Abstract
The current study explored the chemical composition and biological activities of the essential oil and ethanol extract of Salvia lanigera collected from Libya. The ethanol extract obtained from the wild growing S. lanigera was evaluated for the chemical composition via high-performance liquid chromatography with diode-array detection (HPLC–DAD), which demonstrated phenolic and flavonoid contents. A total of 17 compounds, representing phenolic and flavonoid derivatives, have been identified. Besides, gas chromatography–mass spectrometry (GC–MS) analysis of the essential oil obtained from S. lanigera aerial parts identified 24 compounds, accounting for 99.33% of the oil, and the major components were characterized to be 1,8-cineole (27.28%), camphor (25.82%), α-pinene (7.71%), and α-terpineol (7.67%). The results indicated that the oil and ethanol extract exhibited marked radical scavenging capacity toward DPPH (2,2-diphenyl-1-picrylhydrazyl), with IC50 values of 0.1337 and 0.6331 µg/mL, respectively. Further, the oil and ethanol extract demonstrated potent activity against ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)), with IC50 values of 0.17 and 0.0501 µg/mL, respectively. Moreover, S. lanigera oil inhibited more than 76% of the acetylcholinesterase enzyme activity, with an IC50 value of 144 ± 1.04 µg/mL. The in vitro data showed that the ethanol extract has potential antidiabetic properties toward the α-glucosidase enzyme with an IC50 value of 124.6 ± 1.07 µg/mL. Regarding the nature of measurement, assay conditions, and ligand-specific factors such as solubility and stability, we demonstrated a correlation between the IC50 values and in-silico results for antidiabetic assays, where chlorogenic and rosmarinic acids recorded the highest docking scores of -9.4 and − 8.9 kcal/mol, respectively. However, this correlation was not observed in the anti-cholinesterase assay. Further in vivo investigations are essential to confirm the in vitro results of S. lanigera oil and ethanolic extract. This study helps elucidate the potential therapeutic applications of these compounds and their effectiveness in managing diabetes and neurodegenerative disorders.
Similar content being viewed by others
Introduction
The genus Salvia L., commonly known as Sage, is the largest genus in the Lamiaceae family, comprising over 1000 perennial and annual species distributed worldwide. The genus Salvia is represented in Libyan flora with ten species, including S. lanigera, S. fruticosa, S. aegyptiaca, S. verbenaca, S. chudaei, S. spinosa, S. viridis, S. officinals, S. coccinea, and S. splendens. Across the world, many researchers are interested in investigating the potential of Salvia species, attributed to its economic and therapeutic importance, as several Sage species have been traditionally utilized for various ailments, including diarrhoea, gonorrhoea, respiratory problems, wounds, eye diseases, diabetes, inflammation, and impaired cognition. Thus, various studies have documented the biological properties of the essential oils extracted from Salvia species with antibacterial and antiviral activities1,2. Several species of the genus Salvia exhibited antifungal and antibacterial properties toward various food microorganisms. Hence, they are used as natural food preservatives3. Further, bioactive secondary metabolites have been discovered in plants of this genus, including terpenes, diterpene quinones, phenolics, and their derivatives4,5. Among Salvia plants, S. lanigera is a small perennial herb characterized by violet inflorescences, which inhabits low-altitude deserts and sandy loams. Salvia lanigera is well-recognized by its deep violet color of corolla and covered with short-erect hairs giving it its name “Lanigera”, which means “fleecy” or “wool-bearing”. Salvia lanigera has been reported in North Africa, including Egypt and Libya, Turkey and Iran6. It has been reported that S. lanigera is commonly used by Bedouins of the Sinai Peninsula as a condiment for tea7. Previous studies conducted on the essential oil (EO) from S. lanigera have revealed antimicrobial, antioxidant, and antitumor properties3,6.
Alzheimer’s disease (AD) is a brain neurodegenerative disease associated with the deficiency of acetylcholine, a neurotransmitter with crucial role in learning and cognition. This disease affects brain regions, leading to impaired thinking and memory. Currently, one of the most important medications for the management of AD is cholinesterase inhibitors8,9.
Diabetes mellitus (DM) is a disease characterized by hyperglycaemia, and nowadays, it is considered a fatal metabolic disorder with severe complications, including retinopathy, nephropathy, peripheral neuropathy, arterial disease, and infertility. By 2050, about 600 million people will have diabetes, which raises serious concerns on health care systems10. Nowadays, the two most common health issues are AD and DM. It has been noted that DM increases the risk of dementia and cognitive decline, particularly AD and vascular dementia11. Accordingly, there is an urgent need to discover natural-derived products for managing AD and DM. In that context, huge research has been conducted to evacuate the beneficial effects of medicinal plants and their product in treating both AD and DM12,13. Importantly, previous studies have elucidated Salvia herbs’ bioactive compounds and pharmacological potential, which were collected from different geographical regions, including the Sinai Peninsula, Egypt, and Libya14. Furthermore, the observed beneficial biological properties were attributed to several bioactive compounds, including monoterpenes, oxygenated monoterpenoids, sesquiterpenes, phenolic acids, flavonoids, and phenylpropanoids. To the best of our knowledge, limited studies have been conducted on S. lanigera growing Libya. Therefore, this study aimed to evaluate the chemical composition of ethanol extract and EO of the aerial parts of S. lanigera herb, as well as to evaluate the in vitro antioxidant, neuroprotective, and antidiabetic properties. Consequently, the study focused on S. lanigera EOs, phenolic and flavonoid contents that may exhibit strong biological properties and potentially be utilized as therapeutic agents and food preservatives.
Materials and methods
Chemicals and plant material
Gallic acid, ABTS solution, and DPPH (≥ 90%) were sourced from Merck KGaA (Darmstadt, Germany), while all other chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA). The aerial parts of the plant were collected from the Eastern area of Libya during the spring season of 2023. The taxonomist, Dr. Salem Elshatshat, identified the plant as S. lanigera and and a voucher specimen was deposited under the accession number PSL-2406 at herbarium of the Pharmacognosy and Natural Products Laboratory, Faculty of Pharmacy, Assalam University, Libya.
Sample preparation
Extract preparation
The dried aerial parts of S. lanigera (200 g) were extracted three times by maceration with 95% ethanol. The combined ethanol extracts were dried under reduced pressure.
Essential oil extraction
Using the Clevenger apparatus, the fresh aerial parts (500 g) were hydrodistilled for three hours at 75 °C. The oil was then collected, dried over anhydrous sodium sulphate (Na2SO4), and stored in amber glass containers at 4 °C.
Gas chromatography-mass spectrometry (GC-MS) analysis
The oil volatiles were separated using a gas chromatograph (Agilent 8890 System, Delaware, USA) coupled with a mass spectrometer (Agilent 5977B GC/MSD) that had an HP-5MS capillary column (30 m, 0.25 mm i.d., 0.25 mm film thickness). A sample size of 1 µL was injected at 230 °C in split mode (1:50). The oven temperature was initially set at 50 °C and then increased at a rate of 5 °C/min to 200 °C. It was subsequently raised to 280 °C at 10 °C/min, where the temperature was maintained isothermally for 7 min. Mass spectra were acquired in electron impact (EI) mode at 70 eV, covering a mass-to-charge ratio (m/z) range from 39 to 500 amu. Peaks were identified by comparing them to NIST standards and published data. The percentages of detected compounds were calculated based on the GC peak areas. The Kovats index for each compound was determined using the retention times of C6–C26 n-alkanes (Supelco Inc., Bellefonte, PA, USA) and compared to values found in the literature15.
