Abstract
In brain ischaemia (BI), the oxidative and ionic stresses lead slowly but definitely to the injury of neurones. The present investigation is looking to check the status of certain oxidative stress markers and trace elements which are induced in response to brain ischaemia with sodium valproate (VPA), a neuroprotective agent, in the rats exposed to the model of BI. Although VPA is already used for clinical purposes, it deserves careful examination of the potentially detrimental effects of VPA in conditions of ischaemia closely linked to hepatotoxicity and thrombocytopenia. Forty rats were randomly assigned into five groups (n = 8 per group): Control, Sham, Brain Ischaemia (BI), BI + Valproic Acid (VPA), and VPA only. Ischaemia was induced in the BI and BI + VPA groups by bilateral occlusion of the common carotid arteries for 30 min. VPA was administered orally at a dose of 100 mg/kg daily for seven days in the BI + VPA and VPA-only groups. This dose was based on previous preclinical safety and efficacy data. Control, Sham, and BI groups received saline. The Sham group underwent surgical procedures without carotid occlusion. The biochemical investigations pertaining to cortical tissue demonstrated the fact that BI has decreased all antioxidant enzyme levels of SOD, CAT, GPx, and those of trace elements such as Mg, Zn, and Se. At the same time, malondialdehyde (MDA) and neurotoxic metals (Cr, Fe, Cu) increased. These changes brought by BI were restored through treatment with VPA on oxidative stress and the restoration of trace element balance along with reductions in those associated with oxidative damage. The results thus reinforce the capacity of VPA for neuroprotective benefits against ischaemic damage inflicted within brain tissue and hence merit the further exploration of the mechanistic action of this therapeutic A.
Similar content being viewed by others
Introduction
The clinical consequences of ischaemia manifest in terms of poor treatment avenues. This further points out how ischaemia is capable of producing neurological dysfunction, especially in the brain. Ischaemia of the brain (BI) creates complex pathophysiological processes such as oxidative stress and ionic imbalances aggravating neuronal damage. Trace elements comprising magnesium (Mg), zinc (Zn), selenium (Se), iron (Fe), copper (Cu), and chromium (Cr) have vital roles in brain functions by serving as cofactors for antioxidant enzymes and other proteins. However, excess free Fe and Cu can catalyse Fenton reactions, producing reactive oxygen species (ROS) that cause damage to the integrity and energy homeostasis of mitochondria and worsen ischaemic injury1,2.
Oxidative stress becomes inherent in brain ischaemia because of the high lipid content of the organ and its low capacity for antioxidant defences. The antioxidant enzymes superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) will be the main defence against free radical damage. Ischaemia-reperfusion injury will diminish the activity of these enzymes, leading to lipid peroxidation and accumulation of toxic metabolites like malondialdehyde (MDA)3,4,5,6. Further imbalance in trace elements also disturbs this defence and increases oxidative damage.
The well-known neuroprotectant sodium valproate (VPA) is therefore clinically used for neurological disorders. Protective mechanisms of VPA are multiple, including inhibition of HDACs encoding epigenetic regulation of gene expression. HDAC inhibition by VPA is associated with increased neurotrophic factors such as brain-derived neurotrophic factor (BDNF) and glial cell line-derived neurotrophic factor (GDNF), favouring neuronal survival and synaptic plasticity with axonal repair following ischaemic injury7,8,9. Furthermore, VPA modulates microglial activation; it increases the expression of anti-inflammatory genes, helping to mitigate neuroinflammation. Lastly, VPA reduces the effects of glutamate excitotoxicity, the major cause of cell death in ischaemia, by preventing mitochondrial dysfunction, calcium overload, ROS generation, and lysosomal enzyme release10. Thus, all these effects combined contribute towards enhancing brain plasticity, the structure and function adaptation capacity of the central nervous system via neurogenesis, synaptic remodelling, dendrite branching, and axonal sprouting, all critical for post-injury recovery11,12,13,14.
Unfortunately, the adverse effects of VPA, including hepatotoxicity, thrombocytopenia, and gastrointestinal disturbances, require careful dose selection in clinical practice15,16. An intermediate dose of 100 mg/kg has neuroprotective properties with no toxicity or much nervous system depression, according to preclinical studies17,18. Also, VPA is beneficial regarding trace element homeostasis. It can chelate redox-active metals such as Fe and Cu to lower their participation in Fenton reactions and limit ROS formation17. Beneficial trace elements such as Mg, Zn, and Se, which are important cofactors for antioxidant enzymes and critical for neuronal function, can be restored upon VPA treatment19,20,21. This maintenance balance is essential in preventing oxidative damage and enhancing tissue recovery post-ischaemia.
