Abstract
Ferroptosis is a form of iron-dependent cell death of interest for the development of novel anti-cancer therapies. Ferroptosis research uses a process of elimination based on assumed ferroptosis-specific inducers and inhibitors; these molecules however have off-target effects and cannot provide a comprehensive picture of overlapping pathways. We investigated whether pyroptosis—a form of inflammatory cell death—is initiated in cancer cells following treatment with the ferroptosis inducer RSL3. We treated 6 cancer cell lines with RSL3 alone or in combination with inhibitors of ferroptosis (Ferrostatin-1), caspases (zVADfmk), necroptosis (Necrostatin-1), BID (BI-6C9), or STING (H-151). Biomarkers of pyroptosis and ferroptosis were assessed using our novel quantitative multiplex immunoassay. Increased secretion of pyroptosis-associated cytokines (IL-1α, IL-1β, IL-18), and gasdermin D and E (GSDMD/E) cleavage with parallel loss of respective full-length proteins—both hallmarks of pyroptosis—were recorded in 5/6 cell lines following RSL3 treatment. RSL3 cytotoxicity was blocked by Ferostatin-1; BID and STING inhibitors also prevented GSDMD/E cleavage. We conclude that the ferroptosis-inducer RSL3 triggers pyroptosis in cancer cells; further work is required to elucidate the role of mitochondria in this process. Measurement of pathway-specific protein biomarkers is therefore necessary to identify the exact mechanism of action of novel cytotoxic agents.
Similar content being viewed by others
Introduction
Ferroptosis is a caspase-independent form of cell death driven by iron-catalyzed phospholipid peroxidation1; it is regulated by 2 major antioxidant systems: glutathione peroxidase 4 (GPx4), which reduces phospholipid peroxides in a glutathione-dependent reaction2,3; and the ferroptosis suppressor protein (FSP1) that catalyzes the regeneration of Coenzyme Q103,4. Historically, cell death was classified largely by morphological features, but this approach is insufficient given that at least 3 different forms of cell death (ferroptosis, pyroptosis, and necroptosis) induce the so-called ‘type III’ (necrotic) morphology5. Translational research has lagged in the development of definitive ferroptosis biomarkers6. Phospholipid hydroperoxides are predicted to be the most reliable biomarker for ferroptosis, but measurement by LC-MS is technically challenging7. Increased membrane expression of transferrin receptor 1 (TfR), which facilitates cellular iron import, has also been shown to correlate with ferroptosis8. The 2 most commonly used ferroptosis inducers are RSL3, a GPx4 inhibitor9, and erastin, which inhibits cystine uptake by system xC− causing glutathione depletion10. Ferroptosis is presumed if cell death is inhibited by both lipophilic antioxidants (e.g. ferrostatin-1) and iron chelators but not by caspase or Receptor Interacting Protein Kinases (RIPK) inhibitors11,12. Employing a process of elimination dependent on pathway inhibitors is confounded by dose-dependent off-target effects of these compounds13,14, which may explain why different researchers draw distinct conclusions about the induced cell death pathway of a given compound15,16.
Pyroptosis is a form of inflammatory cell death accompanied by release of pro-inflammatory cytokines such as interleukin-1β (IL-1β) and interleukin-18 (IL-18)17. Pyroptosis is executed when gasdermin proteins are cleaved by proteases (primarily, but not exclusively caspases) into N- and C–terminal domains, followed by oligomerization of the N-terminal fragments into membrane pores leading to cell swelling and lysis18,19,20,21. Mechanistically, pyroptosis has been shown to overlap with other forms of cell death such as apoptosis and necroptosis, where caspases play important roles22,23,24, but no co-occurrences have been reported to date with ferroptosis. Pyroptosis and ferroptosis have certain features in common including plasma membrane permeabilization with cell swelling25,26, cyclic GMP-AMP synthase (cGAS)–stimulator of interferon genes (STING) signaling27,28, BH3-interacting domain death agonist (BID) activation29,30, and oxidative stress31,32. Lipid peroxidation has also been linked to GSDMD oligomerization and membrane binding, inflammasome activation, and caspase-1/4/5/11 activation33,34,35; however gasdermin or pro-inflammatory cytokine activation has not been reported to date in ferroptosis36,37,38.
In this study we investigated whether the ferroptosis inducer RSL3 could initiate other forms of cell death. RSL3-triggered pyroptosis is evidenced by cleaved GSDMD and/or GSDME detected by our sensitive sandwich immunoassays39 and release of pyroptosis-associated cytokines (IL-1α, IL-1β and IL-18). We discuss the translational research implications of this observation that a specific ferroptosis inducer also triggers pyroptosis.
Materials & methods
Cell culture
All cell lines were cultured using RPMI-1640 (ATCC modification; Gibco) with 10% FBS (Rockland) in a 37 °C incubator with 5% CO2. BxPC3, SU-DHL-5 and HT-29 cells were purchased from ATCC; MDA-MB-231, NCI-H522, ACHN, UO-31, and THP-1 cells were obtained from the NCI Biological Testing Branch (Frederick, MD). See https://dctd.cancer.gov/drug-discovery-development/reagents-materials/animal-tumor-models/dctd-tumor-repository-catalog for details on quality control and cell line characterization. The HAP1 GSDMD knockout cell line was obtained from Horizon Discovery (Cambridge, UK).
