements at a speed of 60°/sec. At the end of the strength test, a 20-second rest break was taken and the measurement of muscle endurance was started. The endurance of the participants’ leg muscles was measured with four trials and 15 repetitions at a speed of 180°/sec.
Cardiopulmonary exercise testing
Before starting the test, the sports physician took a medical history and performed a physical examination and checked the participant for contraindications to exercise testing. Static and dynamic pulmonary function tests were performed before the test, followed by a resting electrocardiogram (ECG). Participants were informed in detail about the test protocol, the risks and complications of the test were explained, and a consent form was signed.
For the exercise ECG recording systems, 12 leads were recorded using a standard system (Mason-Likar) with 10 electrodes. Before attaching the electrodes, the dead epidermis and fat layer on the skin surface was removed with alcohol. After the electrodes were attached, each lead was monitored on the computer screen and the position of the electrodes was corrected if necessary.
Before the test began, blood pressure was measured while the participants were seated. The measurement was repeated frequently during the test: every 3 min before the participant reached the maximum level and every minute when the participant reached the maximum level. During the recovery phase, measurements were continued at one-minute intervals.
To determine the maximal oxygen consumption and anaerobic threshold values of the participants whose resting ECGs were obtained, a CPET was performed according to the Bruce protocol using a stress test treadmill (Quinton 5000) and a spiroergometry system (Cortex Metalyzer 3B) supported by Metasoft 2.7 software. Oxygen consumption and carbon dioxide production were calculated using the breath-by-breath method. During the test, a Rudolph Mask 7910 2-way mask was used to measure the participants’ metabolic parameters.
The CPET was performed according to the Bruce protocol. The test protocol consists of 7 periods of 3 min each. During the test, participants’ blood pressure was measured in each period and ECG changes were monitored and recorded. The test was continued until the participant was exhausted, unless a pathological condition or finding occurred that would have necessitated termination of the test.
The average V̇O2 value of the last 10 s of exercise was considered as the peak V̇O2. The anaerobic threshold rate (ATHR) was calculated using the V̇-slope method17. Peak V̇O2 and exercise duration were also calculated during CPET. For patients who could not undergo the cardiopulmonary exercise test, a 6-minute walk test was performed instead18.
Design of the exercise prescriptions
The patients practiced individual sports. The exercise cycles were scheduled for 3 days/week, 1 h/day, for a total duration of 12 weeks and were performed under the supervision of a physiotherapist. On days when patients were unable to attend the sports medicine sessions for reasons other than health (e.g. vacations, work, school commitments), the individual training program was continued via online communication systems with the same trainer so that the training schedule was not interrupted.
The patients were given an exercise prescription according to the following scheme:
− 0–2 week: aerobic exercise (30-minute walk).
− 2–4 weeks: aerobic exercises plus isometric core and lower body exercises.
− 4–6 weeks: 2–4 weeks of exercises plus isometric upper body exercises and static balance exercises.
− 6–8 weeks: aerobic exercises, isometric core and upper body exercises, balance exercises plus isotonic lower body exercises.
− 8–10 weeks: aerobic exercises, isometric core, balance exercises, isotonic lower and upper body exercises.
− 10–12 weeks: aerobic exercises, isometric core, isotonic lower and upper body exercises plus dynamic balance exercises.
In week 6, the exercise prescription began to include squats and lunges. The number of repetitions of these exercises was adjusted according to the participants’ tolerance for each exercise.
In the first period (0–12 weeks) of the study, the exercise prescriptions were updated every 2 weeks. However, for patients who agreed to participate in the second period (12–24 weeks), no further updates were made and they continued to follow the exercise prescriptions from weeks 10–12.
Medical nutrition therapy interventions
The nutritional goals for the patients included in the exercise program were set based on literature recommendations. For GSD V patients, medical nutrition therapy consisted of a moderately high-protein (20% of DCI), moderately high-carbohydrate (55% of DCI), and low-fat (25% of DCI) diet19,20 to which 40 g/day of sucrose was added 15 min before exercise21. In GSD III patients, a high-protein (20–25% of DCI), low-carbohydrate (30–35% of DCI) and high-fat (45–50% of DCI) diet was prescribed, supplemented with raw cornstarch (0.5 g/kg/day), with an additional dose (0.2 g/kg/day) on training days22,23.
In FAOD patients with very long-chain acyl-CoA dehydrogenase deficiency (VLCADD), carnitine palmitoyltransferase deficiency type II (CPTIID) and long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency (LCHADD), therapy included a limited intake of long-chain triglycerides (LCT) (max. 35% of DCI) with medium-chain triglycerides (MCT) or triheptanoin (5–25% of DCI), depending on the severity of the disease, as well as adequate protein intake. On training days, pre-exercise snacks were served immediately before training. These snacks consisted of 30 g carbohydrate, 7 g protein and 0.1 g/kg/day MCT/triheptanoin with a macronutrient distribution of 61% carbohydrate, 14% protein and 25% fat, with 96% of the fat content coming from MCT/triheptanoin. Both the carbohydrate content and the MCT/triheptanoin dosage were formulated in accordance with current nutritional guidelines24,25,26,27,28. In multiple acyl-CoA dehydrogenase deficiency (MADD) patients, dietary therapy focused on restricting long-chain fatty acids (max. 25% of DCI), increasing protein intake (17–20% of DCI) and avoiding prolonged fasting29. On training days, a low-fat, high-carbohydrate and high-protein snack was given to cover the increased energy requirement and fluid intake was increased.