Determination of phenolics using High-Performance liquid chromatography (HPLC)
HPLC analysis was carried out using an Agilent 1260 series. The separation was carried out using Zorbax Eclipse Plus C8 column (4.6 mm x 250 mm i.d., 5 μm). The mobile phase consisted of water (A) and 0.05% trifluoroacetic acid in acetonitrile (B) at a flow rate of 0.9 mL/min. The mobile phase was programmed consecutively in a linear gradient as follows: 0 min (82% A); 0–1 min (82% A); 1–11 min (75% A); 11–18 min (60% A); 18–22 min (82% A); 22–24 min (82% A). The multi-wavelength detector was monitored at 280 nm. The injection volume was 5 µl for each of the sample solutions. The column temperature was maintained at 40 °C16.
Antioxidant activity measurements
DPPH radical scavenging assay
A spectrophotometric method using 2,2-diphenyl-1-picrylhydrazyl (DPPH) as a reagent was used to determine the radical scavenging activity of the extract and essential oil obtained from S. lanigera. TROLOX and ascorbic acid was serve as reference standards. The total reaction volume was 3 mL of a methanol solution containing DPPH radicals. The mixture was vigorously shaken and kept in the dark for 30 min. At 517 nm, the absorbance of the mixture was measured thrice for accuracy using a Shimadzu UV-160-IPC spectrophotometer (Kyoto, Japan) against a blank. The result was calculated using the following formula: I%=[(ΔA517C-ΔA517S)/ΔA517S], where ΔA is the average absorbance, C is the control, and S is the sample17.
The ABTS free radical scavenging assay
The second test to evaluate antioxidant activity was the 2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) di-ammonium salt radical cation (ABTS+). A Shimadzu spectrophotometer UV-160-IPC (Kyoto, Japan) measured the absorbance at 734 nm to determine reducing power. Ascorbic acid was used as a positive control, whereas deionized water was used as a blank17.
Quantitative analysis of phenolics and flavonoids
Total phenolic content
Folin-Ciocalteu reagent and gallic acid were used to determine phenolic content. The reaction mixture was incubated at 45 °C for 45 min and measured at 765 nm3. A 100 µL of the sample was mixed with 1.5 mL of the Folin’s phenol reagent (10%). After 5 min, 0.5 mL of saturated sodium carbonate (Na2CO3) (7.5%) was added. The absorbance of the supernatant was measured at 725 nm. TPC was standardized against gallic acid (GA) and expressed in terms of mg GA equivalents (GAE)/mL of dry matter (DM). This assay’s linearity range was determined as 0.02–0.3 mg GA/mL (R2 = 0.99). The analysis was repeated in triplicate.
Estimation of flavonoid content
Using a calibration curve based on rutin, the flavonoid content in the ethanol extract has been measured. A 100 µL of the sample was mixed with 0.1 mL of sodium nitrite (NaNO2) and 0.1 mL of aluminium chloride (AlCl3) solution, and the mixture incubated for 5 min. Then, 2 mL of 4% sodium hydroxide (NaOH) was added, followed by incubation for 10 min. Finally, the absorbance at 510 nm was measured to determine the flavonoid content expressed as milligrams per gram of rutin equivalent17.
Enzyme inhibition activity
α-Amylase inhibition assay
The assay followed the method of Khadayat et al.18. In 96-microwell plates, 20 µL of samples or blanks were combined with 140 µL phosphate buffer (50 mM, 0.9% NaCl, pH 7). Then, 20 µL of amylase enzyme (1 mg/mL) was added, and the mixture was incubated for 15 min at 37 °C. Next, 20 µL of substrate (0.375 mM) was added, followed by an additional 10-minute incubation at 37 °C. Enzyme activity was measured by releasing p-nitrophenol at 405 nm using a microplate reader (Onega, USA). The percentage of α-amylase inhibition was calculated using the formula:
% Inhibition = [(A blank – A sample)/A blank] x 100.
Here, A blank is the absorbance of the control (without inhibitor), and A sample is the absorbance in the presence of the inhibitor.
α-Glucosidase inhibition assay
The assay followed the method of Abdallah et al.19. In 96-microwell plates, 25 µL of samples or blanks were incubated for 10 min at 37 °C with 50 µL of α-glucosidase from Saccharomyces cerevisiae (0.6 U/mL) in phosphate buffer (0.1 M, pH 7). Subsequently, 25 µL of 3 mM p-NPG substrate was added, and the mixture was incubated for an additional 5 min at 37 °C. Enzyme activity was measured by releasing p-nitrophenol at 405 nm using a microplate reader. The percentage of α-glucosidase inhibition was calculated as follows:
% Inhibition = [(A blank – A sample)/A blank] x 100.
A blank is the control absorbance, and A sample is the absorbance with the inhibitor.
Acetylcholine esterase inhibition assay
The assay followed the Elmann et al.20 and Osman et al.21 methods, with minor modifications. In a 96-well plate, 10 µL of indicator solution (0.4 mM in 100 mM tris buffer pH 7.5) was added, followed by 20 µL of enzyme solution (0.02 U/mL acetylcholine esterase in 50 mM tris buffer pH 7.5 with 0.1% BSA) and 20 µL of sample/standard solution. After adding 140 µL of buffer, the mixture was incubated for 15 min at room temperature. Then, 10µL of acetylcholine iodide substrate was added, and the plate was incubated for 20 min in the dark. The color was measured at 412 nm, and data are presented as means ± SD.
Docking study
Enzyme crystal structures were downloaded from the Protein Data Bank (PDB) (https://www.rcsb.org/) on April 24, 2025, including human pancreatic α-amylase (PDB ID: 4GQR), α-glucosidase (PDB ID: 3A4A), and human acetylcholinesterase (PDB ID: 4EY7). Water and ligand molecules were removed and protonated using PyMOL (version 2.5.1). Major phytochemicals as ligands were obtained from PubChem via http://pubchem.ncbi.nlm.nih.gov/ on the same date and optimized with Avogadro (version 1.2.0). The binding potential of the enzyme’s pockets was predicted using CB-DOCK2 and Discovery Studio (ver25.1.0.24284). The docking method was validated by re-docking co-crystallized ligands (Supplementary file) with AutoDock 1.5.6 and Vina22yielding low RMSD values between 0.547 and 0.941 Å. Based on the identified pocket residues and dimensions, docking was performed using AutoDock Vina through CB-DOCK2 (http://clab.labshare.cn/cb-dock/php/) on April 24–25, 202523. The docked complexes were analyzed and visualized with Discovery Studio24.