In conclusion, oxidative stress and trace element imbalance are implicated in the pathogenesis of brain ischaemia. VPA encompasses neuroprotection through multiple actions like epigenetic modulation, antioxidant enzyme action, and metal chelation; thus, it can be harnessed as a potential therapeutic agent. This study will further investigate and elucidate VPA effects on oxidative stress markers and trace element homeostasis following brain ischaemia, thus solving a critical gap in the understanding of the neuroprotective mechanisms.
Materials and methods
Surgery and treatments
For this purpose, a total of thirty-two male albino Wistar rats (220–230 g) were collected from the Elazig Veterinary Control Institute Animal Experiments Department. The animals were kept in controlled conditions (temperature: 18–23 °C, humidity: 40–60%, 12-hour light/dark cycle) with ad libitum access to a rat diet (Table 1) as well as water to drink. The diet was free of any contaminants and pathogens (Arden Research & Experiment Company). Rats were acclimatised for seven days prior to the experiment. Sample size was determined by power analysis (power = 80%, α = 0.05, Cohen’s d = 0.5) and resulted in 8 rats per group for adequate statistical power.
Experimental design
8 were randomly assigned to four groups (n = 8 per group):
-
Control (1.0 ml 0.9% saline orally for 7 days).
-
Brain Ischaemia (BI).
-
Sham (surgical incision without carotid occlusion).
-
BI + valproic acid (VPA) (100 mg/kg oral VPA for 7 days).
-
Administration of Valproic Acid.
Based on previous preclinical studies demonstrating that VPA (Depakin, Sanofi Dogu, Turkey) is administered orally at 100 mg/kg/day for its neuroprotective and antioxidant effects at this dose without CNS depression or toxicity17,18. The preliminary dose-tolerance tests from our lab confirmed that it was safe during 7 days of administration without sedation, motor impairment, or death.
Surgical procedure of induction of brain ischaemia
They were, thus, also anaesthetised with ketamine hydrochloride at 50 mg/kg, i.m. injection, and xylazine: 5 mg/kg, i.m. injection. Rectal temperature was measured and maintained, as well as oxygen was supplied via a paediatric face mask at 200 mL/min. The surgical field was disinfected with 10% povidone-iodine. The bilateral common carotid arteries (CCAs) were exposed through midline cervicotomy for inducing BI after careful dissection from surrounding tissues and occlusion with clamps for 30 min (Fig. 1). The sham group underwent the same surgical exposure but without artery occlusion. Anaesthesia was maintained with intermittent injections of ketamine. Heparinisation (100 IU/kg, i.p.) was done during surgery for the management of coagulation while keeping the active coagulation time (ACT) between 200 and 250 s, as monitored with the Hemochron Jr., every 30 min. After the release of the clamp, prothrombin sulphate (1 mg/kg, i.p.) as well as warm sodium lactate (10 ml, i.p.) were administered to reverse the effects of heparin and control bleeding. The incision was closed using 4 − 0 silk sutures. Postoperative treatment included Cefazolin Na (10 mg/kg; i.m.) and a single injection of Meloxicam (5 mg/kg; s.c.). The rats were monitored for 7 days post-intervention for effects related to reperfusion-induced oxidative stress. At the endpoint of the study, animals were humanely euthanised with a ketamine/xylazine overdose.
Collection of tissues and biochemical analyses
Neocortex tissues from the brain were quickly collected, washed through with ice-cold phosphate-buffered saline, weighed around an average of 200 mg per tissue piece, and homogenised (1:10 w/v) in phosphate buffer (100 mmol/L, pH 7.4) containing 0.05% sodium azide using an Ultra-Turrax T25 homogeniser at 10,000 rpm for 3 min on ice. The homogenate was then centrifuged for 10 min at 5,000 g and at 4 °C, and supernatants were stored at -80 °C for subsequent analytical procedures. All biochemical assays were done in triplicates per sample, from which the mean and standard deviation were calculated.
Assessment of trace elements
Approximately 0.5 g of brain cortex pre-frozen samples were microwave digested in a pressurised system (Mars 5, CEM Corporation, USA) with 65% nitric acid. The digested samples were diluted and analysed for concentration of Mg, Zn, Se, Cr, Cu and Fe by means of ICP-AES (Vista AXE, Varian Inc., Australia). Calibration and quality control during analysis were undertaken with standard reference material (NIST 1577b) and certified blanks. Concentrations were expressed in µg/g tissue:
\({\text{Concentration }}(\mu {\text{g}}/{\text{g}}){\text{ }}={\text{ }}[{\text{Instrument reading }}(\mu {\text{g}}/{\text{L}}) \times {\text{Dilution factor }}\left( {\text{L}} \right)]{\text{ Sample weight }}\left( {\text{g}} \right)\)
Under sterile conditions in a laminar flow cabinet, glassware was acid-washed and ultra-pure water rinsed. Sample handling was completed.