Assessment of metabolic control and exercise-related complications
Throughout the study, patients reported any complaints related to their underlying metabolic myopathy or the prescribed exercise programs via a 24/7 telephone number for urgent questions and an email address for non-urgent inquiries. Patients were informed in detail about symptoms that could be associated with worsening metabolic control, such as muscle pain, muscle weakness, fatigue, palpitations, chest pain, shortness of breath and dark-colored urine. They have been instructed to contact the Department of Metabolism immediately if any of these symptoms occur. In addition, during the visits, conducted every two weeks in the first period and monthly in the second period, the patients were assessed both clinically and by laboratory tests for possible complications that might develop.
If the metabolic control worsened during the follow-up for “non-exercise-related reasons”, such as an infection or a reduced nutrition, the treatment was adjusted accordingly. As exercise had to be temporarily interrupted during this period, the training program was restarted at the beginning of the week within the cycle as soon as the metabolic control was restored.
If the metabolic control deteriorated during the follow-up due to “exercise-related causes”, the necessary adjustments were made to the medical nutrition and pharmacological treatment. In consultation with the sports medicine department, the training program was modified without changing the methodology of the study (e.g. reduction of repetitions, extension of rest periods between exercises). In cases where the patients’ complaints persisted despite these adjustments, the patients were withdrawn from the study.
Assessment of the primary outcomes
The successful primary outcome of the study was defined as the exercise program recommended to the patients, which had an effective and safe profile and was evaluated in three main categories.
Improvement of muscle strength and endurance
In the evaluation of muscle strength, the results of the isokinetic muscle strength and endurance test were assessed on the basis of “peak torque values”. Increases in the “peak torque value ExR, FxR, ExL, FxL” were interpreted as an indication of improved muscle strength16.
“Total work done value ExR, FxR, ExL, FxL” parameters from the same test reflected the endurance of the muscle group. An increase in these parameters was interpreted as an improvement in muscle endurance, which meant that the patient was able to perform strength exercises over a longer period of time16.
Improvement of balance and stability
In the evaluation of balance and stability, the results of the Biodex balance stability system test were assessed on the basis of “bilateral overall, A/P and ML indices”. A reduction in these values was interpreted as an improved balance and postural stability16.
Improvement of aerobic capacity
Based on the CPET results, increases in exercise duration, ATHR and maximal oxygen consumption (peak V̇O2/kg) were determined. An increase in all three parameters indicated an improvement in the patient’s aerobic capacity and overall performance30.
Maintaining metabolic control
While the primary aim of the study was to increase the patients’ muscle strength, the most important condition to ensure was the continuity of metabolic control. The most important indicator of control was the absence of rhabdomyolysis attacks which was measured both clinically (myalgia, weakness, dark-colored urine) and monitored by laboratory parameters (elevated CK levels, abnormal renal functions and electrolyte alterations, hyperuricemia, metabolic acidosis, hyperammonemia, hypoglycemia, disturbance of the plasma acylcarnitine profile).
Assessment of the secondary outcome: quality of life
Self-reported health-related quality of life was assessed with the 36-items Medical Outcomes Study Short-Form Health Survey (SF-36), which was developed for use in a variety of diseases including myopathies. 36 items rated on Likert scales are summed and then converted into the eight SF-36 subscales31.
Patients were administered the SF-36 quality of life questionnaire at weeks 0, 12 and 24. A value was calculated for each subscale, with higher values indicating a better state of health.
Statistical analysis
The Statistical Package for Social Sciences (SPSS 28.0.1, SPSS Inc., Chicago, IL USA) software was used for data analysis. Continuous variables were presented as mean ± standard deviation (SD), while categorical variables were presented as numbers or percentages. The normality of the data distribution was tested using the Kolmogorov-Smirnov test. For normally distributed dependent variables, paired comparisons were performed using the paired samples t-test. The repeated-measures analysis of variance (ANOVA) was used to test the effect of the task on the all-outcome measures in the two groups. Results with p < 0.05 were considered statistically significant.
Results
A total of 18 patients diagnosed with metabolic myopathy were included in the study. Of the patients, 10 (55.6%) were female and 8 (44.4%) were male. The mean age of the patients was 23.5 ± 8.24 years. Grouped by diagnosis, 7 patients (38.9%) were diagnosed with GSD and 11 patients (61.1%) with FAOD. The demographic and molecular characteristics of the patients are shown in Table 1; Fig. 2. Of the 18 patients enrolled in the study, 9 (50%) continued to the second period of the study. No cases of exercise-induced rhabdomyolysis were observed during the entire study period.
During the exercise tests, one patient (patient 15) could not be tested on the treadmill due to his high body weight. For this patient, the 6-minute walk test was performed and the V̇O2/kg peak was calculated based on the 6-minute walk distance32.
The detailed program of the first 12 weeks of the study, including the exercise and dietary adjustments are demonstrated in Fig. 3.
Arrangement of medical nutrition and pharmacological treatments
No significant differences were found in BMI, mid-upper arm circumference or skinfold thicknesses at four different sites and body composition assessment of the entire cohort of patients between measurements at weeks 0, 12 and 24.
Patients diagnosed with GSD V
In the GSD V patients, food intake records collected at baseline revealed that patients generally adhered to a medical nutrition therapy consisting of high carbohydrate (60–65% of DCI), low fat (20–25% of DCI) and adequate protein intake according to age (10–15% of DCI). During the exercise program, medical nutrition therapy was provided with moderately high protein (20% of DCI), moderately high carbohydrate (55% of DCI) and low fat (25% of DCI) to increase muscle strength and lean mass while maintaining CK levels in the desired range19,20.
