Abstract
Linear growth faltering and stunting in children is associated with inflammation of small gut. Intestinal alkaline phosphatase prevents gut inflammation from subclinical bacterial infection that becomes impaired in the presence of neuraminidase-3 (Neu3) activity. We investigated if total Neu3 in children at 15 months can predict their linear growth at later ages (at 18, 21, and 24 months). We collected data from 189 children enrolled in the Malnutrition and Enteric Disease Study (MAL-ED) birth cohort study in Bangladesh. We determined total Neu3 activity in stool samples at 15 months of age and measured its association with length-for-age z-scores (LAZ) at 18, 21, and 24 months of age, in addition to their socio-demographic conditions using bi-variate and multivariable linear regression analyses. We found that total Neu3 at 15 months was negatively associated with the LAZ-score at 18 (regression coefficient -0.004, 95% CI -0.006 to -0.001, p < 0.01), 21 (-0.003, 95% CI -0.006, -0.001, p < 0.01), and 24 (-0.004, 95% CI -0.006, -0.001, p < 0.01) months of age after adjusting the covariates. In conclusion, total neuraminidase-3 in stool is a significant predictor of linear growth in young children and would be key in early detection of linear growth faltering and stunting.
Similar content being viewed by others
Introduction
Stunting is a condition where a child’s height falls below two standard deviations of the World Health Organisation (WHO) Child Growth Standard1. According to the 2020 WHO report, 149.2 million children under the age of five years were stunted2. Despite improvement in the last few decades, the burden of stunting in developing countries is still alarming3. Stunting was thought to be caused by undernutrition and poor hygiene, however, in Bangladesh, a recent report showed that 21% of children from the privileged part of the population were stunted as well4. Stunting is also associated with an increased risk of developing learning deficits (due to poor cognitive development) as well as non-communicable diseases like diabetes and hypertension5,6. Complications caused by stunting are irreversible if not addressed quickly7. Thus, stunting is an issue that impacts young children in all socioeconomic conditions, that requires early intervention to prevent long-term sequelae.
A recent report showed that environmental enteric dysfunction (EED) – a subclinical chronic inflammatory disorder of the small intestine – underlies 40% of all stunting cases8, even when diarrhea is controlled9,10. EED is still highly prevalent in low- and middle-income countries, especially in children who dwell in slums8. During EED the microvilli of the gut epithelium become flattened and the crypts become shallow11,12. Also, the intestinal wall loses its capacity to absorb nutrients while simultaneously compromising the barrier function13 leading to bidirectional leakage of fluid, pathogens, and toxins across the intestinal wall11,12,14. This functional impairment of the gut epithelium is the major driver of the gut inflammation seen in EED cases and may be a mechanism by which EED contributes to stunting. One of the enzymes involved in mitigating this inflammatory response in the gut is intestinal alkaline phosphatase (IAP).
IAP is a dephosphorylating enzyme found in the apical brush border of the small intestine. It neutralizes intestinal pathogens and ensures optimal intestinal functionality. Although IAP produced exclusively by the duodenal enterocytes, the activity is highest in the terminal part of the ileum15,16,17. IAP dephosphorylates the lipopolysaccharide-phosphate (LPS-P) endotoxin thereby preventing the activation of the TLR-4-complex mediated gut inflammation18,19,20,21. However, IAP loss of function (caused by decreased catalytic activity with/without decreased enzyme expression) can result in reduced detoxification of LPS-dependent TLR-4 agonist activity22. Activation of TLR4, for example in recurrent subclinical Salmonella enterica Typhimurium infection, can lead to Toll-like receptor 4 (TLR4)-dependent expression of neuraminidase-3 (Neu3), triggering the onset of gut inflammation23. Although Salmonella – a major human foodborne pathogen—does not code for neuraminidase, it is widely encoded by diverse organisms, including the human gut microbiota23,24. Neuraminidases, also known as sialidases, is also expressed by human cells and cleaves sialic acid from glycan moieties25. Neu3 was reported to trigger intestinal inflammation and colitis in human23. An increased neuraminidase activity can hydrolyze the IAP, reducing its half-life and leading to an acquired deficiency26. Impaired expression of IAP has also been found to be associated with inflammatory bowel disease and celiac disease17. Given the impact of Neu3 on IAP it is worth investigating whether it can be used as a marker to indicate intestinal health and functionality. Figure 1 presents the graphical summary indicating the relationship EED, Neu-3 and stunting.