Ethical approval
Each procedure was approved by the Local Ethics Committee of Animal Experiments of the Elazig Veterinary Control Institute, representing ARRIVE guidelines and 3R principles (Replacement, Reduction, Refinement): 2023/08, EVKEM.
Diet composition
During the experiment, the animals were given commercial feed supplied by Arden Research & Experiment Company. Details of the feed composition are given in Table 1, which is called “Standard Rat Diet”. There was no chance of the feed being contaminated or harbouring pathogens, so one could rest assured that the diet would not interfere with the results of the study.
In pre-frozen brain cortex tissue samples, elemental concentrations were determined after subjecting the material to a clear solution preparation process in a pressurised microwave oven (Mars 5, CEM Corporation, USA). A Vista AXE ICP-AES (Inductively Coupled Plasma–Atomic Emission Spectrometer, Varian Inc., Australia) was utilised for the analysis. Approximate sample weight (ca. 0.5 g) was measured on a balance for each specimen that was kept in 10% nitric acid for 24 h, rinsed with deionised water, transferred into 10 ml polypropylene tubes, and marked. Collection of samples was achieved by means of sterilised instruments, and all procedures were performed in a laminar flow cabinet. Glassware was then thoroughly washed with 65% HNO₃ before being rinsed with ultrapure water. The combustion process was initiated by adding 10 ml of 65% HNO₃ (w/w, Merck, Germany) to each XP-1500 (Tetrafluorometaxyl, CEM Corporation, USA) tube and then heating until a pressure of 150 PSI and a temperature of 180 °C were attained. After cooling, the samples were transferred to 25 balloon-shaped tubes. Deionised water (resistivity: 18 Ω cm⁻¹, P.Nix UP 900, Human Corporation, Korea) was added, the resulting suspension homogenised by shaking for 10 min, and thereafter re-transferred to 10 ml polypropylene tubes and numbered. NIST 1577b (National Institute of Standards and Technology, Gaithersburg, Maryland, USA) was used as a standard reference material. Prior to the analysis, the ICP-AES instrument was calibrated with single- and multi-element standards (Merck, Darmstadt, Germany). Measurement uncertainty was verified with certified reference matrices and blanks. Blank measurements were performed to omit interferences due to contamination.
Concentration for Mg, Zn, Se, Cr, Cu, and Fe was measured and recorded in micrograms per gram (µg/g). Fundamentally, despite being useful, the trace element analyses become confounded owing to environmental circumstances and methodology-related limitations. It has been shown in previous research that environmental contamination occurring during sample collection can cause discrepancies in the results22. Considering this, solid standardisation and repeatability should be strictly adhered to.
The measurement of the SOD, CAT, GPx activities and MDA level in brain cortex tissue
Superoxide dismutase activity
Using the Superoxide Dismutase Assay Kit (Cayman Chemical, Ann Arbour, MI, USA), superoxide dismutase activity in brain cortex tissue supernatants was measured in conjunction with a Bio-Tek ELx-800 microplate reader (Winooski, VT, USA). The method relies upon detection of superoxide radicals produced through the combined use of xanthine oxidase and hypoxanthine. By definition, one unit of SOD activity is that amount required for 50% dismutation of superoxide radical in 37 °C conditions. Units per milligram of protein (U/mg protein) were used to standardise the results.
Catalase activity
Measurement of catalase activity in brain cortex was done using the Catalase Assay Kit (Cayman Chemical Co., Ann Arbour, MI, USA). The method is based on the catalase-mediated reaction of hydrogen peroxide presence in methanol, resulting in the formation of formaldehyde. The generated formaldehyde acts in concert with 4-amino-3-hydrazino-5-mercapto-1,2,4-triazole to yield a chromophoric compound and is quantified for spectrophotometric measurement at 540 nm using a Thermo Scientific Multiskan Spectrum microplate reader (Ann Arbour, MI, USA). This was then expressed in U/mg protein.
Glutathione peroxidase activity
GP activities were measured using the Glutathione Peroxidase Assay Kit (Cayman Chemical Co., Ann Arbour, MI, USA). The assay is based on the reduction of hydroperoxides by GPx through a reducing substrate, that is, GSH, which is oxidised via glutathione reductase, resulting in an oxidation of NADPH to NADP. This oxidation is verified through the decrease of absorbance recorded at 340 nm, which is proportional to the activity of GPx. The GPx activity was expressed as U/mg protein.
Malondialdehyde level
MDA levels were measured in brain cortex tissue supernatants using the Thiobarbituric Acid Reactive Substances (TBARS) Assay Kit by Cayman Chemical Company (Ann Arbour, MI, USA) according to the instructions given by the manufacturer. An aspect of MDA is that it transfuses with thiobarbituric acid into a pink chromogen, which was read spectrophotometrically at 532 nm. Results are expressed in nanomoles of MDA per milligram of tissue (nmol/mg tissue).