For adherent cell lines, biomarker experiments were performed using T-175 tissue culture–treated flasks (Corning) (Supplemental Table S1). Approximately 2 days after seeding, the growth media was replaced with media containing RSL3 and 5% FBS (final DMSO concentration 0.1%). In inhibitor combination experiments, cells were pre-treated with inhibitors (Supplemental Table S2) for 1 h before the growth media was replaced with media containing both RSL3 and inhibitor. SU-DHL-5 suspension cells were treated at 0.25 × 106 cells/mL in media with 10% FBS and the 1 h inhibitor pre-treatment was not performed to minimize cell handling. Cell type-specific seeding densities and compound concentrations are outlined in Supplemental Table S1; drug and inhibitor concentrations are shown in Supplemental Table S2. RSL3 concentrations were selected to reliably induce a strong cytotoxic effect after 24 h.
After approximately 24 h of treatment, cells were collected by scraping into media on ice, washed twice by resuspension in ice-cold DPBS, and pelleted at 500 x g, 4 °C for 5 min, and then washed a final time with ice-cold DPBS containing protease and phosphatase inhibitors (Roche). Cells were pelleted at 10,000 x g, 4 °C for 5 min. DPBS was aspirated, and cell pellets were frozen on dry ice, and then stored at −80 °C until protein extraction.
THP-1 cells were used as a positive control of pyroptosis40; for this purpose 1 × 106 cells/mL were differentiated into adherent cells with 50 ng/mL phorbol 12-myristate 13-acetate (PMA; Sigma Aldrich) for 3 days, then primed with 10 µg/mL lipopolysaccharide (LPS) from E. coli (Sigma Aldrich) overnight. The next day, cells were treated with vehicle (0.1% ethanol) or 20 µM nigericin (Cayman Chemicals) for 30 min. Samples were collected as described above.
HT-29 cells were used as a positive control for necroptosis and MLKL phosphorylation; 1.7 × 106 cells were seeded in T-175 flasks the day before treatment with 10 µM emricasan for 30 min to inhibit caspases followed by addition of 20 ng/mL TNFα and 1 µM tolinapant. Cell pellets were collected after 6 h of treatment.
Total protein extraction
Whole cell lysates were generated from cell pellets as previously reported41. Briefly, cold Cell Extraction Buffer (Invitrogen) containing protease and phosphatase inhibitors (Roche) was added to cell pellets on ice; pellets were resuspended by pipetting and vortexing, and then incubated on ice for 1 h with shaking and vortexed every 20 min. Samples were centrifuged at 13,000 x g, 4 °C for 5 min, and supernatants were collected and stored at −80 °C. Protein concentrations were determined using the Bradford method (Bio-Rad).
ATP measurement in cell cultures
Cells were seeded in 96-well white-walled CELLSTAR plates (Greiner Bio-One) in a total volume of 0.1 mL/well and treated as described above (see Supplemental Table S1 for seeding densities). Inhibitors were added at the same time as RSL3 to minimize handling, without pre-treatment. Relative quantities of ATP were assayed as a measure of cell viability using CellTiter Glo-2.0 (Promega) according to the manufacturer’s instructions, and luminescence was read with a Tecan Infinite M1000 multimode plate reader.
Cytokine measurement in cell culture supernatants
Cells were seeded in 12- or 24–well plates (Corning) to a final volume of 1 mL/well and 0.5 mL/well respectively, and treated with RSL3 for 4–24 h as described above. After removing cells and debris by centrifugation (800 x g, 4 °C for 10 min), supernatants were frozen on dry ice and stored at −80 °C. Supernatants were concentrated approximately 3-6-fold using Amicon Ultra devices with a 10 kDa nominal molecular weight limit (EMD Millipore) according to the manufacturer’s instructions and sample concentration factors determined for each sample (starting sample volume ÷ eluted volume). Cytokines secreted in concentrated cell culture supernatants were assayed with a customized MILLIPLEX MAP Human Cytokine panel A (EMD Millipore) according to the manufacturer’s instructions.
Ferroptosis and pyroptosis multiplex assay
Assay details are provided in the Supplemental Methods.
Statistical analysis
Multiplex biomarker data were generated using Luminex xPONENT software. Relative transferrin receptor quantitation was performed using blank-subtracted assay signals. For the remaining targets, analyte concentrations in samples were interpolated from calibrator standard curves fitted to 5-parameter logistic regressions (Bio-Plex Manager v6.1 or newer). Analyte concentrations were normalized to total protein load (pg analyte/µg total protein), and then to the experiment-matched vehicle sample average (expressed as % of vehicle). Every biomarker data point represents the vehicle-normalized value obtained from 1 sample (flask), and each treatment was performed on a minimum of 3 different days with 2 flasks per treatment per experiment, unless otherwise specified. Cell viability data was normalized to the vehicle group average for each experiment with 3 technical replicates per group and 3 independent experiments; each data point represents the replicate-averaged value obtained from 1 experiment. Similarly, secreted cytokine data from 2 to 3 independent experiments for each cell line were divided by sample concentration factors and then normalized to vehicle group averages. Null hypothesis testing of treatment compared to vehicle control was performed with 1-sample t-tests using µ = 100% (representing no change from vehicle) and a cutoff significance level of α = 0.05. The effect of inhibitors on RSL3-induced changes in ATP or biomarker levels was evaluated by one-way ANOVA with correction for multiple comparisons and each biomarker was considered independently. All statistical analyses were performed in GraphPad Prism v.10. Probability values are indicated in the figures (* p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001).