Results and discussion
Phytochemical analysis of Salvia lanigera extract and essential oil
The current study investigated the chemical profile of S. lanigera extract and essential oil (EO). In the study, the aerial parts of S. lanigera were extracted via maceration with ethanol (95%) and hydrodistilled by the Clevenger apparatus. Further, the extract was phytochemically analyzed using HPLC-DAD against specific phenolics and flavonoids standards. Additionally, the distilled EO was phytochemically profiled using gas chromatography-mass spectrometry (GC-MS) analysis. The family Lamiaceae and Salvia species are aromatic plants known for their production of essential oils25,26. These plants are a valuable source of biologically active phenolic acids and flavonoids26. However, the plant production of the essential oils, phenolic acids, and flavonoids is highly affected by several abiotic and biotic factors. These factors are also affecting the plants’ biological activities and therapeutic indications27.
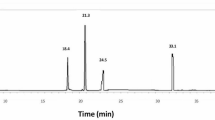
In HPLC analysis, the chemical compounds were determined based on comparing their retention times with those of reference standards, and they were characetrized as gallic acid, chlorogenic acid, catechin, methyl gallate, caffeic acid, syringic acid, ellagic acid, rutin, coumaric acid, vanillin, ferulic acid, naringenin, rosmarinic acid, daidzein, quercetin, cinnamic acid, kaempferol, and hesperitin as shown in Table 1 and Figs. 1 and 2. Notably, marked higher contents of phenolic acids, totally calculated as 8387.68 µg/g of the plant extract, were revealed via HPLC analysis. Moreover, phenolic acid derivatives, including gallic acid and chlorogenic acid, were determined at contents more than 1000 µg/g (1675.66 and 1073.21 µg/g of the plant extract, respectively. Additionally, the study outcomes revealed the presence of coumaric acid, caffeic acid, ellagic acid, syringic acid, ferulic acid, vanillin, and rosmarinic acid in considerable amounts of 798.00, 553.82, 213.39, 234.05, 96.61, 155.76, and 3451.75 µg/g of the plant extract, respectively. Flavonoids, including kaempferol, naringenin, and hesperetin were determined at contents of 406.91, 371.34, and 152.61 µg/g, respectively. The findings in Table 1 and Fig. 1 are consistent with the reported phenolic acids and flavonoids of plants grown in various regions, such as Egypt1 and also revealed variations in polyphenolic nature, which may reflect the effect of the growing environment on the plant.
Chemical structures of the identified chemical compounds: gallic acid (1), chlorogenic acid (2), methyl gallate (3), caffeic acid (4), syringic acid (5), rutin (6), ellagic acid (7), coumaric acid (8), vanillin (9), ferulic acid (10), naringenin (11), rosmarinic acid (12), daidzein (13), quercetin (14), cinnamic acid (15), kaempferol (16), and hesperetin (17).
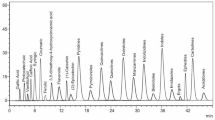
The volatile constituents of S. lanigera were identified via GC-MS analysis (Figs. 3 and 4). The identification was confirmed by comparison with the retention indices, the mass spectrum of authentic compounds, and the NIST mass spectra library data. As shown in Table 2, GC–MS analysis was used to identify 24 volatile compounds in the S. lanigera sample, accounting for 99.33% of all compounds detected. The compounds 1–24 identified were categorized into different chemical classes, including monoterpene, sesquiterpene, phenylpropene, and fatty alcohol derivatives.
In the present study, monoterpenes derivatives were characterized, including oxygenated bicyclic hydrocarbon (1,8-cineole 10, borneol 16, camphor 14, 55.6%), representing the major oil constituents, cyclic hydrocarbon (α-terpinene 7, D-limonene 9, γ-terpinene 11, terpinolene 12, 4.22%), oxygenated cyclic hydrocarbon (δ-terpineol 15, terpinen-4-ol 17, α-terpineol 18, 11.34%), oxygenated acyclic hydrocarbon (linalool 13, citronellol 19, 1.56%), acyclic hydrocarbon (β-myrcene 6, 1.27%), and bicyclic hydrocarbon (α-thujene 1, α-pinene 2, camphene 3, β-pinene 5, 17.47%). p-Cymene 8 (2.62%) was detected as an aromatic hydrocarbon, whereas methyl eugenol 20 (1.84%) was determined as phenylpropene. Moreover, 1-octen-3-ol 4 (1.39%) was determined as fatty alcohol derivative in the essential oil of S. lanigera. Additionally, four sesquiterpene compounds were determined, classified as bicyclic hydrocarbons, including caryophyllene 21, caryophyllene oxide 22, tau-cadinol 23, and α-betulenol 24, 1.92%.
The literature claimed that the chemical profile of EOs exhibits variability depending on the maturity stage of the plant, the time of harvest, geographical location, and prevailing environmental conditions28. Previous studies revealed that monoterpene derivatives were the main chemical class of the essential oil reported in S. lanigera with 71.7%, followed by sesquiterpenes with 21.7% and phenylpropanoid derivatives with (3.5%). The high content of monoterpenes was mainly due to the presence of thymol (54.9%). Other constituents included cedrol (8.9%), methyl chavicol (3.5%) and spathulenol (3.4%). The reported essential oil was mainly composed of oxygenated derivatives (85.9%), classified as alcohols, ketones, aldehydes and phenols29.
Total phenolics and flavonoids
The phenolic and flavonoid contents of the plant EO and extract were also measured, and their quantities in equivalents to gallic and rutin are presented in Table 3. The findings indicated comparable results for the phenolic contents of both EO and the plant extract. The phenolic contents in EO are mainly related to the phenolic volatile oils, such as methyl eugenol, which was detected at a relative concentration of 1.84% (Table 1). However, phenolic contents of the extract are consistent with the presence of gallic acid, chlorogenic acid, and rosmarinic acid at higher concentrations, as demonstrated in Table 1. The flavonoid contents were only determined in the plant extract and revealed the presence of 65.08 rutin equivalent per g of the extract. The current findings for the plant’s phenolic and flavonoid contents do not agree with the literature, which measures S. lanigera phenolic and flavonoid contents at 43.04–67.39 mg GAE/g and 26.42–51.59 mg QE/g, respectively6. This could be due to a variety of factors, including the plant’s collection time and variations in the flavonoid standard used to determine total flavonoid content.
The antioxidant potential of Salvia lanigera oil and ethanol extract [DPPH and ABTS radical scavenging assay
This study delved into the antioxidant activity of S. lanigera EO and ethanol extract as radical scavenger potential against DPPH and ABTS free radicals. The study outcomes demonstrated that S. lanigera EO and ethanol extract had marked radical scavenging capacity in a dose-dependent manner. Importantly, S. lanigera oil and ethanol extract showed marked radical scavenging activity toward DPPH, with IC50 values of 0.1337 and 0.6331 µg/mL, respectively (Table 3). Similar scavenging capacity patterns were discovered in the ABTS assay. Notably, EO and ethanol extract demonstrated potent activity at a concentration of 150 µg/mL, with IC50 values of 0.17 and 0.0501 µg/mL, respectively. These findings highlight the potent antioxidant activity of S. lanigera EO and its ethanol extract. These results are inconsistent with the reported antioxidant activity of the plant6,30,31 and can be correlated to the presence of several antioxidant compounds in the plant EO, e.g., 1,8-cineole32,33. The presence of several well-known antioxidant phenolic acids, e.g., caffeic acid, gallic acid, rosmarinic acid, and coumaric acid, and flavonoids, e.g., rutin and quercetin, is also attributed to the antioxidant activity of the plant extract. This suggests that the overall antioxidant capacity may be influenced not only by the individual compounds but also by their synergistic interactions.