Bio-concentration measures in brain cortex tissue
Total protein concentration in the homogenate of brain cortex was estimated using the Bio-Rad DC Protein Assay Kit (San Francisco, CA, USA). This test is based on the colourimetric detection of protein in which a blue-coloured complex is formed following the interaction with assay reagents, resulting in light to dark variations in the intensity of colour in proportion to the amount of protein present. Absorbance was measured at 595 nm using a Thermo Scientific Multiskan Spectrum microplate reader. The endogenous control was the use of that protein concentration for the normalisation of enzymatic activities.
Results
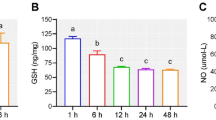
As illustrated in Fig. 2, the antioxidant enzymes SOD, CAT, GPx, and the level of lipid peroxidation MDA increased significantly in the control, BI, and BI + VPA rat groups. When compared with the control group, MDA levels were significantly higher in the brain cortical tissue of the BI rats by the ischaemic insult (α: p < 0.05). In contrast, VPA treatment arrested this increase and lowered MDA levels significantly (p < 0.05 vs. BI group). Antioxidant enzymes SOD, CAT, and GPx were also inhibited significantly in the BI group in comparison to controls (α: p < 0.05). VPA treatment restored more or less the normal activity (*: p < 0.05 vs. BI group), thus confirming its neuroprotective modality by inhibiting oxidative stress and stimulating antioxidant defence in the brain.
-
SOD activity: decreased markedly in BI due to enhanced formation of free radicals; increased significantly after VPA treatment.
-
CAT and GPx: No different behaviour from SOD for the pattern shown, but there was an enhancement by VPA.
-
MDA levels: decrease in MDA gives an idea of lipid peroxidation due to VPA treatment.
The activities of (A) SOD, (AB CAT, (C) GPx (units per mg protein), and (D) MDA were then plotted in this figure. The average value ± standard error of the mean was given from the data (n = 8/group) and analyzed using Kruskal–Wallis ANOVA followed by Fisher’s LSD post hoc test comparison. p < 0.05 vs. Control; p < 0.05 vs. BI. BI brain ischaemia, VPA sodium valproate, SOD superoxide dismutase, CAT catalase, GPx glutathione peroxidase, MDA malondialdehyde.
Collectively, these observations clearly confirm that VPA has a decent ability to counteract oxidative injury and substantiates its function in neuronal protection against ischaemia.
As for the effect of VPA on major status of natural elements in the brain cortex tissue, significant decreases in Mg, Zn and Se levels were recorded for the BI versus controls (α: p < 0.05). Trace elements—specifically, all the above—were increased significantly with VPA treatment (p < 0.05 vs. BI group). This suggests its restorative activity on trace element homeostasis linked to injury from ischaemia; in contrast, Cr, Cu and Fe (Fig. 4a) were significantly elevated in the BI group when compared with controls (p < 0.05) most likely resulting from metal-associated oxidative stress. Values were subsequently reduced significantly by treatment with VPA (p < 0.05 vs. BI group) thereby confirming chelating properties and possible curative actions against metal toxicity.
Specific observations include
-
Mg, Zn, Se: Their conspicuous rise after VPA treatment highlights their role in antioxidant defence and cellular repair mechanisms.
-
Cr, Cu, Fe: Reduction by VPA suggests its ability to prevent Fenton-reaction-mediated oxidative damage. The difference was taken as significant at p < 0.05. α p < 0.05 vs. the control group, *p < 0.05 vs. the BI group, BI: Brain Ischaemia, VPA: Sodium Valproate (Figs. 3, 4).
Fig. 3 The levels of essential elements—(A) magnesium, (B) zinc, and (C) selenium—in the tissue of the brain cortex before and after treatment are displayed in this figure. Results of the experiment have been expressed as mean SD. In this study, the Kruskal–Wallis one-way analysis of variance (ANOVA) statistical technique was employed, followed by Fisher’s least significant difference tests for applied analysis. Statistical difference is said to be significant at p < 0.05. α p < 0.05 vs. control group, *p < 0.05 vs. BI group, BI: Brain Ischaemia, VPA: Sodium Valproate.
Fig. 4 Levels of essential elements (A) chromium (Cr), (B) copper (Cu), (C) and iron (Fe) in brain cortex tissue before and after treatment. Results were expressed in mean S.D. The statistical analysis carried out in this study was the Kruskal–Wallis test, a one-way ANOVA, which was later followed by Fisher’s least significant difference test. p < 0.05 was considered significant. α p < 0.05 vs. control group, *p < 0.05 vs. BI group, BI: Brain Ischaemia, VPA: Sodium Valproate.