Results
RSL3 induces Fer-1–preventable necrotic morphologies and ATP depletion that are not blocked by caspase and necroptosis inhibitors
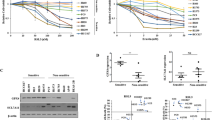
The morphologies, ATP content (as a surrogate for cell viability), and biomarkers of pyroptosis and ferroptosis were evaluated in 6 cancer cell lines treated with the ferroptosis inducer RSL3, alone or in combination with the ferroptosis inhibitor Fer-1, for 24 h (Supplemental Tables S1, S2). When treated with RSL3, each cell line exhibited necrotic morphologies observable by brightfield microscopy (Fig. 1A), which were prevented by Fer-1. These features include cell rounding and swelling into a large bubble of thinned plasma membrane with organelles compacted to one side (Fig. 1A). The appearance is indistinguishable from the morphology seen in the commonly used model of pyroptosis in which THP-1 macrophages are primed with LPS and treated with nigericin40 (Fig. 1B). Intracellular ATP content was reduced by > 75% in all 6 cell lines following RSL3 treatment, and the effect was completely prevented by Fer-1 (Fig. 2A). Using our custom immunoassay39 we evaluated GPx4 and full-length TfR levels following RSL3 and inhibitor treatment as putative adjacent markers of ferroptosis. As expected, RSL3 reduced GPx4 protein levels in all cell lines, except for NCI-H522 (Supplemental Fig. S1), in which GPx4 levels increased by 13% (p = 0.026). Levels of full-length TfR decreased in 3 cell lines and increased in 2 cell lines (Supplemental Fig. S1) following RSL3 treatment. Co-treatment with Fer-1 counteracted loss of GPx4 only in SU-DHL-5 and UO-31 cells and increased GPx4 in NCI-H522 cells to 46% over vehicle. Fer-1 treatment prevented TfR modulation by RSL3 in all cell lines (Supplemental Fig. S1).
RSL3 Induces Necrotic Morphologies in Cancer Cell Lines. (A) RSL3 induces necrotic morphology in 6 cancer cell lines which is prevented by the lipophilic antioxidant and ferroptosis inhibitor Fer-1. This morphology is characterized by a swollen, rounded appearance with cellular organelles compressed to one side. Scale bars for SU-DHL5: 100 μm; all other scale bars: 200 μm. (B) THP-1 macrophages primed with LPS and treated with 20 µM nigericin for 30 min to induce pyroptosis display the same basic morphological features as RSL3-treated cells.
RSL3 Induces Gasdermin Cleavage in Cancer Cells and Pyroptosis is Partially Prevented by Fer-1 and Nec-1, but Not by Caspase Inhibition. (A) ATP levels relative to vehicle control following 1 day of RSL3 treatment with or without ferroptosis inhibitor Fer-1, pan-caspase inhibitor (zVADfmk), or RIPK1 and IDO1/2 inhibitor (Nec-1) co-treatment. All N ≥ 3. Error bars: SEM. Probability values: * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001. (B) Full-length and cleaved GSDMD and GSDME levels relative to vehicle control following 1 day of RSL3 treatment with or without ferroptosis inhibitor Fer-1, pan-caspase inhibitor (zVADfmk), or RIPK1 and IDO1/2 inhibitor (Nec-1) co-treatment. All N ≥ 3. Error bars: SEM. Probability values: * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001.
To investigate contributions from other cell death pathways, we also evaluated cellular morphologies, biomarkers, and ATP content in cells treated with RSL3 in combination with a pan-caspase inhibitor (zVADfmk), or a necroptosis inhibitor (Necrostatin-1, Nec-1). Nec-1 partially rescued 5/6 cell lines from RSL3-induced ATP depletion, whereas caspase inhibitor zVADfmk did not prevent RSL3-induced ATP depletion (Fig. 2A). Neither inhibitor reduced the prevalence of cells with necrotic morphologies (Supplemental Fig. S2). Co-treatment with zVADfmk did not substantially affect GPx4 or TfR levels compared to RSL3 treatment alone (Supplemental Fig. S1). Nec-1 co-treatment followed a pattern similar to that observed following Fer-1 co-administration (Supplemental Fig. S1). Although ferroptosis does not require caspase activity, we detected an increase in active caspase-3 levels in 2/6 cell lines following RSL3 treatment (Supplemental Fig. S1), which was completely blocked by Fer-1 and partially prevented by zVADfmk.
RSL3 treatment induces gasdermin cleavage, which is partially affected by caspase inhibitors
Using our custom multiplex immunoassay39 we detected significant (p < 0.001) RSL3-induced cleavage of GSDMD in 5/6 cell lines and of GSDME in 3/5 cell lines, accompanied by a reduction in full-length GSDMD and GSDME (Fig. 2B). Fer-1 co-treatment inhibited the effects of RSL3 on cleaved and full-length GSDMD/E levels. SU-DHL-5 cells do not express GSDME and are not included in GSDME-related plots (Supplemental Fig. S3A). GSDMD protein is not detectable in NCI-H522 cells by Western blot, although low amounts could be detected by our assay (Supplemental Fig. S3B). See Supplemental Materials for complete WB pictures. Only the ACHN cell line did not have significant induction of either GSDMD or GSDME cleavage following RSL3 treatment, although the full-length respective protein levels decreased significantly (p < 0.0001).