Evaluation of the anticholinesterase potential of Salvia lanigera extract and essential oil
Acetylcholine (ACh) is a brain neurotransmitter that has an important role in managing Alzheimer’s disease (AD). It plays a crucial role in learning and memory development. It is important to note that cholinesterase inhibitors are medications most frequently recommended to manage AD. Traditionally, plants of the genus Salvia are well-recognized for their memory-enhancing properties34. Thus, the current study tested the inhibition capacity of S. lanigera EO toward acetylcholinesterase enzyme (AChE). At a concentration of 500 µg/mL, S. lanigera EO inhibited more than 76% of AChE activity with an IC50 value of 144 ± 1.04 µg/mL as seen in Table 4. These findings are consistent with previous reports in the literature, which revealed a significant activity of various Salvia species, particularly rosmarinic acid, which showed neuroprotective properties34.
Salvia species have been associated with neuroprotective properties. Importantly, the lipophilicity of EO components making them capable of passing the blood–brain barrier and thus could be utilized as a significant strategy for the treatment of neurodegenerative disorders35. EOs are widely applied in food, hair, and skin treatments, indicating that they are generally well tolerated. Compared to synthetic drugs, EOs contain a variety of compounds that perform their action via synergism to reduce the risk of drug resistance and improve the efficacy of treatment36.
It is worth mentioning that the EO composition of S. lanigera growing in Libya has not been fully investigated yet. To the best of our information, the investigation of inhibitory activity of S. lanigera EO against acetylcholinesterase enzyme is reported here for the first time and those of S. sharifii, S. santolinifolia, S. reuterana, S. spinosa, S. palaestina, S. virgata, S. hypoleuca, S. mirzayanii, S. sclarea, S. verticillata, S. multicaulis, and S. syriaca EOs were previously evaluated37. Besides, literature survey indicated that the AChE inhibition activity of ethanol and water extracts of S. fruticosa and S. lanigera at concentrations 25–100 µg/mL was evaluated. The ethanol extract of S. lanigera (100 µg/mL) exhibited a weaker inhibition activity (31.03 ± 0.43%) than positive control, galanthamine (57.11%)6.
Previous studies have discovered that α-pinene, β-pinene, 1,8-cineole, camphor, borneol, α-thujone and β-thujone, thymol, caryophyllene, and caryophyllene oxide have been characterized as predominant components in EOs obtained from Salvia species. Besides, caryophyllene oxide was reported as the most abundant compound in all EOs of various Salvia plants37.
Previous investigation of the acetylcholinesterase inhibition activity EO obtained from S. verticillata demonstrated a weak effect with 20.4% inhibition, which was consistent with results reported by Gharehbagh and her team37. Additionally, S. syriaca EO exhibited a potent AChE inhibition activity with an IC50 value of 1.90 ± 0.1 mg/mL, as compared to the positive drug, galantamine (IC50 = 2.59 ± 0.01 mg/mL). Conversely, Gharehbagh and her team reported that S. syriaca EO (500 µg/mL) demonstrated a weak AChE inhibition activity with an IC50 value of 15.8 ± 0.5%, as compared to donepezil (IC50 = 89.9 ± 0.12)37.
The presence of α-pinene, 1,8-cineole, linalool, limonene, and myrtenyl acetate in the EO of Myrtus communis leaves have been correlated with anti-cholinesterase, antioxidant and neuroprotective effects38. Further, α-pinene and 1,8-cineole of S. leriifolia exerted promising BChE inhibition activities with IC50 values of 0.87 and 0.93 mM, respectively39. Additionally, α-pinene showed a marked inhibition activity against AChE with 76.3 ± 1.27% inhibition40. Caryophyllene oxide from S. verticillate showed a strong inhibition effect against AChE and BuChE (61.03 ± 3.81% and 41.46 ± 2.66%, respectively)41. Noteworthy, 1,8-cineole, a monoterpenoid compound reported in many plant EOs has been shown to possess anticholinesterase activity and beneficial neuroprotective properties, as it could modulates tau phosphorylation through down-regulating the activity of GSK-3β and inhibiting the BACE1 activity and consequently reducing Aβ production. Hence, 1,8-cineole was suggested as a therapeutic agent in the treatment of AD42,43. Furthermore, the acetylcholinesterase inhibitory activity for the Cinnamomum camphora EO at concentration of 1 mg/mL revealed 53.61 ± 2.66% inhibition, as compared to standard drug physostigmine 97.53 ± 0.63% at 100 ng/mL. Apparently, the EO detected in C. camphora was found rich in 1,8-cineole (55.84%), sabinene (14.37%), and α-terpineol (10.49%), that might contribute to AChE inhibition activity44. Interestingly, 1,8-cineole and α-terpineol were characterized here in S. lanigera EO with 27.28 and 7.67%, respectively.
In the present study, GC–MS analysis of S. lanigera EO revealed that monoterpenes, phenyl propene and sesquiterpenes were determined in EO from S. lanigera. Among them, 1,8-cineole, camphor, α-pinene, α-terpineol, caryophyllene oxide and other sesquiterpenes have been determined. In this work, the EO of S. lanigera growing in Libya, exerted strongest activity toward AChE. In this regard, it seems that α-pinene, 1,8-Cineole, α-terpineol, linalool, limonene, sesquiterpenes, and other volatile constituents detected in EO of S. lanigera contribute to the acetylcholinesterase inhibition properties. Thus, S. lanigera might be a potential and safe source for new medicinal agents to control AD.
Evaluation of the α-glucosidase and α-amylase inhibitory activities of Salvia lanigera extract and essential oil
Alzheimer’s disease (AD) is the most prevalent form of dementia, accounting for 60–80% of all cases. Apparently, AD is one of the main reasons why older people worldwide are becoming less functional in their day-to-day activities. Synaptic dysfunction, neuronal death, behavioural changes and impaired cognitive functions are the main characteristics of AD45. Notably, impaired control of blood sugar levels may elevate the risk of developing AD. Hence, AD is currently regarded as type 3 diabetes as impaired glucose metabolism has been shown to be an important regulatory factor in the onset and progression of AD46.