Statistical analysis and biological implications
The Kruskal-Wallis one-way ANOVA proved statistically significant across the groups for each of the measured parameters. Efficacy of VPA in reversing the changes due to ischaemia on trace elements and antioxidant enzyme activities was confirmed in the post hoc tests. These findings acquire biological relevance, especially on the grounds of the ability of VPA to restore metal homeostasis and ameliorate the generation of antioxidant defences, which is crucial in combating the ischaemic damage of the brain. Power analysis was employed to estimate the minimal sample size required so as to yield adequate statistical power in the experimental study. Power analysis is the notion of determining how many animals are needed to maximise the probability (power) of a statistical test giving a correct result. Statistical power in this study was set at 80% (β = 0.2), and the significance level (α) was 5% (0.05). Effect size (Cohen’s d value) was determined as 0.5 (moderate effect). Calculation of sample size requirements resulted in a minimum of 8 required for each group, and therefore the experiment might be sufficiently powerful to yield reliable results. Power analysis was conducted using G*Power software.
Discussion
Our studies strongly support the claim that valproic acid (VPA) has a strong neuroprotective effect in a rat model of transient bilateral carotid occlusion. The neuroprotective properties of VPA seem to be mediated largely via restoration of endogenous antioxidant defence, correction of trace element deficits, and reduction of accumulation of neurotoxic metals23,24,25,26.
Our study showed ischaemia to significantly decrease the enzyme activity of antioxidant enzymes (SOD, CAT, GPx), while it increased MDA levels, which indicated lipid peroxidation. VPA treatment reverted these changes, restoring enzyme activities and significantly reducing MDA levels23,24,25,26,27,28. These results suggest that VPA may be enhancing the endogenous antioxidant defences via epigenetic mechanisms involving inhibition of HDACs or activation of the Nrf2 pathway7.
We also found that ischaemia leads to significant depletion of essential trace elements (Mg, Zn, Se), all of which were significantly prevented by treatment with VPA. These elements act as cofactors for essential antioxidant enzymes and functionally participate in mitochondrial and synaptic activities. Restoration of their levels may contribute to the recovery of the antioxidant activity seen in VPA-treated rats29,30,31,32,33,34,35.
The present study also shows that ischaemia significantly induces the elevations of redox-active metals like iron (Fe), copper (Cu), and chromium (Cr), all of which were significantly reduced by VPA treatment. Excess of these metals might aggravate oxidative stress via Fenton-type reactions causing neuronal damage36,37,38,39,40,41. This mitigation might arise from putative metal-chelating properties of VPA, or it could be an alteration of metal transport pathways.
Such results correlate with observations made on the basis of neurodegenerative disorders. Jankovic and Sherer8 pointed out how VPA may modulate metal-induced oxidative stress in Alzheimer’s and Parkinson’s diseases. Thus, all our data suggest that VPA is able to provide multimodal neuroprotective effects that enhance endogenous antioxidant defences, restore the balance of trace elements, and decrease toxic metal burden levels. Such comprehensive mechanism distinguishes VPA from single-pathway-monotherapeutic agents.
Clinically, evidence does support this. The neurotherapeutic potential of VPA has been demonstrated in several human studies besides its conventional use as an antiepileptic. For instance, Karimi et al.39 stated that VPA provides better cognitive and emotional outcomes in post-stroke patients, thereby implicating it in the context of neurorehabilitation. In parallel, Suda et al.40 stated that VPA administration reduced cerebral oedema and improved neurological scores in acute ischaemic stroke patients. In addition, Zhou et al.41 showed that VPA reduces proinflammatory cytokine levels, thus promoting motor recovery over a period of 3 months, providing additional evidence for its neuroprotective efficacy in clinical settings.
To complement the evidence from clinical studies, mechanistic studies demonstrate the epigenetic influence of VPA in neurodegenerative and neuropsychiatric disorders. For instance, Pavlou and Outeiro42 proposed the role of VPA as a potential modulator for the epigenetic dysregulation in the aging brain, drawing attention to its importance with regards to histone acetylation changes in Parkinson’s disease. Similar to this, Pal et al.43 examined the role of histone deacetylase inhibitors, including VPA, as cognitive enhancers as well as modulators of mood and behaviour, suggesting applications in disorders ranging from stroke to mood disorders. In agreement with these perspectives, Chiu et al.44 proposed that VPA and lithium have therapeutic effects beyond bipolar disorder via mechanisms of neurotrophic support, mitochondrial stabilisation, and anti-inflammatory activity. Thus, mechanistic insight combined with clinical trial data proposes the plausibility of repositioning VPA as a prevalent agent for ischaemic brain injury through modulation of oxidative stress and epigenetic regulation and neuronal plasticity.