Caspase inhibitor treatment had various effects on the cell lines, mostly inhibiting GSDME cleavage in 4/5 cell lines relative to RSL3 treatment (Fig. 2B; p < 0.05). Paradoxically, zVADfmk increased GSDMD cleavage in 3 cell lines (Fig. 2B; p < 0.05), including the ACHN cell line where no significant GSDMD cleavage was detected after treatment with RSL3 alone. In the NCI-H522 and SU-DHL-5 cell lines, caspase inhibition suppressed RSL3-induced GSDMD cleavage to vehicle levels or lower (Fig. 2B). Nec-1 co-treatment did not markedly affect GSDMD/E cleavage compared to RSL3 single-agent treatment, but it significantly inhibited loss of full-length GSDMD in 3/6 cell lines tested (p < 0.05), and prevented loss of full-length GSDME in MDA-MB-231 cells (Fig. 2B).
Western blot assays confirmed that RSL3 induced GSDME cleavage in BxPC3, NCI-H522, and MDA-MB-231 cell lines, which closely matched the results seen with our Luminex assay (Supplemental Figure S4; see also Supplemental Materials for complete WB pictures). However, we were unable to detect cleaved GSDMD in RSL3-treated cancer cells by Western blot, while a positive control (LPS-primed THP-1 macrophages treated with nigericin) showed the predicted cleaved GSDMD bands (Supplemental Fig. S5A). We established lower limits of detection (LODs) for GSDMD-FL and clGSDMD in our assay using HAP1 GSDMD knockout (KO) cell lysate to confirm that GSDMD cleavage levels are not due to noise or non-specific binding (Supplemental Fig. S5B). See Supplemental Materials for complete WB pictures. To further rule-out non-specific contributions to the cleaved GSDMD Luminex assay signal, we performed additional validations including cleaved GSDMD peptide competition with antigen peptide and removal of the GSDMD detection antibody from the multiplex. Cleaved GSDMD Luminex assay signal was reduced by an average of 100% with peptide competition (range: 97 − 107%) and by an average of 76.5% when removing GSDMD detection antibody (range: 40–99%, 7/8 samples > 70%), thus confirming that cleaved GSDMD signals in the RSL3-treated samples are not due to non-specific binding (Supplemental Fig. S5B).
RSL3-induced cytotoxicity and gasdermin cleavage is prevented by STING or BID Inhibition
We next investigated the involvement of BID and STING signaling in RSL3-induced cytotoxicity due to their reported roles in both ferroptosis42,43 and pyroptosis44,45. In all 6 cell lines tested, inhibitors of BID (BI-6C9) or STING (H-151) prevented the deleterious effects of RSL3 on ATP levels (Fig. 3A) and the appearance of necrotic cells (Supplemental Fig. S6). Inhibiting either pathway also reduced GSDMD/E cleavage to vehicle levels in all cell lines, except for NCI-H522 cells, in which STING inhibition only slightly reduced GSDMD and GSDME cleavage despite restoration of full-length protein levels (Fig. 3B). We note that 3 of the 6 cell lines in our study express very little STING (NCI-H522, ACHN, and SU-DHL-5), and 2 cell lines express no measurable cGAS protein (ACHN, UO-31) on Western blot (Supplemental Fig. S7; see also Supplemental Materials for complete WB pictures). Although BID inhibition effectively prevented cell death from RSL3 we did not detect truncated BID in RSL3-treated BxPC3 cells by Western blot (data not shown); however, multiple studies have reported that BID is involved in ferroptosis and full-length BID can localize to the outer mitochondrial membrane in a process that is inhibited by BI-6C942,46.
BID Activation and STING Signaling Are Critical in RSL3-induced Pyroptosis. (A) ATP levels relative to vehicle control following 1 day of RSL3 treatment with or without STING inhibitor (H-151) or BID inhibitor (BI-6C9) co-treatment. All N ≥ 3. Error bars: SEM. (B) Full-length and cleaved GSDMD and GSDME levels relative to vehicle control following 1 day of RSL3 treatment with or without H-151 or BI-6C9 co-treatment. All N ≥ 3. Error bars: SEM. Probability values: * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001.
Both inhibitors prevented RSL3-induced loss of GPx4 (Supplemental Fig. S8) to > 80% of vehicle levels in UO-31 and SU-DHL-5 cells (p < 0.05), but had little effect in MDA-MB-231 cells and no effect in BxPC3 and ACHN cells. In NCI-H522, the only cell line where GPx4 protein levels did not decrease following RSL3 treatment, these inhibitors nearly doubled (BID inhibitor) or tripled (STING inhibitor) GPx4 protein levels relative to vehicle (p < 0.05, Supplemental Fig. S8). Both inhibitors partially or completely prevented TfR modulation caused by RSL3 treatment in 4/6 cell lines (Supplemental Fig. S8). Both BID and STING inhibition blocked RSL3-induced caspase-3 activation in MDA-MB-231 and SU-DHL-5 cells and partially prevented loss of active caspase-3 in UO-31 cells (Supplemental Fig. S8). Only the STING inhibitor partially prevented loss of active caspase-3 in BxPC3 and induced caspase-3 activation in NCI-H522 cells (Supplemental Fig. S8).