Diabetes is a chronic metabolic disease characterized by high levels of blood glucose resulting from defects in the secretion of insulin, its action, or both, which finally leads to serious damage to various organs in the human body. The prevalence of type 2 diabetes (T2D) has risen dramatically in many countries around the world, accounting for 90–95%47. Lowering the elevated blood glucose levels is critical for preventing diabetes and associated complications, as it can lead to life-threatening issues such as cerebrovascular and cardiovascular disorders48. Carbohydrate molecules are digested to their respective monosaccharides by intestinal enzymes until they are absorbed, causing an increase in blood glucose levels postprandially. α-glucosidase is the most important enzyme in carbohydrate digestion49. As a result, inhibiting α-glucosidase allows for a slower rate of carbohydrate digestion, resulting in lower blood sugar levels and suppression of postprandial hyperglycaemia.
In this study, the in vitro data exhibited that the ethanol extract of S. lanigera had potential antidiabetic inhibition activity toward α-glucosidase enzyme with an IC50 value of 124.6 ± 1.07 µg/mL (Table 4). Conversely, the ethanol extract showed a weak inhibition activity toward α-amylase with 18.08 ± 1.42% inhibition, whereas the EO revealed inhibition activity of 27.22 ± 1.90%.
Inhibition of carbohydrate metabolism is a useful therapeutic approach in the management of diabetes and other metabolic disorders. Herein, the results obtained demonstrated that the ethanol extract of S. lanigera showed important inhibition effect against α-glucosidase enzyme.
It was observed that phenolic derivatives, particularly flavonoids and phenolic acids, have been attributed to managing the complications of metabolic diseases. These compounds can inhibit digestive enzymes’ activity associated with carbohydrate and lipid metabolism, including α-glucosidase, α-amylase and pancreatic lipase. The Salvia species, including S. officinalis, S. greggii, and S. elegans were evaluated for their inhibition potency of enzymes responsible for carbohydrate metabolism. There is a potential correlation between the phenolic content of these plants and the inhibition effect toward α-glucosidase enzyme50,51. This possibility could be attributed to caffeic acid and its derivatives. Moreover, a molecular docking screening of polyphenols, such as caffeic acid, daidzein, hesperetin, naringenin, quercetin, and kaempferol toward the α-glucosidase activity suggested significant inhibition of the α-glucosidase enzyme52. Besides, a literature survey indicated that rosmarinic acid could efficiently inhibits the α-glucosidase enzyme activity with lower EC50 value than acarbose. Importantly, the antidiabetic activity of Salvia species is related to the inhibition of α-glucosidase enzyme due to the presence of rosmarinic acid53,54. In the current study, marked higher contents of phenolic acid derivatives, including coumaric acid, caffeic acid, gallic acid, chlorogenic acid, ellagic acid, syringic acid, ferulic acid, vanillin, and rosmarinic acid, as well as flavonoids, including kaempferol, naringenin, and hesperetin, were determined. These bioactive molecules characterized in S. lanigera support its potential utilization as a significant source of antidiabetic therapies.
Molecular Docking study
The study utilized a molecular docking assay to assess the inhibitory effects of major volatiles, phenolic acids, and flavonoids on α-amylase, α-glucosidase, and acetylcholinesterase, complementing in vitro studies. The chosen crystal structures were 4GQR for α-amylase, 3A4A for α-glucosidase, and 4EY7 for acetylcholinesterase55,56,57. Chlorogenic, ellagic, and rosmarinic acids exhibited potent inhibition against the enzymes, with docking scores from − 8.1 to −9.4 kcal/mol, outperforming the control acarbose (−7.6 and − 7.9 kcal/mol) as seen in Fig. 5. Flavonoids followed with scores between − 7.7 and − 8.7 kcal/mol, while monoterpenes from Salvia lanigera oil showed weaker binding energies of −5.3 and − 6.6 kcal/mol. The same trend was observed for the docking against acetylcholinesterase, where chlorogenic, ellagic, rosmarinic acids, and flavonoids have the highest comparable binding energies among the examined ligands, ranging from − 9.9 to −10.9 kcal/mol. However, all the ligands examined were lower than the control, donepezil (−12.2 kcal/mol). To our knowledge, nothing has been reported for in-silico studies with S. lanigera oil or extract constituents. However, many studies have revealed the interaction through molecular docking for essential oils and botanical extracts with a common trend of higher binding affinities for phenolics compared to terpenes against the same enzymes illustrated in the current study58,59,60,61.
Figure 6 revealed the types of binding interactions of 4GQR-rosmarinic acid, 3A4A-chlorogenic acid, and 4EY7-camphor complexes, which showed the highest docking scores among all phytochemicals examined. Eleven conventional hydrogen bonds between the hydroxyl, imino, and amino groups of rosmarinic acid, THR A:6, ARG A:421, ARG A:252, and ARG A:398, as proton donors, and hydroxyl, carbonyl, and carboxylic of rosmarinic acid, ARG A:10, GLN A:8, SER A:289, and ASP A:402 as proton acceptors, were responsible for such higher docking score (Fig. 6A). Surprisingly, the same number of conventional H-bonding observed in 3A4A-chlorogenic complex, also between donors such as amino, imino, and hydroxyl groups of ILE A:272, HIS A:295, SER A:298, and chlorogenic acid, and acceptors as hydroxyl and carbonyl groups of chlorogenic acid, THR A:290, ASP A:341, and CYS A:342 (Fig. 6B). Finally, only one bond observed between the hydroxyl group of TYR A:124 as a donor and the camphor carbonyl group as an acceptor (Fig. 6C).
N–H⋯O interactions were more prevalent than O–H⋯O and N–H⋯N interactions, with neutral and charged hydrogen bonds occurring equally. Proteins primarily acted as hydrogen bond donors, with glycine frequently serving as both an acceptor and donor due to its flexibility. Arginines formed more hydrogen bonds than lysines, likely because of the three nitrogen atoms in arginine’s guanidinium group. Charged O–H⋯O interactions, mainly between alcohols and carboxylic acids, were three times more common than neutral ones, with ligands more often acting as donors. Aspartic acids were the main acceptors for charged bonds, while asparagine, glycine, and glutamine were typical in neutral interactions. Serine was the most common donor62.
Carbon-hydrogen bonds could be observed from the methine groups of PHE A:335, HIS A:295, and chlorogenic acid to the carbonyl groups of rosmarinic acid, chlorogenic acid, and ASN A:259 (Figs. 6A and B). Aryl rings are important for hydrophobic interactions in proteins, particularly with amino acids like Trp, which often present their aromatic side chains at binding sites. Aromatic rings’ unique shape and electronic properties allow for favorable interaction geometries, primarily T-shaped edge-to-face and parallel-displaced stacking63. Hydrophobic π-π T-shaped interaction was observed between TRP A:15 and the pi-orbitals of chlorogenic acid (Fig. 6B). Similarly, amide.π-stacking interaction was observed in 3A4A-chlorogenic acid complex, where the π-surface of the amide bond (SER A:291 and ALA A:292) stacks against the π-surface of the ligand aromatic ring (Fig. 6B). Finally, hydrophobic π-alkyl interactions could be observed between the π-orbitals of rosmarinic acid and the alkyl group of PRO A:4 (Fig. 6A). The π- σ hydrophobic interaction from the C-H of ALA A:292 to π-orbitals of chlorogenic acid agreed with the ranking of the donor residues reported by Brandl et al.64.