In our study, we could not perform behavioural tests, but the normalisation of critical elements such as Mg and Zn, associated with synaptic plasticity, supports the view that VPA may bring about functional recovery27,28,29,30,31, which still remains to be confirmed in the immediate future experiments. Our study is one of few to consider oxidative enzymes, trace elements, and neurotoxic metals in unison in a model of cerebral ischaemia. Such an integrated biochemical profile paints a fuller picture of the neuroprotective profile of VPA. Histopathological examinations (e.g. GFAP, caspase-3) and behavioural tests (e.g. rotarod, Morris water maze) were not done in our study to confirm neurofunctional results; future studies should take these into account.
Additionally, we administered only one dose of VPA and one time point after ischaemia in our study. Further studies of multiple dosing and extended time schedules will better map the therapeutic window of VPA. From a clinical perspective, long-term administration of VPA in patients with epilepsy and bipolar disease has been correlated with cognitive stability and structural brain protection45, thus adding support for its repurposing for managing ischaemic stroke under proper safety monitoring.
In summary, our study demonstrates valproic acid as ameliorating ischaemia-induced oxidative stress, restoring the balance of trace elements, and reducing the burden of redox-active metals. These effects validate its activity as a potent multi-targeted neuroprotective agent. In conjunction with encouraging clinical data, VPA stands out as a promising candidate for the treatment of ischaemic brain injury. However, further translational studies and controlled clinical trials are needed to ascertain its efficacy and long-term safety in human populations.
Data availability
Data Availability Data sets generated and/or analysed during the current study are available from the corresponding author upon reasonable request.
References
Zhang, L. et.al. Role of mitochondrial calcium uniporter-mediated Ca2 + and iron accumulation in traumatic brain injury. J. Cell Mol. Med. 23,2995–3009 https://doi.org10.1111/jcmm.14206. (2019).
Valko, M., Rhodes, C. J., Moncol, J., Izakovic, M. & Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 160,1–40 https://doi.org10.1016/j.cbi.2005.12.009. (2006).
Luo, Y. Human serum Albumin-enriched clopidogrel bisulfate nanoparticle alleviates cerebral Ischemia- reperfusion injury in rats. Pharm. Res. 40, 1821–1833. https://doi.org/10.1007/s11095-023-03543-8 (2023).
Alizadeh, A., Dyck, S. M. & Karimi-Abdolrezaee, S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front Neurol. 10,282 https://doi.org10.3389/fneur.2019.00282. (2019).
Luo, J., Wang, T., Gao, P., Krings, T. & Jiao, L. Endovascular treatment of intracranial atherosclerotic stenosis: current debates and future prospects. Front. Neurol. 9, 666. https://doi.org10.3389/fneur.2018.00666 (2018).
Chen, S. et al. Valproic acid attenuates traumatic spinal cord injury-induced inflammation via STAT1 and NF-κB pathway dependent of HDAC3. J. Neuroinflammation. 15 (150). https://doi.org10.1186/s12974-018-1193-6 (2018).
Shukla, S. & Tekwani, B. L. Histone deacetylases inhibitors in neurodegenerative diseases, neuroprotection and neuronal differentiation. Front. Pharmacol. 11,537 https://doi.org10.3389/fphar.2020.00537 (2020).
Jankovic, J. & Sherer, T. The role of epigenetics in neurodegenerative diseases. Neurother 12, 659–670. https://doi.org10.3389/fphar.2020.00537 (2015).
Wu, X. Histone deacetylase inhibitors up-regulate astrocyte GDNF and BDNF gene transcription and protect dopaminergic neurons. Int. J. Neuropsychopharmacol. 11, 1123–1134. https://doi.org/10.1017/S1461145708009024 (2008). https://doi.org/DOI
Terzioğlu Bebitoğlu, B., Oğuz, E. & Gökçe, A. Effect of valproic acid on oxidative stress parameters of glutamate-induced excitotoxicity in SH-SY5Y cells. Exp. Ther. Med. 20, 1321–1328. https://doi.org/10.3892/etm.2020.8802 (2020).
Phiel, C. J. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J. Biol. Chem. 276, 36734–36741. https://doi.org/10.1074/jbc.M101287200 (2001).
Romoli, M. Valproic acid and epilepsy: from molecular mechanisms to clinical evidences. Curr. Neuropharmacol. 17, 926–946. https://doi.org/10.2174/1570159X17666181227165722 (2019).
Park, S. W. Thiazolidinedione class of peroxisome proliferator-activated receptor gamma agonists prevents neuronal damage, motor dysfunction, Myelin loss, neuropathic pain, and inflammation after spinal cord injury in adult rats. J. Pharmacol. Exp. Ther. 320, 1002–1012. https://doi.org/10.1124/jpet.106.113472 (2007).