RSL3 induces secretion of pyroptosis-associated cytokines
We used a commercial Luminex assay to measure pyroptosis-associated cytokines (IL-1α, IL-1β, IL-18)17,47 in cell culture supernatant after 4 h and 24 h of RSL3 treatment (a ‘high’ RSL3 concentration used in all experiments and a ‘low’ concentration only used in cytokine experiments; see Supplemental Table S1). Increased IL-18 was detected in the supernatants from RSL3-treated cells of all cell lines except SU-DHL-5, IL-1α was detected in 3 cell lines (BxPC3, MDA-MB-231, UO-31) and IL-1β in 2 cell lines (BxPC3, MDA-MB-231) (Fig. 4). RSL3-induced cytokine secretion was greatest for ACHN (IL-18) and UO-31 (IL-1α, IL-18).
RSL3 Induces Secretion of Pyroptosis-Associated Cytokines. Cells treated with 2 concentrations of RSL3 (see Supplemental Table S1) for 4 h and 24 h secrete at least 1 pyroptosis-associated cytokine in all but the SU-DHL-5 cell line. N ≥ 3 except for IL-1β plots where N = 2 for 4 h treatments (MDA-MB-231 and BxPC3) and 24 h treatments (MDA-MB-231) due to IL-1β being below detection limits in vehicle samples. Error bars: SEM. Probability values: * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001.
Discussion
Vulnerability to ferroptosis has been identified in multiple therapy-resistant cancer cells12,48, making this pathway an attractive target for drug development. However, better tools are required to more precisely understand overlapping cell death pathways. In this study, we apply novel multiplex immunoassays to interrogate biomarkers of ferroptosis and pyroptosis and provide evidence that a commonly used ferroptosis inducer, the GPx4 inhibitor RSL3, also induces pyroptosis in cancer cells. Our data show that RSL3 induced the appearance of necrotic cells (Fig. 1A), cleavage of both GSDMD/E (Fig. 2B), and release of pyroptosis-associated cytokines (Fig. 4). The results are summarized in Table 1. Reversal of both ATP depletion and GSDMD/E cleavage following treatment with BID and STING inhibitors (Fig. 3) suggests that mitochondria may link ferroptosis and pyroptosis following RSL3-induced cell death, but further work is needed to distinguish the relative contributions and time courses followed by the 2 independent cell death pathways.
A swollen, balloon-like morphology has been historically associated with unregulated necrosis49, which in ferroptosis is assumed to be caused by nanopores formed when phospholipid peroxidation damages the plasma membrane50. Pores formed during ferroptosis25,51 and pyroptosis20,52 are similar in size. Distinguishing between forms of cell death therefore requires evidence of involvement of pathway-specific activated protein executioner(s), e.g., cleaved gasdermins (for pyroptosis), phosphorylated or oligomerized Mixed Lineage Kinase Domain Like (MLKL, for necroptosis), and blebbing-localized cleaved caspase-3 (for apoptosis). No protein executioner biomarker has been described for ferroptosis, but recent research suggests protein lipid modifications are attractive targets for biomarker development53.
Using the common approach of combining a cytotoxic compound (RSL3) with inhibitors of lipid oxidation (Fer-1), caspases (zVADfmk), or necroptosis (Nec-1) to identify the cell death pathway, we observe a pattern consistent with ferroptosis: ATP depletion was completely prevented by Fer-1, not significantly blocked by zVADfmk, and the effect of Nec-1 varied by cell line (Fig. 2A). However, we have not detected evidence of necroptosis by Western blot in select cell lines where Nec-1 either partially (NCI-H522) or markedly (ACHN, SU-DHL5) blocked cell death (Supplemental Fig. S9; see also Supplemental Materials for complete WB pictures). A recent report concluded that Nec-1 inhibits ferroptosis by upregulating antioxidant genes, rather than by inhibiting RIPK1 or indoleamine 2,3-dioxygenase 1/2 (IDO1/2)13, suggesting that Nec-1 is not a useful tool for distinguishing necroptosis from other forms of cell death.
Our biomarker data suggest that the role of caspases in RSL3-induced ferroptosis and in pyroptosis may be more complex than previously reported. Unexpectedly (but not unprecedented54), RSL3 induces caspase-3 activation in 2 cell lines (Supplemental Fig. 1), which along with GSDME cleavage, is inhibited by zVADfmk. GSDME cleavage is also inhibited in cell lines without RSL3-induced caspase-3 activation. This suggests that GSDME may be cleaved by other proteases sensitive to zVADfmk, or that caspase-3 activity is altered in the context of excessive lipid peroxidation through protein modifications such as carbonylation, glutathionylation, etc. Paradoxically, caspase inhibition dramatically increases GSDMD cleavage in 3 cell lines (Fig. 2B); the mechanisms remain elusive, but a possible explanation is that caspase inhibition relaxes a negative feedback signal on an unidentified protease. Recent studies have identified non-caspase proteases capable of cleaving GSDMD or GSDME55,56,57. Interestingly, caspase inhibition by zVADfmk has been reported to increase intracellular ROS in glutamate-induced neurotoxicity, a form of oxytosis/ferroptosis58. Finally, recent studies have reported either an association between ferroptosis and pyroptotic gene expression59 or promotion of GSDMD-N oligomerization by cysteine oxidation60 or lipid peroxidation33,61, providing a potential link between ROS and gasdermin activation.