To optimize energy availability and support physical performance before the onset of the second wind, a dose of 40 g/day of sucrose was added to the patients’ medical nutrition therapy 15 min before exercise21. Sucrose is preferred due to its lower hyperosmolar impact compared to glucose, making it more tolerable for the gastrointestinal system. Additionally, to promote recovery after exercise, a carbohydrate and protein-containing snack was also included in the post-exercise meal, and patients’ fluid intake was increased compared to normal days. As patients were closely monitored throughout the study, their treatments were individually and dynamically adjusted based on complaints during exercise and plasma CK levels. When exercise programs that involved more of the proximal muscle groups of the lower extremities resulted in elevated CK levels and complaints of muscle weakness/cramps, these were controlled by increasing the protein content of the diet above standard recommendations. All GSD V patients in our study received low-dose creatine supplementation19. For patients starting creatine treatment for the first time, the initial dose was 30 mg/kg/day and was gradually increased to 50 mg/kg/day. In patients who were already taking creatine before the study, the dose was increased to 50 mg/kg/day and maintained at this level.
Patients diagnosed with GSD III
The two GSD III patients who participated in our study were diagnosed with remarkable hypoglycemia and hepatomegaly in childhood. Therefore, their medical nutrition therapy consisted of a high-carbohydrate (50–55% of DCI), adequate protein (10–15% of DCI) and adequate fat (25–30% of DCI). To prevent hypoglycemia, raw cornstarch (at a dose of 0.5–1 g/kg/day) was also administered throughout the day and at night33. In adolescence and early adulthood, however, the hepatomegaly had regressed and hypoglycemia no longer occurred even after the dose of cornstarch was reduced. However, both patients experienced severe myopathic symptoms, which led to a reduced quality of life. Creatine kinase levels were significantly elevated, and similar to GSD V patients, they had difficulty with activities such as climbing hills and stairs, carrying heavy objects, or engaging in high-intensity exercise. Due to the existing high-carbohydrate medical nutrition therapy, these patients developed hepatic steatosis, and in combination with the exercise program, there was no improvement in their physical performance or muscle strength. Given the myopathic features, medical nutrition therapy was changed to a high-protein (20–25% of DCI), low-carbohydrate (30–35% of DCI) and high-fat (45–50% of DCI) diet22,23. The nocturnal dose of raw cornstarch remained at 0.5 g/kg/day. To ensure adequate energy intake during exercise and to prevent possible hypoglycemia, 0.2 g/kg/day of raw cornstarch was added before exercise. To improve post-exercise recovery and prevent catabolism, a protein and carbohydrate snack was provided and adequate hydration was ensured. Finally, a creatine supplement of 30 mg/kg/day was administered to improve the patients’ muscle strength similar to GSD V patients.
In one patient (Patient 9), significant muscle pain and elevated CK levels during exercise led to the administration of a sucrose supplement, similar to the protocol used in GSD V patients. In the other patient (Patient 6), an enteral product containing hydroxymethylbutyrate (HMB), was administered due to inadequate protein intake noted in the dietary records. The enteral product provided 2.4 g of HMB/day and 0.6 g/kg/day of protein, increasing the protein content of the patient’s medical nutrition therapy to 24% of DCI. This change in medical nutrition therapy resulted in a significant increase in the patient’s lean body mass based on anthropometric measurements, and an improvement in physical endurance was noted.
Patients diagnosed with FAOD
In patients with VLCADD, CPTIID and LCHADD, medical nutrition therapy with small amounts of LCT (max. 35% of DCI) to avoid prolonged fasting and, depending on the severity of the disease, MCT or triheptanoin (5–25% of DCI) and adequate protein intake24,25,26. On exercise days, snacks containing 0.1–0.3 g/kg/day MCT/triheptanoin plus carbohydrates and protein were added and should be taken immediately before exercise. In patients with MADD, medical nutrition therapy was designed to restrict LCT (max. 25% of DCI) and increase protein intake (17–20% of DCI) while avoiding prolonged fasting29. On exercise days, a low-fat snack with a high carbohydrate and protein content was served to cover the increased energy requirements during exercise, and fluid intake was increased. All patients diagnosed with FAOD received carnitine supplementation as needed according to the free carnitine level in the acylcarnitine profile.
Assessment of primary outcomes in the first period of the study
When evaluating the muscle strength of the patients, the peak torque values were compared. The analyses revealed a statistically significant increase in muscle strength between weeks 0–12 (for the peak torque values ExR, FxR, ExL, FxL, p = 0.000, p = 0.000, p = 0.000, p = 0.000 respectively). In the analysis of the data by subgroups, statistical significance was also observed in patients with GSD (for the peak torque values ExR, FxR, ExL, FxL, p = 0.006, p = 0.013, p = 0.005, p = 0.017 respectively) and FAOD (for the peak torque values ExR, FxR, ExL, FxL, p = 0.000, p = 0.000, p = 0. 000, p = 0. 000 respectively).
When evaluating the endurance of the patients, the total work done values were compared. The analyses revealed a statistically significant increase in endurance between weeks 0–12 (for the total work done value ExR, FxR, ExL, FxL, p = 0.000, p = 0.000, p = 0.000, p = 0.000 respectively). In the analysis of the data by subgroups, statistical significance was also observed in patients with GSD (for the total work done values ExR, FxR, ExL, FxL, p = 0.019, p = 0.003, p = 0.005, p = 0.001 respectively) and FAOD (for the total work done values ExR, FxR, ExL, FxL, p = 0.000, p = 0.000, p = 0. 000, p = 0. 000 respectively).
When evaluating the balance and postural stability of the patients, the bilateral overall, A/P and ML indices were compared. The analyses revealed a statistically significant increase in the balance and postural stability between weeks 0–12 (for the bilateral overall, A/P, ML; p = 0.004, p = 0.007, p = 0.023 respectively). In the analysis of the data by subgroups, statistical significance was also observed in patients with GSD (for the bilateral overall, A/P, ML; p = 0.012, p = 0.019, p = 0.013 respectively). However no significant difference was found in patients with FAOD (for the bilateral overall, A/P, ML; p = 0.111, p = 0.152, p = 0.231 respectively).