Lushchak, V. I. Free radicals, reactive oxygen species, oxidative stress. Biochem 3 (1), 11–26. https://doi.org/10.2478/s11658-014-0185-5 (2014).
Murphy, M. P. How mitochondria produce reactive oxygen species. Biochem. J. 417, 1–13. https://doi.org/10.1042/BJ20081386 (2009).
Li, P., Stetler, R. A. & Leak, R. K. Oxidative stress and mitochondrial dysfunction in ischemic brain injury and neurodegeneration. Neuropharmacol 134, 208–217. https://doi.org/10.1016/j.neuropharm.2017.11.011 (2018).
Ulas, M. & Argadal, O. G. Trace element, antioxidant and oxidant levels in spinal cord injury: different perspective on the effects of valproic acid. Eur. Rev. Med. Pharmacol. Sci. 27, 3892–3905 (2023).
Chu, T. Valproic acid- mediated neuroprotection and neurogenesis after spinal cord injury: from mechanism to clinical potential. Regen Med. 10, 193–209. https://doi.org/10.2217/rme.14.86 (2015).
Lin, M. C., Liu, C. C., Lin, Y. C. & Hsu, C. W. Epigallocatechin gallate modulates essential elements, zn/cu ratio, hazardous metal, lipid peroxidation, and antioxidant activity in the brain cortex during cerebral ischemia. Antioxid. (Basel). 11, 396. https://doi.org/10.3390/antiox11020396 (2022).
Adibhatla, R. M. & Hatcher, J. F. Lipid oxidation and peroxidation in CNS health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 12, 125–169. https://doi.org/10.1089/ars.2009.266 (2010).
Singh, D. Hidden Pharmacological activities of valproic acid: A new insight. Biomed. Pharmacother. 142 (112021). https://doi.org/10.1016/j.biopha.2021.112021 (2021).
Jomova, K. & &Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicol 283, 65–87. https://doi.org/10.1016/j.tox.2011.03.001 (2011).
Ulas, M. & Cay, M. The effects of 17beta-estradiol and vitamin E treatments on oxidative stress and antioxidant levels in brain cortex of diabetic ovariectomized rats. Acta Physiol. Hung. 97, 208–215. https://doi.org/10.1556/aphysiol.97.2010.2.7 (2010).
Fang, K. M. Trace element, antioxidant activity, and lipid peroxidation levels in brain cortex of gerbils after cerebral ischemic injury. Biol. Trace Elem. Res. 152, 66–74. https://doi.org/10.1007/s12011-012-9596-1 (2013).
Ro, J. H., Liu, C. C. & Lin, M. C. Resveratrol mitigates cerebral ischemic injury by altering levels of trace elements, toxic metal, lipid peroxidation, and antioxidant activity. Biol. Trace Elem. Res. 199, 3718–3727. https://doi.org/10.1007/s12011-020-02497-x (2021).
Todorova, A., Vonderheid-Guth, B. & Dimpfel, W. Effects of tolterodine, trospium chloride, and Oxybutynin on the central nervous system. J. Clin. Pharmacol. 41, 636–644. https://doi.org/10.1177/00912700122010528 (2001).
Bhattacharya, P., Pandey, A. K. & Paul, S. Minocycline and magnesium in combination May be a good therapeutic intervention for cerebral ischemia. Med. Hypotheses. 77, 1129–1131. https://doi.org/10.1016/j.mehy.2011.09.019 (2011).
Li, W. Magnesium sulfate for acute traumatic brain injury. J. Craniofac. Surg. 26, 393–398. https://doi.org/10.1097/SCS.0000000000001339 (2015).
Akintoye, O. O. Zinc supplement reverses short-term memory deficit in sodium benzoate-induced neurotoxicity in male Wistar rats by enhancing anti-oxidative capacity via Nrf 2 up-regulation. Behav. Brain Res. 437, 114–163. https://doi.org/10.1016/j.bbr.2022.114163 (2023).
Akbari, G. Role of zinc supplementation on ischemia/reperfusion injury in various organs. Biol. Trace Elem. Res. 196, 1–9. https://doi.org/10.1007/s12011-019-01892-3 (2020).
Prakash, A., Bharti, K. & Majeed, A. B. Zinc: indications in brain disorders. Fundam Clin. Pharmacol. 29, 131–149. https://doi.org/10.1111/fcp.12110 (2015).
Staneviciene, I. et al. Effect of organic selenium on the homeostasis of trace elements, lipid peroxidation, and mRNA expression of antioxidant proteins in mouse organs. Int. J. Mol. Sci. 2, 9704. https://doi.org/10.3390/ijms24119704 (2023).