The N-terminal domains of cleaved gasdermins oligomerize to form pores in the cell membrane leading to the release of inflammatory molecules and pyroptotic cell death21. Here we report the release of at least 1 pyroptosis-associated interleukin (IL-1α, IL-1β, IL-18) in 5/6 cell lines following treatment with the ferroptosis inducer RSL3 (Fig. 4; Table 1). SU-DHL-5, the only cell line where we do not detect interleukin release, also does not express detectable levels of GSDME and experiences limited GSDMD cleavage compared to other cell lines following RSL3 treatment. High levels of secreted interleukins correlate with increased GSDMD and/or GSDME cleavage in 3/5 cell lines (UO-31, BXPC3 and MDA-MB-231), whereas increased GSDMD/E cleavage does not correlate with substantial release of interleukins following RSL3 treatment in NCI-H522 cells. Overall, our results are consistent with pyroptotic cell death following RSL3 treatment, although the exact mechanism of action remains to be determined.
Our findings confirm prior studies reporting involvement of STING and the apoptosis-associated protein BID, in ferroptosis46,62 and pyroptosis28,30. ATP depletion by RSL3 is almost completely prevented by inhibiting either of these pathways (Fig. 3A), independent of the variable levels of STING protein in the cell lines. Both inhibitors restore full-length GSDMD/E levels to approximate vehicle controls compared to RSL3 treatment. The inhibitors also prevent cleavage of GSDMD in 4/6 cell lines, and of GSDME in 3/5 cell lines (Fig. 3B). We believe that STING may connect pyroptosis and ferroptosis through lysosomes and/or the endoplasmic reticulum28,62,63 culminating in the activation or release of proteases such as calpains58,64,65 or cathepsins57,65,66.
Overall, although our results strongly indicate that pyroptosis is induced in cancer cells following RSL3 treatment, the relative contributions of ferroptosis and pyroptosis to RSL3-induced cell death is currently unknown. Similar claims were made in a recent study which demonstrates GSDMD cleavage in RSL3-treated neuron-like rat cells and brain tissue67. Our study emphasizes the critical need to develop reliable biomarkers that better distinguish between different cell death pathways. Increased TfR expression is reported to be a specific biomarker for ferroptosis8, but our data do not support this finding. Based on our observations, we recommend using a combination of morphology and measurement of pathway-specific protein biomarkers with validated antibodies or reagents to identify the mechanism of action of novel cytotoxic agents. When small molecule inhibitors are used, it is critical to consider their concentrations and possible off-target effects; such observations need to be corroborated by additional evidence to facilitate translational drug development for patients with cancer and other diseases.
Data availability
The original data presented in the study are included in the article/supplementary material. For further inquiries contact the corresponding authors.
References
Dixon, S. J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 (2012).
Toppo, S., Flohe, L., Ursini, F., Vanin, S. & Maiorino, M. Catalytic mechanisms and specificities of glutathione peroxidases: variations of a basic scheme. Biochim. Biophys. Acta. 1790, 1486–1500 (2009).
Yan, H. F. et al. Ferroptosis: mechanisms and links with diseases. Signal. Transduct. Target. Ther. 6, 49 (2021).
Doll, S. et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 575, 693–698 (2019).
Galluzzi, L. et al. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell. Death Differ. 25, 486–541 (2018).
Chen, X., Comish, P. B., Tang, D. & Kang, R. Characteristics and biomarkers of ferroptosis. Front. Cell. Dev. Biol. 9, 637162 (2021).
Kagan, V. E. et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Chem. Biol. 13, 81–90 (2017).
Feng, H. et al. Transferrin receptor is a specific ferroptosis marker. Cell. Rep. 30, 3411–3423e3417 (2020).
Yang, W. S. et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 156, 317–331 (2014).
Dixon, S. J. et al. Pharmacological Inhibition of cystine-glutamate exchange induces Endoplasmic reticulum stress and ferroptosis. Elife 3, e02523 (2014).
Soriano-Castell, D., Currais, A. & Maher, P. Defining a Pharmacological inhibitor fingerprint for oxytosis/ferroptosis. Free Radic Biol. Med. 171, 219–231 (2021).
Jiang, X., Stockwell, B. R. & Conrad, M. Ferroptosis: mechanisms, biology and role in disease. Nat. Rev. Mol. Cell. Biol. 22, 266–282 (2021).
Yuk, H., Abdullah, M., Kim, D. H., Lee, H. & Lee, S. J. Necrostatin-1 prevents ferroptosis in a RIPK1- and IDO-Independent manner in hepatocellular carcinoma. Antioxidants (Basel) 10, 1347 (2021).
Chauvier, D., Ankri, S., Charriaut-Marlangue, C., Casimir, R. & Jacotot, E. Broad-spectrum caspase inhibitors: from myth to reality? Cell. Death Differ. 14, 387–391 (2007).
Lachaier, E. et al. Sorafenib induces ferroptosis in human cancer cell lines originating from different solid tumors. Anticancer Res. 34, 6417–6422 (2014).
Zheng, J. et al. Sorafenib fails to trigger ferroptosis across a wide range of cancer cell lines. Cell. Death Dis. 12, 698 (2021).