Aerobic capacity was assessed in three dimensions: increase in exercise duration, ATHR and peak V̇O2/kg. In the first period of the study, there was a statistically significant increase in exercise duration (p = 0.000) and ATHR (p = 0.000). In the analysis of the data by subgroups, statistical significance was also observed in patients with GSD (for the exercise duration and ATHR, p = 0.000) and FAOD (for the exercise duration and ATHR, p = 0.000 and p = 0.005, respectively). No significant difference was found in mean peak V̇O2/kg values in all groups (p = 0.752, p = 0.089, p = 0.658 for all patients, GSD patients and FAOD patients respectively).
The statistical data on muscle strength, endurance, balance, postural stability and aerobic capacity of the patient cohort in the first period are shown in Table 2.
Assessment of primary outcomes in the second period of the study
Nine patients contributed to the second period of the study. In the second period, in which the exercise intensity was not increased and the study continued with the exercise program of week 10–12, no significant increase in balance and postural stability parameters was observed (for the bilateral overall, A/P, ML; p = 0.143, p = 0.145, p = 0.216 respectively), while the significant increase in strength (for the peak torque values ExR, FxR, ExL, FxL, p = 0.001, p = 0.002, p = 0.001, p = 0.005 respectively) and endurance parameters (for the total work done value ExR, FxR, ExL, FxL, p = 0.001, p = 0.002, p = 0.001, p = 0.001 respectively) persisted. Regarding aerobic capacity, the significant increase in exercise duration (p = 0.000) and ATHR (p = 0.000) persisted, while no difference was found in peak V̇O2/kg (p = 0.255).
When comparing the GSD and FAOD patients as groups for all of the above parameters, no significant difference was found between the two groups. The statistical data on muscle strength, endurance, balance, postural stability and aerobic capacity of the patient cohort in the second period are shown in Supplementary Material 1.
Evaluation of metabolic control and exercise-induced metabolic complications
Plasma CK levels were monitored biweekly in the first period and monthly in the second period of the study. Although there was a transient increase in plasma CK levels after the adjustment of exercise at week 6, including isotonic exercise, no significant changes in plasma CK levels were observed in patients throughout the study (p = 0.193).
Throughout the study, no patient developed rhabdomyolysis as indicated by clinical and laboratory findings induced by exercise. Patients were asked to rate any muscle pain they experienced during or after exercise, and no patient reported a severe pain. In cases where mild pain occurred during exercise that did not interfere with daily life, adjustments were made to both exercise protocols and dietary strategies. Exercise-related modifications included longer rest periods between exercise sets and a reduction in the number of repetitions to avoid excessive muscle strain. Since the main cause of exercise-induced pain in these patients was thought to be a lack of energy in the muscle, dietary modifications were made to optimize energy availability6,7. In GSD V patients, sucrose intake was increased prior to exercise to provide an immediate energy source, while in FAOD patients, carbohydrate and calorie intake was increased prior to exercise and prolonged fasting was avoided to ensure a continuous energy supply.
Evaluation of the quality of life
The assessment of quality of life was calculated in eight subscales. In the first period, quality of life improved in the subscales role limitations due to emotional problems (p = 0.018), energy/fatigue (p = 0.056), pain (p = 0.022) and general health (p = 0.006). In the evaluation of the 9 patients whose study duration was extended to 24 weeks, the improvement in role limitations due to emotional problems (p = 0.02), energy/fatigue (p = 0.04) remained. A significant difference was also found in the social functioning subscale in patients who contributed to the second period (p = 0.059). The data on the overall quality of life parameters of the SF-36 test are shown in Table 3 and Supplementary Material 2.
Discussion
This study evaluated the results of an individualized and supervised strength exercise program in a group of patients with metabolic myopathy consisting of GSD III, GSD V and FAOD. Through the program, patients experienced significant improvements in muscle strength, endurance, balance and postural stability parameters in the first 12 weeks. The significant increases in muscle strength and endurance parameters continued in patients whose exercise time was extended to 24 weeks. Although the program focused primarily on strength exercises rather than aerobic exercises, a significant increase in aerobic capacity parameters, particularly exercise duration and ATHR values, was observed in the first 12 weeks and was sustained in the patients whose exercise period was extended to 24 weeks. This change was considered a positive improvement in aerobic capacity. The results of the SF-36 quality of life scale showed an objective improvement who started regular exercise. No patient experienced exercise-induced metabolic attacks or rhabdomyolysis. This study evaluated the exercise experience of GSD III patients for the first time in the literature, emphasizing that the treatment of the disease to improve muscle strength after adolescence may have similarities with GSD V. The use of HMB and creatine supplementation in the management of GSD III patients was also discussed. A key factor in the success of this program was not only the structured exercise program itself, but also the dynamic, multidisciplinary supervision provided throughout the intervention. In contrast to standard training programs, nutritional and medical adjustments were made every two weeks in this study, tailored to the physiological responses of individual patients. The individualized modifications, including adjustments to caloric intake, macronutrient composition, pre-exercise sucrose administration in GSD V, creatine supplementation in GSD III, and metabolic support strategies in FAOD patients, played a critical role in ensuring safe and effective exercise participation. Based on these results, it is suggested that a dynamic, multidisciplinary and individualized exercise program-integrating exercise prescription with tailored medical nutrition therapy and pharmacological treatments- is an effective and reliable profile for patients with metabolic myopathy.