Ramírez-Acosta, S. The role of selenium in shaping mice brain metabolome and selenoproteome through the gut-brain axis by combining metabolomics, metallomics, gene expression, and amplicon sequencing. J. Nutr. Biochem. 117, 109323. https://doi.org/10.1016/j.jnutbio.2023.109323 (2023).
Yang, Q., Zhong, Y., Zhang, Z. & Dang, Z. Simultaneous degradation of sulfamethazine and reduction of Cr(VI) by flexible self-supporting Fe-Cu-Al2O3 nanofibrous membranes as heterogeneous catalysts: insights into synergistic effects and mechanisms. J. Chem. Eng. 472, 144984. https://doi.org/10.1016/j.cej.2023.144984 (2023).
Al-Naama, L. M., Hassan, M. K. & Mehdi, J. K. Association of erythrocytes antioxidant enzymes and their cofactors with markers of oxidative stress in patients with sickle cell anemia. Qatar Med. J. 14 (2016). https://doi.org/10.5339/qmj.2015.14 (2015).
Travacio, M., Polo, J. M. & Llesuy, S. Chromium (VI) induces oxidative stress in the mouse brain. Toxicol 162, 139–148. https://doi.org/10.1016/S0300-483X(00)00254-7 (2001).
Wise, J. P., Jr, Young, J. L., Cai, J. & Cai, L. [cr(vi)]urrent Understanding of hexavalent chromium [Cr(VI)] neurotoxicity and new perspectives. Environ. Int. 158, 106877. https://doi.org/10.1016/j.envint.2021.106877 (2022).
Strausak, D., Mercer, J. F., Dieter, H. H., Stremmel, W. & Multhaup, G. Copper in disorders with neurological symptoms: alzheimer’s, menkes, and Wilson diseases. Brain Res. Bull. 55, 175–185. https://doi.org/10.1286/S40795-023-00690-4 (2001).
Karimi, E. et al. The effects of Royal jelly supplementation on oxidative stress, inflammatory mediators, mental health, cognitive function, quality of life, and clinical outcomes of patients with ischemic stroke: study protocol for a randomized controlled trial. BMC Nutr. 9 (1), 32. https://doi.org/10.1016/S0361-9230(01)00454-3 (2023).
Suda, S. Valproic acid ameliorates ischemic brain injury in hyperglycemic rats with permanent middle cerebral occlusion. Brain Res. 1606, 1–8. https://doi.org/10.1016/j.brainres.2015.02.013 (2015).
Zhou, J., Fangma, Y., Chen, Z. & Zheng, Y. Post-stroke neuropsychiatric complications: types, pathogenesis, and therapeutic intervention. Aging Disease. 14 (6), 2127. https://doi.org/10.1111/cns.13993 (2023).
Pavlou, M. A. S. & Outeiro, T. F. Epigenetics in Parkinson’s disease (ed. Martins-de-Souza, D.) in Neuroepigenomics Aging Dis., 363–390 (Munich Germany 2017).
Pal, D., Sahu, P., Mishra, A. K., Hagelgans, A. & Sukocheva, O. Histone deacetylase inhibitors as cognitive enhancers and modifiers of mood and behavior. Curr. Drug Targets. 24 (9), 728–750. https://doi.org/10.2174/1389450124666221207090108 (2023).
Chiu, C. T., Wang, Z., Hunsberger, J. G. & Chuang, D. M. Therapeutic potential of mood stabilizers lithium and valproic acid: beyond bipolar disorder. Pharmacol. Rev. 65, 105–142. https://doi.org/10.1124/pr.111.005512 (2013).
Perucca, E. Pharmacological and therapeutic properties of valproate: a summary after 35 years of clinical experience. CNS Drugs. 16, 695–714. https://doi.org/1172-7047/02/0010-0695 (2002).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conceptualization, M.U., E.B.Ö.; methodology, M.U.; software, M.U.; validation, M.U., formal analysis, M.U., E.B.O and O.G.A.; investigation, M.U., E.B.Ö.; resources, M.U., E.B.O. and O.G.A.; data curation, M.U., writing-original draft preparation, M.U.; writing-review and editing, M.U., E.B.O.; visualization, M.U. and O.G.A.; supervision, M.U.; project administration, M.U.; funding acquisition, O.G.A.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The protocol number 2023/08, EVKEM, was approved by the Ethics Committee of Elazig University Local Ethics Committee of Animal Experiments and Center Elazig Veterinary Control Institute.
AI disclosure
No artificial intelligence or assisted technologies were used in the study’s production.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ulaş, M., Gökay Argadal, Ö. & Bardaş Özkan, E. Targeting oxidative stress and metal imbalance in cerebral ischemia: the neuroprotective role of sodium valproate. Sci Rep 15, 27529 (2025). https://doi.org/10.1038/s41598-025-12425-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-12425-5