Tan, Y. et al. Pyroptosis: a new paradigm of cell death for fighting against cancer. J. Exp. Clin. Cancer Res. 40, 153 (2021).
Liu, X. et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535, 153–158 (2016).
Aglietti, R. A. et al. GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proc. Natl. Acad. Sci. U S A. 113, 7858–7863 (2016).
Chen, X. et al. Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell. Res. 26, 1007–1020 (2016).
Yu, P. et al. Pyroptosis: mechanisms and diseases. Signal. Transduct. Target. Ther. 6, 128 (2021).
Malireddi, R. K. S., Kesavardhana, S. & Kanneganti, T. D. ZBP1 and TAK1: master regulators of NLRP3 inflammasome/pyroptosis, apoptosis, and necroptosis (PAN-optosis). Front. Cell. Infect. Microbiol. 9, 406 (2019).
Bedoui, S., Herold, M. J. & Strasser, A. Emerging connectivity of programmed cell death pathways and its physiological implications. Nat. Rev. Mol. Cell. Biol. 21, 678–695 (2020).
Fritsch, M. et al. Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature 575, 683–687 (2019).
Riegman, M. et al. Ferroptosis occurs through an osmotic mechanism and propagates independently of cell rupture. Nat. Cell. Biol. 22, 1042–1048 (2020).
Sborgi, L. et al. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 35, 1766–1778 (2016).
Li, C. et al. Mitochondrial DNA stress triggers autophagy-dependent ferroptotic death. Autophagy 17, 948–960 (2021).
Gaidt, M. M. et al. The DNA inflammasome in human myeloid cells is initiated by a STING-Cell death program upstream of NLRP3. Cell 171, 1110–1124e1118 (2017).
Neitemeier, S. et al. BID links ferroptosis to mitochondrial cell death pathways. Redox Biol. 12, 558–570 (2017).
de Torre-Minguela, C., Gomez, A. I., Couillin, I. & Pelegrin, P. Gasdermins mediate cellular release of mitochondrial DNA during pyroptosis and apoptosis. FASEB J. 35, e21757 (2021).
Zhou, B. et al. Tom20 senses iron-activated ROS signaling to promote melanoma cell pyroptosis. Cell. Res. 28, 1171–1185 (2018).
Yang, W. S. & Stockwell, B. R. Ferroptosis: death by lipid peroxidation. Trends Cell. Biol. 26, 165–176 (2016).
Kang, R. et al. Lipid peroxidation drives gasdermin D-Mediated pyroptosis in lethal polymicrobial Sepsis. Cell. Host Microbe. 24, 97–108e104 (2018).
Fan, R., Sui, J., Dong, X., Jing, B. & Gao, Z. Wedelolactone alleviates acute pancreatitis and associated lung injury via GPX4 mediated suppression of pyroptosis and ferroptosis. Free Radic Biol. Med. 173, 29–40 (2021).
Chen, R. et al. AGER-Mediated lipid peroxidation drives Caspase-11 inflammasome activation in Sepsis. Front. Immunol. 10, 1904 (2019).
Tang, L. et al. Induction mechanism of ferroptosis, necroptosis, and pyroptosis: A novel therapeutic target in nervous system diseases. Int J. Mol. Sci 24, 10127 (2023).
Xu, C. et al. The glutathione peroxidase Gpx4 prevents lipid peroxidation and ferroptosis to sustain Treg cell activation and suppression of antitumor immunity. Cell. Rep. 35, 109235 (2021).
Zhang, Y. et al. Ferroptosis inhibitor SRS 16–86 attenuates ferroptosis and promotes functional recovery in contusion spinal cord injury. Brain Res. 1706, 48–57 (2019).
Herrick, W. G., Govindharajulu, J., Parchment, R. E., Doroshow, J. H. & Srivastava, A. K. in AACR Annual Meeting 2023. (American Association for Cancer Research).
Okondo, M. C. et al. DPP8 and DPP9 Inhibition induces pro-caspase-1-dependent monocyte and macrophage pyroptosis. Nat. Chem. Biol. 13, 46–53 (2017).
Srivastava, A. K. et al. Effect of a Smac mimetic (TL32711, Birinapant) on the apoptotic program and apoptosis biomarkers examined with validated multiplex immunoassays fit for clinical use. Clin. Cancer Res. 22, 1000–1010 (2016).
Jelinek, A. et al. Mitochondrial rescue prevents glutathione peroxidase-dependent ferroptosis. Free Radic Biol. Med. 117, 45–57 (2018).
Kuang, F., Liu, J., Li, C., Kang, R. & Tang, D. Cathepsin B is a mediator of organelle-specific initiation of ferroptosis. Biochem. Biophys. Res. Commun. 533, 1464–1469 (2020).
Zhang, W. et al. Cytosolic escape of mitochondrial DNA triggers cGAS-STING-NLRP3 axis-dependent nucleus pulposus cell pyroptosis. Exp. Mol. Med. 54, 129–142 (2022).
Yu, J. et al. Inflammasome activation leads to Caspase-1-dependent mitochondrial damage and block of mitophagy. Proc. Natl. Acad. Sci. U S A. 111, 15514–15519 (2014).