Studying molecular docking between major phytochemicals and target enzymes can help reveal interactions in the ligand-receptor complex. However, deviations from in vitro or in vivo results are expected due to factors not considered in in-silico studies. For example, IC50 values in in-vivo studies account for absorption, distribution, metabolism, and excretion (ADME), which docking does not. A compound may show a high docking score but fails to reach the target in vivo, resulting in a high IC50. Moreover, proteins are flexible, and environments are dynamic, while docking typically uses rigid protein structures and idealized conditions65.
Docking may overlook factors like water, ions, or buffer components that affect in vitro binding. Assay conditions (pH, ionic strength) can change enzyme-ligand interactions, leading to deviations from docking predictions. Issues like poor solubility or degradation of phytochemicals can reduce efficacy, and non-specific inhibition from aggregation is not captured in docking. Moreover, in vitro assays may include substrates or cofactors not considered in simulations66,67,68. For example, AChE activity is pH-sensitive, with an optimum around pH 8. Docking simulations typically assume idealized protonation states, while experimental pH affects ligand/protein charge and hydrogen bonding. In vitro assays may involve substrates or co-factors that compete with inhibitors, which docking often overlooks. Additionally, cations or detergents can influence ligand binding stability, and kinetic assays are necessary for irreversible or slow-binding inhibitors, which docking cannot mimic.
In agreement with our findings, Benayad et al.69 studied the effect of various solvent extracts and essential oils of Moroccan Citrus aurantium (L) peel against α-amylase and α-glucosidase. Ethanol, acetone, and chloroform extracts inhibited the two enzymes by approximately 98%. In contrast, the essential oil was inactive among the examined extracts. There were strong correlations between the phenolics identified and enzyme inhibition, which was verified by molecular docking. In the same line, the best inhibitory activity on acetylcholinesterase was exhibited by the Thymus algeriensis oil. On the other hand, Teucrium polium oil was more efficient against butyrylcholinesterase, whereas ethanol extracts of the previous plants showed weak or no inhibitory effect, particularly against acetylcholinesterase69.
Conclusion
In summary, according to the results of the HPLC, Salvia lanigera growing in Libya was found to contain higher polyphenol and flavonoid components in its polar ethanol extract. Among phenolics and flavonoids, rosmarinic acid, gallic acid, chlorogenic acid, caffeic acid, coumaric acid, kaempferol, naringenin, and hesperitin were dominant. Moreover, this research explored the oil composition of S. lanigera and indicated the presence of various hydrocarbons and their oxygenated derivatives. Salvia lanigera polar ethanol extract and essential oil showed potent antioxidant activity. Only S. lanigera essential oil at a concentration of 500 µg/mL demonstrated inhibition activity toward AChE. Whilst the ethanol extract displayed an inhibition activity toward the α-glucosidase enzyme. The obtained results revealed that S. lanigera, which originated from Libya, is a promising source of natural constituents, including polyphenols, flavonoids, and volatile constituents, and possesses beneficial biological properties that can be potentially employed to create novel therapeutic agents for medicinal applications. We found a correlation between IC50 values and in-silico results for antidiabetic assays, but not with the anticholinesterase assay. It is well-known that the nature of assays and ligands, reaction environmental conditions, and ADMET may be responsible for the deviation of the in-silico results compared to in-vitro or in-vivo findings. In vivo studies are necessary to confirm the in vitro findings of S. lanigera oil and its ethanolic extract. These investigations will provide a clear understanding of the therapeutic potential and efficacy of these compounds in treating diabetes and related conditions, ultimately guiding future drug development efforts. Ultimately guiding future drug development efforts, it is essential to explore the mechanisms of action of S. lanigera oil and its extracts.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
Change history
24 October 2025
The original online version of this Article was revised: Esra El Naili and Mohamed A. Sharkasi were incorrectly affiliated. The correct Information now accompanies the original Article.
Abbreviations
- ABTS:
-
(2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid))
- AChE:
-
Acetylcholinesterase enzyme
- AD:
-
Alzheimer’s disease
- ADMET:
-
Absorption, distribution, metabolism, and excretion
- BACE1:
-
Beta-site amyloid precursor protein cleaving enzyme 1
- BChE:
-
Butyrylcholinesterase
- DPPH:
-
2,2-diphenyl-1-picrylhydrazyl
- DM:
-
Diabetes mellitus
- EO:
-
Essential oil
- GC–MS:
-
Gas Chromatography–Mass Spectrometry
- GSK-3β:
-
Glycogen synthase kinase-3 beta
- HPLC–DAD:
-
High-Performance Liquid Chromatography with Diode-Array Detection
- RI:
-
Retention indices
- LRI:
-
Retention indices according to literature
- SL:
-
Salvia lanigera
- T2D:
-
Type 2 diabetes
- TPC:
-
Total phenolic content
- TFC:
-
Total flavonoid content
- GAE/g:
-
Gallic acid equivalent per gram
References
Farid, M. M. et al. Metabolomic profiling and DNA-Fingerprinting of newly recorded White-Flowered populations of Salvia lanigera poir. In Egypt. Chem Biodivers. 21, 1–11 (2024).
Mousa, S. A., Lamlom, S. H. & Al-Barghathi, M. F. The impact of altitude and soil properties on the essential oil components of wild Libyan Salvia fruticosa mill. From Al-Jabal Al-Akhdar area. Int. J. Adv. Sci. Res. 8, 63–67 (2023).
Nasr, A., Yosuf, I., Turki, Z. & Abozeid, A. LC-MS metabolomics profiling of Salvia aegyptiaca L. and S. lanigera poir. With the antimicrobial properties of their extracts. BMC Plant. Biol. 23, 1–16 (2023).
Tepe, B., Sokmen, M., Akpulat, H. A. & Sokmen, A. Screening of the antioxidant potentials of six Salvia species from Turkey. Food Chem. 95, 200–204 (2006).
El-Lakany, A. M. Two new diterpene Quinones for the roots of Salvia lanigera Poir. Die Pharm. Int. J. Pharm. Sci . 34, 75–76 (2003).
Duletić-Laušević, S. et al. Composition And biological activities of Libyan Salvia fruticosa mill. And S. lanigera poir. Extracts. South. Afr. J. Bot. 117, 101–109 (2018).
Bailey, C. & Danin, A. Bedouin plant utilization in Sinai and the Negev. Econ. Bot. 35, 145–162 (1981).
Mukherjee, P. K., Kumar, V., Mal, M. & Houghton, P. J. Acetylcholinesterase inhibitors from plants. Phytomedicine 14, 289–300 (2007).
Othman, A., Sayed, A. M., Amen, Y. & Shimizu, K. Possible neuroprotective effects of amide alkaloids from Bassia indica and agathophora alopecuroides: in vitro and in Silico investigations. RSC Adv. 12, 18746–18758 (2022).
Matoori, S. Diabetes and its complications. ACS Pharmacol. Transl Sci. 5, 513–515 (2022).
Kale, M. B. et al. Navigating the intersection: diabetes and alzheimer’s intertwined relationship. Ageing Res. Rev. 100, 102415 (2024).