Throughout the study, patients’ medical nutrition therapies were adjusted based on their personal preferences, their discomfort during exercise and the results of laboratory tests. The literature covers different aspects of medical nutrition therapies recommended for GSD V patients. Previous data generally support the view that a high-carbohydrate diet (20% fat, 15% protein, 65% carbohydrate of DCI) is the primary dietary modality. When comparing a high-protein, low-fat diet (55% protein, 15% fat and 30% carbohydrate of DCI) with a high-carbohydrate diet, it was found that the high-carbohydrate diet resulted in increased maximal oxygen uptake during exercise and a significant decrease in heart rate. In addition, a decrease in CK levels and an increase in muscle strength were observed. A high-carbohydrate diet improves glucose mobilization by increasing glycogen stores in the liver, making more glucose available for the muscles. However, no significant improvement in exercise tolerance was observed when a modified ketogenic diet was administered. Therefore, according to the Cochrane systematic review, a high-carbohydrate medical nutrition therapy is recommended19,20. Recent studies investigating the modified ketogenic diet have shown that patients with GSD V can utilize alternative energy sources such as fats and ketones during exercise, which may alleviate some of the exercise intolerance associated with the disease. It has been reported that a low carbohydrate ketogenic diet (LCKD) may improve exercise tolerance in these patients and reduce the frequency of exercise-induced symptoms33,34. When evaluating nutrition with regard to the second wind phenomenon, it is recommended to consume a drink containing 75 g of sucrose 40 min before exercise in order to improve exercise capacity21. Due to tolerance issues and the long wait time before exercise, recent publications recommend consuming 37 g of sucrose 5 min before exercise. This dosage and timing have been shown to improve exercise tolerance and prevent a rise in CK levels35,36,37. In our study, medical nutrition therapies of the GSD V patients were carried out in accordance with the current literature and guidelines. However, during the exercise program, special care was taken to increase the protein content of the diet (18–20% of DCI) rather than focusing on a high-carbohydrate diet to increase muscle strength. The rationale behind increasing protein intake was based on its potential role in improving muscle energy metabolism and mitigating muscle damage in metabolic myopathies. As reported by Jensen et al.38, a high-protein diet improved energy kinetics and endurance in a patient with GSD V, suggesting that protein intake may contribute to a more efficient utilization of muscle energy during exercise. In our study, we observed increased CK levels and muscular complaints during the weeks when isotonic exercise was introduced and the proximal muscle groups of the lower extremities were more involved in the exercise program. As protein plays a crucial role in muscle repair and recovery, dietary protein intake was increased above standard recommendations to support muscle metabolism and potentially reduce CK elevations. This adjustment was aimed at optimizing muscle energy availability and minimizing metabolic stress caused by exercise. To optimize energy availability and support physical performance before the onset of the second wind phenomenon, 40 g of sucrose were administered 5 min before exercise, a strategy that is strongly supported in the literature. It was observed that the administration of sucrose prior to exercise helped to prevent muscle stiffness in our patients. In GSD V patients, creatine is the only treatment that has been shown to increase muscle strength. Creatine supplementation, especially at doses up to 60 mg/kg/day, has been reported to have beneficial effects on increasing muscle strength, while toxic effects have been observed at higher doses up to 150 mg/kg/day19,39. In our study, all GSD V patients received low-dose creatine supplementation, which is documented in the literature.
The two GSD III patients in our study showed a hepatic phenotype in childhood, but developed a more pronounced muscular phenotype after adolescence and did not report hypoglycemia. The muscular involvement of GSD III patients is characterized by weakness and exercise intolerance due to distal myopathy. Rhabdomyolysis does not occur in the patients. Medical nutrition therapy with a high protein content is recommended in the literature for GSD III patients with a predominant muscular phenotype22,32. Increased dietary protein intake may improve muscle function by increasing muscle protein synthesis, and replacing some carbohydrates with protein may reduce unnecessary glycogen storage. High-protein medical nutrition therapy has been associated with improvements in hepatomegaly, an increase in lean body mass and improved exercise capacity23. In our study, high protein (20–25% of DCI), low carbohydrate (30–35% of DCI) and high fat (45–50% of DCI) medical nutrition therapy was introduced for the GSD III patients, with the nightly dose of raw corn starch maintained at 0.5 g/kg/day. Before exercise, an additional 0.2 g/kg/day of raw cornstarch was administered, along with a protein and carbohydrate-containing snack. Similar to the literature, an improvement in exercise tolerance was also observed with high-protein medical nutrition therapy23. In our study, one of the GSD III patients who was unable to achieve adequate protein intake orally had her medical nutrition therapy modified with a dietary supplement containing HMB, arginine and glutamine. Hydroxymethylbutyrate is a product of leucine metabolism that is produced endogenously in the body in small quantities and accounts for 0.66% of the total leucine conversion. It is a dietary supplement with proven efficacy in the literature for the prevention of sarcopenia and muscle weakness, particularly in geriatric cohorts and cancer patients. This compound can be administered as an isolated supplement or in combination with amino acids such as arginine and glutamine (HMB/Arg/Gln) or HMB-enriched enteral nutrition products. The use of HMB has been reported to lead to an increase in muscle mass and strength40,41. Studies investigating the combination of HMB and exercise have shown significant improvements in muscle strength and physical performance42. Similar to the literature, a significant increase in muscle strength and mass was observed with HMB supplementation in our study. In addition to the change in medical nutrition therapy, creatine treatment was initiated at a dose of 30 mg/kg/day to increase muscle strength, similar to GSD V patients. It was observed that patients under creatine supplementation had a better exercise tolerance. Since there are no exercise studies in the literature that address GSD III patients, we believe that the effective profile obtained by using sucrose supplementation prior to training, creatine supplementation and HMB supplementation in one of our GSD III patients will contribute to the literature.