Tobaben, S. et al. Bid-mediated mitochondrial damage is a key mechanism in glutamate-induced oxidative stress and AIF-dependent cell death in immortalized HT-22 hippocampal neurons. Cell. Death Differ. 18, 282–292 (2011).
Tsuchiya, K. et al. Gasdermin D mediates the maturation and release of IL-1alpha downstream of inflammasomes. Cell. Rep. 34, 108887 (2021).
Viswanathan, V. S. et al. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature 547, 453–457 (2017).
Laster, S. M., Wood, J. G. & Gooding, L. R. Tumor necrosis factor can induce both apoptic and necrotic forms of cell Lysis. J. Immunol. 141, 2629–2634 (1988).
Agmon, E., Solon, J., Bassereau, P. & Stockwell, B. R. Modeling the effects of lipid peroxidation during ferroptosis on membrane properties. Sci. Rep. 8, 5155 (2018).
Pedrera, L. et al. Ferroptotic pores induce Ca(2+) fluxes and ESCRT-III activation to modulate cell death kinetics. Cell. Death Differ. 28, 1644–1657 (2021).
Fink, S. L. & Cookson, B. T. Caspase-1-dependent pore formation during pyroptosis leads to osmotic Lysis of infected host macrophages. Cell. Microbiol. 8, 1812–1825 (2006).
Amoscato, A. A. et al. Formation of protein adducts with Hydroperoxy-PE electrophilic cleavage products during ferroptosis. Redox Biol. 63, 102758 (2023).
Yu, Y. et al. The ferroptosis inducer Erastin enhances sensitivity of acute myeloid leukemia cells to chemotherapeutic agents. Mol. Cell. Oncol. 2, e1054549 (2015).
Zhang, Z. et al. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature 579, 415–420 (2020).
Karmakar, M. et al. N-GSDMD trafficking to neutrophil organelles facilitates IL-1beta release independently of plasma membrane pores and pyroptosis. Nat. Commun. 11, 2212 (2020).
Burgener, S. S. et al. Cathepsin G Inhibition by Serpinb1 and Serpinb6 prevents programmed necrosis in neutrophils and monocytes and reduces GSDMD-Driven inflammation. Cell. Rep. 27, 3646–3656 (2019). e3645.
Elphick, L. M. et al. Opposing roles for caspase and Calpain death proteases in L-glutamate-induced oxidative neurotoxicity. Toxicol. Appl. Pharmacol. 232, 258–267 (2008).
De Backer, J. et al. Cytoglobin Silencing promotes melanoma malignancy but sensitizes for ferroptosis and pyroptosis therapy response. Antioxidants (Basel) 11, 1548 (2022).
Devant, P. et al. Gasdermin D pore-forming activity is redox-sensitive. Cell. Rep. 42, 112008 (2023).
Tang, Y. et al. Cardiolipin oxidized by ROS from complex II acts as a target of gasdermin D to drive mitochondrial pore and heart dysfunction in endotoxemia. Cell. Rep. 43, 114237 (2024).
Hu, X. et al. Emerging role of STING signalling in CNS injury: inflammation, autophagy, necroptosis, ferroptosis and pyroptosis. J. Neuroinflammation. 19, 242 (2022).
Jia, M. et al. Redox homeostasis maintained by GPX4 facilitates STING activation. Nat. Immunol. 21, 727–735 (2020).
Davis, M. A. et al. Calpain drives pyroptotic vimentin cleavage, intermediate filament loss, and cell rupture that mediates immunostimulation. Proc. Natl. Acad. Sci. U S A. 116, 5061–5070 (2019).
Yamashima, T. Can ‘calpain-cathepsin hypothesis’ explain alzheimer neuronal death? Ageing Res. Rev. 32, 169–179 (2016).
Nagakannan, P., Islam, M. I., Conrad, M. & Eftekharpour, E. Cathepsin B is an executioner of ferroptosis. Biochim. Biophys. Acta Mol. Cell. Res. 1868, 118928 (2021).
Bai, H. et al. GPX4 Inhibition contributes to NLRP3-Mediated pyroptosis and cognitive impairment in Ketamine-Exposed neonatal rats. Mol Neurobiol (2025).
Acknowledgements
We thank Lauren Beaumont, Adam Johnson, Vijaya Gowda, Vanessa Wall, Carissa Grose, and Jane Jones for recombinant protein production.
Funding
Open access funding provided by the National Institutes of Health. This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract Number HHSN261201500003I. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Author information
Authors and Affiliations
Contributions
W.G.H., D.E., J.H.D and A.K.S. performed study concept and design; W.G.H., H.L.T., F.R.T., B.B., J.G. and D.E developed methodology and generated data; W.G.H. performed statistical analysis; W.G.H., L.K., R.E.P and A.K.S. wrote, reviewed and revised the paper; W.G.H. and A.K.S. provided analysis and interpretation of data; R.E.P. and J.H.D provided technical and material support. All authors read and approved the final paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Herrick, W.G., Tran, HL., Tomaino, F.R. et al. Potent ferroptosis agent RSL3 induces cleavage of Pyroptosis-Specific gasdermins in Cancer cells. Sci Rep 15, 25249 (2025). https://doi.org/10.1038/s41598-025-11368-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-11368-1
Keywords
This article is cited by
-
Emerging small molecule strategies target cancer stem cells through ferroptosis and metabostemness
Discover Oncology (2025)