Paşayeva, L. et al. Evaluation of the chemical composition, antioxidant and antidiabetic activity of rhaponticoides Iconiensis flowers: effects on key enzymes linked to type 2 diabetes in vitro, in Silico and on alloxan-induced diabetic rats in vivo. Antioxidants 11, 2284 (2022).
Paşayeva, L. et al. Optimizing health benefits of walnut (Juglans regia L.) agricultural by-products: impact of maceration and Soxhlet extraction methods on phytochemical composition, enzyme inhibition, antioxidant, antimicrobial, and cytotoxic activities. Food Biosci. 64, 105923 (2025).
Alonazi, M. A. et al. Evaluation of the in vitro anti-inflammatory and cytotoxic potential of ethanolic and aqueous extracts of Origanum syriacum and Salvia lanigera leaves. Environ. Sci. Pollut Res. 28, 19890–19900 (2021).
Adams, R. P. Identification of essential oil components by gas chromatography/mass spectrometry. 5 online ed. Gruver, TX USA Texensis Publ. (2017).
Kim, K. H., Tsao, R., Yang, R. & Cui, S. W. Phenolic acid profiles and antioxidant activities of wheat Bran extracts and the effect of hydrolysis conditions. Food Chem. 95, 466–473 (2006).
Abdel-Razek, A. G. et al. Assessment of the quality, bioactive compounds, and antimicrobial activity of egyptian, ethiopian, and Syrian black Cumin oils. Molecules 29, 4985 (2024).
Khadayat, K., Marasini, B. P., Gautam, H., Ghaju, S. & Parajuli, N. Evaluation of the alpha-amylase inhibitory activity of Nepalese medicinal plants used in the treatment of diabetes mellitus. Clin. Phytoscience. 6, 1–8 (2020).
Abdallah, H. M. et al. Phenolics from chrozophora oblongifolia aerial parts as inhibitors of α-glucosidases and advanced glycation end products: in-vitro assessment, molecular Docking and dynamics studies. Biology (Basel). 11, 762 (2022).
Ellman, G. L., Courtney, K. D., Andres Jr, V. & Featherstone, R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 7, 88–95 (1961).
Osman, H., Kumar, R. S., Basiri, A. & Murugaiyah, V. Ionic liquid mediated synthesis of mono-and bis-spirooxindole-hexahydropyrrolidines as cholinesterase inhibitors and their molecular Docking studies. Bioorg. Med. Chem. 22, 1318–1328 (2014).
Morris, G. M. et al. AutoDock4 and AutoDockTools4: automated Docking with selective receptor flexibility. J. Comput. Chem. 30, 2785–2791 (2009).
Liu, Y. et al. CB-Dock2: improved protein–ligand blind Docking by integrating cavity detection, Docking and homologous template fitting. Nucleic Acids Res. 50, W159–W164 (2022).
Gharzouli, M. et al. Bio-preservative potential of marjoram and fennel essential oil nano-emulsions against toxigenic fungi in citrus: integrating in-vitro, in-vivo, and in-silico approaches. Food Addit. Contam. - Part. A. https://doi.org/10.1080/19440049.2025.2473551 (2025).
Mohammed, H. A. et al. Salvia officinalis L., constituents, hepatoprotective activity, and cytotoxicity evaluations of the essential oils obtained from fresh and differently timed dried herbs: A comparative analysis. Molecules 26, 5757 (2021). Sage.
Wu, Y. B. et al. Constituents from Salvia species and their biological activities. Chem. Rev. 112, 5967–6026 (2012).
Mohammed, H. A. et al. Factors affecting the accumulation and variation of volatile and Non-Volatile constituents in rosemary, Rosmarinus officinalis L. J. Appl. Res. Med. Aromat. Plants 100571 (2024).
Huang, B. et al. Comparative analysis of essential oil components and antioxidant activity of extracts of Nelumbo nucifera from various areas of China. J. Agric. Food Chem. 58, 441–448 (2009).
Flamini, G., Cioni, P. L., Morelli, I. & Bader, A. Essential oils of the aerial parts of three Salvia species from jordan: Salvia lanigera, S. spinosa and S. syriaca. Food Chem. 100, 732–735 (2007).
Tenore, G. C. et al. Antimicrobial and antioxidant properties of the essential oil of Salvia lanigera from Cyprus. Food Chem. Toxicol. 49, 238–243 (2011).
Loizzo, M. R. et al. Salvia leriifolia Benth (Lamiaceae) extract demonstrates in vitro antioxidant properties and cholinesterase inhibitory activity. Nutr. Res. 30, 823–830 (2010).
Mohammed, H. A. et al. Essential Oils Pharmacological Activity: Chemical Markers, Biogenesis, Plant Sources, and Commercial Products (Process Biochem, 2024).
Mohammed, H. A., Mohammed, S. A. A., Khan, O. & Ali, H. M. Topical eucalyptol ointment accelerates wound healing and exerts antioxidant and Anti-Inflammatory effects in rats’ skin burn model. J. Oleo Sci. 71, 1777–1788 (2022).
Mervić, M. et al. Comparative antioxidant, Anti-Acetylcholinesterase and Anti-α-Glucosidase activities of mediterranean Salvia species. Plants 11, 625 (2022).
Savelev, S. U., Okello, E. J. & Perry, E. K. Butyryl- and Acetyl-cholinesterase inhibitory activities in essential oils of Salvia species and their constituents. Phyther Res. 18, 315–324 (2004).
Tisserand, R. & Young, R. Essential Oil Safety: A Guide for Health Care Professionals (Elsevier Health Sciences, 2013).
Gharehbagh, H. J. et al. Chemical composition, cholinesterase, and α-glucosidase inhibitory activity of the essential oils of some Iranian native Salvia species. BMC Complement. Med. Ther. 23, 1–16 (2023).
Hussein, B. A., Karimi, I. & Yousofvand, N. Chemo- and bio-informatics insight into anti-cholinesterase potentials of berries and leaves of Myrtus communis L., myrtaceae: an in vitro/in Silico study. BMC Complement. Med. Ther. 23, 1–16 (2023).
Loizzo, M. R. et al. In vitro biological activity of Salvia leriifolia Benth essential oil relevant to the treatment of alzheimer’s disease. J. Oleo Sci. 58, 443–446 (2009).
Orhan, I., Şener, B., Kartal, M. & Kan, Y. Activity of essential oils and individual components against Acetyl-and butyrylcholinesterase. Z. fur Naturforsch - Sect. C J. Biosci. 63, 547–553 (2008).
Karakaya, S. et al. A caryophyllene oxide and other potential anticholinesterase and anticancer agent in Salvia verticillata subsp. Amasiaca (Freyn & Bornm.) Bornm.(Lamiaceae). J. Essent. Oil Res. 32, 512–525 (2020).
An, F. et al. 1,8-Cineole ameliorates advanced glycation end Products-Induced alzheimer’s Disease-like pathology in vitro and in vivo. Mol. 2022. 27, 3913 (2022).