In the literature, low-fat (not > 35% of DCI) and the substitution of MCT or triheptanoin is recommended for medical nutrition therapy for LC-FAODs24,25,26. A guideline for medical nutrition therapy in combination with exercise is only provided for VLCADD among FAOD patients26. The guidelines for VLCADD recommend the use of MCT as an energy source at a dose of 0.1–0.3 g/kg/day 20 min before exercise and ensuring adequate hydration. In addition, pre-exercise meals should be rich in carbohydrates to ensure adequate glycogen stores, while post-exercise recovery should focus on replenishing glycogen stores and providing protein for muscle repair43. In our study, similar changes to medical nutrition therapy were made for the FAOD patients as in the VLCADD guidelines with the exception of MADD. In addition, all FAOD patients consumed a low-fat, high-carbohydrate snack before exercise to meet increased energy requirements and ensured adequate hydration.
Studies focusing on strength exercises in patients with metabolic myopathies are very limited in the literature. For GSD V patients, there is only one clinical study in which muscle strength was monitored after strength exercises. This study involved seven adult patients who, after a 24-minute warm-up exercise, performed strength exercises for large muscle groups with 5 to 6 repetitions at a rate of perceived exertion (RPE) of 6 to 7. The exercise program led to a significant increase in the lean body mass of both the whole body and the lower extremities. The increase in muscle strength was analyzed based on the maximum power measured during the exercises, and a significant increase was recorded at the end of the study. It was also found that the disease scores, which reflect the patients’ quality of life, fell to a lower level in all patients44. No studies have been conducted in this area with pediatric patients and only one case report is available. In this case, a 15-year-old male patient performed light to moderate intensity strength exercises twice a week for 6 weeks. Following the program, maximal bench press performance increased by 27% and squat performance increased by 6% without the development of myoglobinuria, and the patient moved to a lower disease severity class45. Although it is known that GSD III patients, especially in the adult age group, present with exercise intolerance and muscle weakness, there are no exercise studies for this patient group11. Similarly, the effects of exercise on muscle strength in patients with FAOD have not been studied. In our study, statistically significant improvements in muscle strength, endurance, postural stability and balance were observed with a personalized and supervised strength exercise program in both GSD and FAOD patients. While similar research has been conducted in GSD V patients, our study extends previous work by including pediatric patients with GSD V and is one of the first to include GSD III and FAOD patients. In addition, it is one of the few studies to assess muscle strength using isokinetic dynamometry and to analyze endurance, balance and postural stability in addition to muscle strength. It is suggested that these results will contribute novel data to the existing literature.
In the literature, most studies conducted in patients with metabolic myopathies focus on aerobic exercise programs and their outcomes. In the studies conducted on GSD V patients, walking or cycling exercises were performed for 30–45 min at 65–70% of maximum heart rate, and the increase in aerobic capacity was calculated using peak V̇O2/kg. Post-exercise studies showed that maximal oxygen consumption increased by 14–111%, and positive changes in functional capacity and general well-being were observed in quality-of-life assessments9,36,3746,47,48,,. Similarly, a significant correlation between aerobic exercise and an increase in maximal oxygen consumption was found in a patient with CPTIID12. In our study, statistically significant improvements in exercise duration and ATHR were observed in both GSD and FAOD patients. However, no statistically significant increase was seen in peak V̇O2/kg. The study was primarily aimed at patients diagnosed with metabolic myopathies characterized by a reduction in muscle strength, a condition that can also affect functionality. For this reason, patients were primarily given strength exercises to increase muscle strength. The lack of a significant increase in peak V̇O2/kg in our study was attributed to the fact that the exercises selected were not aerobic. Although the focus was not on aerobic exercise, regular implementation of a program consisting solely of strength exercises led to an increase in exercise duration, and ATHR values also showed an improvement. The increase in ATHR with regular exercise indicates better compensation for lactic acid buildup and suggests that the individual experiences fatigue later during exercise. In this context, the increase in ATHR was considered an indicator of improved aerobic capacity.
This study has some limitations. The first limitation is the small sample size, which is due to the frequency of these diseases, as they belong to the group of rare metabolic diseases. The second limitation is due to the precautions taken to ensure patient safety and to avoid episodes of rhabdomyolysis. Due to these safety measures, we were not able to progressively increase the intensity of aerobic exercise based on ATHR levels, and the study focused solely on strength exercises. For future research, we aim to conduct a large-scale, multicenter study that focuses on a single subgroup of metabolic myopathy, allowing for a larger sample size and a more comprehensive investigation of exercise interventions. In addition, we plan to include aerobic exercise protocols alongside strength training, ensuring patient safety to better assess their impact on individuals with metabolic myopathies. Importantly, future studies should also aim to identify disease-specific exercise modalities, as tailoring interventions to the underlying metabolic disorder could improve both the safety and efficacy of exercise-based management strategies in patients with metabolic myopathies.
Conclusion
Our study highlights that an individualized and supervised strength exercise program guided by a multidisciplinary approach leads to significant improvements in muscle strength, endurance, postural stability and balance in patients with metabolic myopathy. Although the focus was on strength exercises rather than aerobic exercises, improvements in exercise duration and ATHR indicate improved aerobic capacity. These changes were also reflected in improved quality of life, as evidenced by objective measures of well-being.
The individualized and dynamic interventions, including tailored medical nutrition therapy and targeted medical treatments such as creatine and HMB supplementation, played a critical role in optimizing muscle function, preventing metabolic attacks and reducing the risk of rhabdomyolysis. This emphasizes the need for continuous, patient-specific monitoring and adaptive modifications by a multidisciplinary team. In contrast to standardized protocols, this approach allowed for real-time adjustments to both nutritional and pharmacological strategies based on each patient’s metabolic status, exercise tolerance, and physiological response. The ability to proactively tailor interventions to individual needs was fundamental to ensuring the overall safety, feasibility and long-term efficacy of the program.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Lilleker, J. B., Keh, Y. S., Roncaroli, F., Sharma, R. & Roberts, M. Metabolic myopathies: A practical approach. Pract. Neurol.18, 14–26 (2018).
Berardo, A., DiMauro, S. & Hirano, M. A diagnostic algorithm for metabolic myopathies. Curr. Neurol. Neurosci. Rep.10, 118–126 (2010).
Finsterer, J. Update review about metabolic myopathies. Life (Basel). 10 (43). https://doi.org/10.3390/life10040043 (2020).
Karazi, W. et al. Toward an Understanding of GSD5 (McArdle disease): how do individuals learn to live with the metabolic defect in daily life. J. Neuromuscul. Dis. 11, 103–116 (2024).
Ollivier, K. et al. Exercise tolerance and daily life in McArdle’s disease. Muscle Nerve31, 637–641 (2005).
Alghannam, A. F., Ghaith, M. M. & Alhussain, M. H. Regulation of energy substrate metabolism in endurance exercise. Int. J. Environ. Res. Public. Health. 18 (4963). https://doi.org/10.3390/ijerph18094963 (2021).
Hearris, M. A., Hammond, K. M., Fell, J. M. & Morton, J. P. Regulation of muscle glycogen metabolism during exercise: Implications for endurance performance and training adaptations. Nutrients10, 298. https://doi.org/10.3390/nu10030298 (2018).
Noury, J. B., Zagnoli, F., Petit, F., Marcorelles, P. & Rannou, F. Exercise efficiency impairment in metabolic myopathies. Sci. Rep.10, 8765. https://doi.org/10.1038/s41598-020-65770-y (2020).
Bordoli, C., Murphy, E., Varley, I., Sharpe, G. & Hennis, P. A. Systematic review investigating the effectiveness of exercise training in glycogen storage diseases. Ther. Adv. Rare Dis. 3 (26330040221076497). https://doi.org/10.1177/26330040221076497 (2022).
Batten, K., Bhattacharya, K., Simar, D. & Broderick, C. Exercise testing and prescription in patients with inborn errors of muscle energy metabolism. J. Inherit. Metab. Dis.46, 763–777 (2023).
Preisler, N. et al. Exercise intolerance in glycogen storage disease type III: Weakness or energy deficiency?. Mol. Genet. Metab.109, 14–20 (2013).
Parimbelli, M. et al. Nutrition and exercise in a case of carnitine palmitoyl-transferase II deficiency. Front. Physiol.12, 637406. https://doi.org/10.3389/fphys.2021.637406 (2021).
Herrera-Olivares, A. M., Fernández-Luque, J. A., Paradas, C., Lucia, A. & Santalla, A. Combined HIIT and resistance training in very Long-Chain Acyl-CoA dehydrogenase deficiency: A case report. Front. Physiol. 10 (650). https://doi.org/10.3389/fphys.2019.00650 (2019).
Crisafulli, O. et al. The first case of a competitive basketball player affected by carnitine palmitoyl transferase II deficiency presenting an undescribed compound heterozygous genetic mutation. Eur. J. Appl. Physiol.125, 1311–1322 (2025).
Arnold, B. L. & Schmitz, R. J. Examination of balance measures produced by the Biodex stability system. J. Athl. Train.33, 323–327 (1998).
Gaines, J. M. & Talbot, L. A. Isokinetic strength testing in research and practice. Biol. Res. Nurs.1, 57–64 (1999).
Wasserman, K., Whipp, B. J., Koyl, S. N. & Beaver, W. L. Anaerobic threshold and respiratory gas exchange during exercise. J. Appl. Physiol.35, 236–243 (1973).
Ross, R. M., Murthy, J. N., Wollak, I. D. & Jackson, A. S. The six minute walk test accurately estimates mean peak oxygen uptake. BMC Pulm. Med.10, 31. https://doi.org/10.1186/1471-2466-10-31 (2010).
Quinlivan, R., Martinuzzi, A. & Schoser, B. Pharmacological and nutritional treatment for mcardle disease (Glycogen storage disease type V). Cochrane Database Syst. Rev. 11 https://doi.org/10.1002/14651858.CD003458.pub5 (2014).
Andersen, S. T. & Vissing, J. Carbohydrate- and protein-rich diets in McArdle disease: Effects on exercise capacity. J. Neurol. Neurosurg. Psychiatry.12, 1359–1363 (2008).
Vissing, J. & Haller, R. G. The effect of oral sucrose on exercise tolerance in patients with McArdle’s disease. N. Engl. J. Med.349, 2503–2509 (2003).
Kalkan Uçar, S. et al. Long-term personalized high-protein, high-fat diet in pediatric patients with glycogen storage disease type IIIA: Evaluation of myopathy, metabolic control, physical activity, growth, and dietary compliance. J. Inherit. Metab. Dis.https://doi.org/10.1002/jimd.12741 (2024).
Massimino, E., Amoroso, A. P., Lupoli, R., Rossi, A. & Capaldo, B. Nutritional management of glycogen storage disease type III: a case report and a critical appraisal of the literature. Front. Nutr. 10 (1178348). https://doi.org/10.3389/fnut.2023.1178348 (2023).
Negro, M. et al. Exercise, nutrition, and supplements in the muscle carnitine palmitoyl-transferase II deficiency: New theoretical bases for potential applications. Front. Physiol.12, 704290. https://doi.org/10.3389/fphys.2021.704290 (2021).
Mozrzymas, R., Konikowska, K. & Regulska-Ilow, B. Energy exchangers with LCT as a precision method for diet control in LCHADD. Adv. Clin. Exp. Med.26, 515–525 (2017).
Bleeker, J. C. et al. Proposal for an individualized dietary strategy in patients with very long-chain acyl-CoA dehydrogenase deficiency. J. Inherit. Metab. Dis.42, 159–168 (2019).
Van Calcar, S. C. et al. Nutrition management guideline for very-long chain acyl-CoA dehydrogenase deficiency (VLCAD): An evidence- and consensus-based approach. Mol. Genet. Metab.131, 23–37 (2020).