Hoch, C. C. et al. 1,8-cineole (eucalyptol): A versatile phytochemical with therapeutic applications across multiple diseases. Biomed. Pharmacother. 167, 115467 (2023).
Rawat, A. et al. Comparative chemical composition and acetylcholinesterase (AChE) inhibitory potential of Cinnamomum camphora and Cinnamomum tamala. Chem. Biodivers. 20, e202300666 (2023).
Guzman-Martinez, L., Maccioni, R. B., Farías, G. A. & Fuentes, P. Navarrete, L. P. Biomarkers for alzheimer’s disease. Curr. Alzheimer Res. 16, 518–528 (2019).
González, A., Calfío, C., Churruca, M. & Maccioni, R. B. Glucose metabolism and AD: evidence for a potential diabetes type 3. Alzheimer’s Res. Ther. 2022 141 14, 1–11 (2022).
Li, W. L., Zheng, H. C., Bukuru, J. & De Kimpe, N. Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J. Ethnopharmacol. 92, 1–21 (2004).
Mwakalukwa, R., Amen, Y., Nagata, M. & Shimizu, K. Postprandial hyperglycemia Lowering effect of the isolated compounds from Olive mill Wastes – An inhibitory activity and kinetics studies on α-Glucosidase and α-Amylase enzymes. ACS Omega. 5, 20070–20079 (2020).
Borges, P. H. O. et al. Inhibition of α-glucosidase by flavonoids of Cymbopogon citratus (DC) Stapf. J. Ethnopharmacol. 280, 114470 (2021).
Mamache, W., Amira, S., Ben Souici, C., Laouer, H. & Benchikh, F. In vitro antioxidant, anticholinesterases, anti-α-amylase, and anti-α-glucosidase effects of Algerian Salvia aegyptiaca and Salvia verbenaca. J. Food Biochem. 44, e13472 (2020).
Pereira, O. R., Catarino, M. D., Afonso, A. F., Silva, A. M. S. & Cardoso, S. M. Salvia elegans, Salvia greggii and Salvia officinalis decoctions: antioxidant activities and Inhibition of carbohydrate and lipid metabolic enzymes. Mol. 2018. 23, 3169 (2018).
Rasouli, H., Hosseini-Ghazvini, S. M. B., Adibi, H. & Khodarahmi, R. Differential α-amylase/α-glucosidase inhibitory activities of plant-derived phenolic compounds: a virtual screening perspective for the treatment of obesity and diabetes. Food Funct. 8, 1942–1954 (2017).
Adımcılar, V. et al. Rosmarinic and carnosic acid contents and correlated antioxidant and antidiabetic activities of 14 Salvia species from Anatolia. J. Pharm. Biomed. Anal. 175, 112763 (2019).
Ngo, Y. L., Lau, C. H. & Chua, L. S. Review on Rosmarinic acid extraction, fractionation and its anti-diabetic potential. Food Chem. Toxicol. 121, 687–700 (2018).
Ibrahim, M. A., Bester, M. J., Neitz, A. W. & Gaspar, A. R. M. Rational in Silico design of novel α-glucosidase inhibitory peptides and in vitro evaluation of promising candidates. Biomed. Pharmacother. 107, 234–242 (2018).
Abdelgawad, M. A. et al. Synthesis and characterization of novel pyrazoline derivatives as dual α-amylase /α-glucosidase inhibitors: molecular modeling and kinetic study. J. Mol. Struct. 1339, 142350 (2025).
Ajala, A., Uzairu, A., Shallangwa, G. A. & Abechi, S. E. Structure-Based drug design of novel piperazine containing hydrazone derivatives as potent alzheimer inhibitors: molecular Docking and drug kinetics evaluation. Brain Disord. 7, 100041 (2022).
Assaggaf, H. et al. GC/MS Profiling, In Vitro Antidiabetic Efficacy of Origanum compactum Benth. Essential Oil and In Silico Molecular Docking of Its Major Bioactive Compounds. Catalysts 13, (2023).
Rants’o, T. A., Koekemoer, L. L., Panayides, J. L. & van Zyl, R. L. Potential of essential Oil-Based anticholinesterase insecticides against Anopheles vectors: A review. Molecules 27, (2022).
Mahnashi, M. H. et al. HPLC-DAD phenolics analysis, α-glucosidase, α-amylase inhibitory, molecular Docking and nutritional profiles of Persicaria hydropiper L. BMC Complement. Med. Ther. 22, 1–20 (2022).
Hussein, H. et al. The Valorization of Spent Coffee Ground Extract as a Prospective Insecticidal Agent against Some Main Key Pests of Phaseolus vulgaris in the Laboratory and Field. Plants 11, (2022).
de Freitas, R. F. & Schapira, M. A systematic analysis of atomic protein–ligand interactions in the PDB. Medchemcomm 8, 1970–1981 (2017).
Bissantz, C., Kuhn, B. & Stahl, M. A medicinal chemist’s guide to molecular interactions. J. Med. Chem. 53, 5061–5084 (2010).
Brandl, M., Weiss, M. S., Jabs, A., Sühnel, J. & Hilgenfeld, R. C-H···π-interactions in proteins. J. Mol. Biol. 307, 357–377 (2001).
Macip, G. et al. Haste makes waste: A critical review of docking-based virtual screening in drug repurposing for SARS-CoV-2 main protease (M-pro) Inhibition. Med. Res. Rev. 42, 744–769 (2022).
Mikra, C., Rossos, G., Hadjikakou, S. K. & Kourkoumelis, N. Molecular Docking and structure activity relationship studies of nsaids. What do they reveal about IC50? Lett Drug Des. Discov. 14, 949–958 (2017).
Huang, S. Y. & Zou, X. Advances and challenges in Protein-ligand Docking. Int. J. Mol. Sci. 11, 3016–3034 (2010).
Chen, Y. C. Beware of docking! Trends Pharmacol. Sci. 36, 78–95 (2015).
Benayad, O. et al. Phytochemical profile, α-glucosidase, and α-amylase inhibition potential and toxicity evaluation of extracts from citrus aurantium (L) peel, a valuable by-product from Northeastern Morocco. Biomolecules 11, (2021).
Acknowledgements
The Researchers would like to thank the Deanship of Graduate Studies and Scientific Research at Qassim University for financial support (QU-APC-2025).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conceptualization, F.A.E., A.F., H.A.M.; Methodology, Visualization and Experimental design, F.A.E., A.F., H.A.M., A.O., E.M., N.A.A.; Writing original manuscript, F.A.E., A.F., H.A.M., A.O., E.M., N.A.A., N.E., E.E., and M.A.S.;, Statistical Analysis, Formal analysis, and Interpretation of biological data, A.F., A.O., E.M., N.A.A. Data curation, Validation, Writing – Review, Revision & editing, H.A.M., F.A.E.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elshibani, F.A., Farouk, A., Naili, E.E. et al. GC-MS and HPLC chemical profile, antioxidant, anti-acetylcholinesterase, and anti-diabetic activities of Libyan Salvia lanigera herb extract and essential oil. Sci Rep 15, 31853 (2025). https://doi.org/10.1038/s41598-025-12233-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-12233-x