Thomas, D. T., Erdman, K. A. & Burke, L. M. Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and athletic performance. J. Acad. Nutr. Diet.116, 501–528 (2016).
Prasun, P. Multiple Acyl-CoA dehydrogenase deficiency. In GeneReviews® [Internet] (eds Adam, M. P. et al.) (University of Washington, 2020).
Riley, M. S., Nicholls, D. P. & Cooper, C. B. Cardiopulmonary exercise testing and metabolic myopathies. Ann. Am. Thorac. Soc. 14, S129–S139 (2017).
Demiral, Y. et al. Normative data and discriminative properties of short form 36 (SF-36) in Turkish urban population. BMC Public Health6, 247. https://doi.org/10.1186/1471-2458-6-247 (2006).
Kishnani, P. S. et al. Glycogen storage disease type III diagnosis and management guidelines. Genet. Med.12, 446–463 (2010).
Nogales-Gadea, G. et al. Exercise and preexercise nutrition as treatment for McArdle disease. Med. Sci. Sports Exerc.48, 673–679 (2016).
Løkken, N. et al. Can a modified ketogenic diet be a nutritional strategy for patients with McArdle disease? Results from a randomized, single-blind, placebo-controlled, cross-over study. Clin. Nutr.42, 2124–2137 (2023).
Maté-Muñoz, J. L. et al. Favorable responses to acute and chronic exercise in McArdle patients. Clin. J. Sport Med.17, 297–303 (2007).
Perez, M. et al. Exercise capacity in a 78 year old patient with McArdle’s disease: It is never too late to start exercising. Br. J. Sports Med.40, 725–726 (2006).
Andersen, S. T., Haller, R. G. & Vissing, J. Effect of oral sucrose shortly before exercise on work capacity in McArdle disease. Arch. Neurol.65, 786–789 (2008).
Jensen, K. E., Jakobsen, J., Thomsen, C. & Henriksen, O. Improved energy kinetics following high protein diet in mcardle’s syndrome. A 31P magnetic resonance spectroscopy study. Acta Neurol. Scand. 81, 499–503. https://doi.org/10.1111/j.1600-0404.1990.tb01007.x (1990).
Vorgerd, M. et al. Effect of high-dose creatine therapy on symptoms of exercise intolerance in McArdle disease: Double-blind, placebo-controlled crossover study. Arch. Neurol.59, 97–101 (2002).
Prado, C. M., Orsso, C. E., Pereira, S. L., Atherton, P. J. & Deutz, N. E. P. Effects of β-hydroxy β-methylbutyrate (HMB) supplementation on muscle mass, function, and other outcomes in patients with cancer: A systematic review. J. Cachexia Sarcopenia Muscle13, 1623–1641 (2022).
Cruz-Jentoft, A. J. et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age. Ageing. 43, 748 – 579 (2014).
Din, U. S. U. et al. A double-blind placebo controlled trial into the impacts of HMB supplementation and exercise on free-living muscle protein synthesis, muscle mass and function, in older adults. Clin. Nutr.38, 2071–2078 (2019).
Yoo, H. W. Inborn errors of mitochondrial fatty acid oxidation: Overview from a clinical perspective. J. Lipid Atheroscler.10, 1–7 (2021).
Santalla, A. et al. Feasibility of resistance training in adult mcardle patients: clinical outcomes and muscle strength and mass benefits. Front. Aging Neurosci. 6 (334). https://doi.org/10.3389/fnagi.2014.00334 (2014).
García-Benítez, S., Fleck, S. J., Naclerio, F., Martín, M. A. & Lucia, A. Resistance (weight lifting) training in an adolescent with McArdle disease. J. Child Neurol.28, 805–808 (2013).
Haller, R. G., Wyrick, P., Taivassalo, T. & Vissing, J. Aerobic conditioning: An effective therapy in McArdle’s disease. Ann. Neurol.59, 922–928 (2006).
Lucia, A. et al. Double trouble (McArdle’s disease and myasthenia gravis): How can exercise help?. Muscle Nerve35, 125–128 (2007).
Porcelli, S., Marzorati, M., Morandi, L. & Grassi, B. Home-based aerobic exercise training improves skeletal muscle oxidative metabolism in patients with metabolic myopathies. J. Appl. Physiol. (1985). 121, 699–708 (2016).
Funding
Study was funded by the Scientific and Technological Research Council of Turkey (TÜBİTAK) under the 1001 Research Support Program (project number:321S360).
Author information
Authors and Affiliations
Contributions
TZ, SY, GM and ÇAZ conceptualized and designed the study, drafted the initial manuscript, and critically reviewed and revised the manuscript.FY, EU, AO, ŞD and EK designed the data collection instruments, collected data, carried out the initial analyses, and critically reviewed and revised the manuscript.TŞ, MŞC, Eİ, NYS and MGG designed the data collection instruments, collected data, carried out the initial analyses, and critically reviewed and revised the manuscript.EG, BÖH and MCB conceptualized and designed the study, coordinated, and supervised data collection, and critically reviewed and revised the manuscript for important intellectual content.All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.Tanyel ZUBARIOGLU is the corresponding author of this study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was designed in accordance with the current revision of the Helsinki declaration and was approved by the local Ethical Committee of Istanbul University-Cerrahpasa, Cerrahpasa Medical Faculty (E-22686390-050.01.04-10987).
Informed consent
Informed consent was obtained from all patients for being included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
ZUBARIOGLU, T., YAKAL, S., YEGİN, F. et al. Impact of individualized and supervised strength training on muscle physiology, metabolic control and quality of life in metabolic myopathies. Sci Rep 15, 26125 (2025). https://doi.org/10.1038/s41598-025-11361-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-11361-8