Graphical summary indicating the relationship between stunting and EED. (Stunting is a form of linear growth faltering in children, which is often associated with EED (environmental enteric dysfunction). In EED, the microvilli of the gut epithelium become flattened, and crypts become shallow. Recurrent subclinical enteric infection ensues higher expression of a sialidase, neuraminidase 3 (Neu3). It cleaves off the sialic acid residue from IAP, increasing its internalization and degradation. IAP is capable of inactivating LPS-P and attenuating gut inflammation. However, in the absence of IAP, TLR4-complex becomes activated, which results in the release of pro-inflammatory cytokines, through activation of MyD88-dependent and MyD88-independent signaling pathways 25–28. EED – Environmental Enteric Dysfunction; TLR4—Toll-like receptor 4; Neu3 – Neuraminidase 3; IAP – Intestinal Alkaline Phosphatase; LSP-P – Phosphorylated Lipopolysaccharide; TRIF—TIR-domain-containing adapter-inducing interferon-β; MyD88—Myeloid differentiation primary response 88; IKK—inhibitor of nuclear factor-κB kinase; TBK—TANK-binding kinase; NF-κB—Nuclear factor kappa B; IRF3—IKK, and interferon regulatory factor 3).
In this context, we hypothesize that the Neu3 activity in young children will negatively impact their nutritional status, leading to stunting. To test our hypothesis, we measured the total Neu3 in children at 15 months and their linear growth status at the ages of 18, 21, and 24 months, to determine if Neu3 has the potential to be used as a predictor of linear growth.
Methods
Study site, study design, participants, and ethical consideration
In this study, we used the data and the biological samples collected from the children of the Malnutrition and Enteric Disease Study (MAL-ED) in Bangladesh. In the MALED study, children enrolled at birth at the Bauniabadh area of Mirpur, Dhaka and the adjacent community were followed up to 24 months of age. The study protocol (ethical approval number: PR-18068) was approved by the Institutional Review Board of International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b). Informed written consent was taken from one of the parents of each study participant. All the methods were performed following the relevant guidelines and regulations. A detailed description of the MAL-ED study methodology has been published elsewhere27.
Data collection, anthropometry, specimen collection and storage
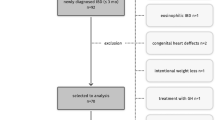
Baseline sociodemographic and household information of the children was collected at enrollment after birth. Following standard anthropometric methodology, trained field workers measured the recumbent length of the children to the nearest 1 mm using Seca infantometer (model no: 417, Hamburg, Germany) every month until 24 months of age28. The length-for-age z-score (LAZ-score) was calculated according to the WHO 2006 child growth standards using the lambda-mu-sigma method29. The LAZ-score was used to assess the linear growth status. Length was measured using commercial measuring boards (Seca Infantometer; model no. 417). Stored stool samples from 188 children in the MAL-ED fifteen-month cohort were used to assess the levels of Neu3. Stool samples were collected without a fixative, and were aliquoted and stored at –70 °C. The 15 month time point was chosen as it had the maximum number of stool samples available for analysis.
Determination of total neuraminidase activity
The total neuraminidase activity was evaluated with the product technical sheet obtained from the neuraminidase activity assay kit of Sigma-Aldrich. Briefly, 480 uL of water was added to 20 uL of the 10 mM standard (standard enzyme, provided with the kit) to make a 400-uM standard working solution. Appropriate volumes of water were added to make 0 (blank), 120, 240, and 400 uM standards. Then 20 µl of standards were added to separate wells of a 96-well clear flat-bottom plate in four replicates. 80 µl of the reaction mixture with substrate was added to 2 wells and 80 µl reaction mixture alone (control) was added to the remaining 2 wells. The plate was sealed and incubated at 37 °C in the dark. Absorbance was measured at 570 nm at 20 min and 50 min. Absorbance measured at 50 min was plotted for each standard against the standard concentrations. The linearity of standard curve was evaluated, slope of the linear regression was determined, and the activity of each sample was calculated according to the manufacturer’s protocol.
Explanatory and outcome variables
Mean values of morbidity (diarrhea and fever) episodes and total calorie intake during the preceding three months of assessment of outcome variables were used as indicators of morbidity and energy intake. Trained field workers visited the participant’s household twice a week and used a surveillance assessment form (SAF) to document illness status of the child. During every such visits, field workers asked the caregiver whether the children experienced any morbidity symptoms listed in SAF on each day since the last visit. Diarrhoea is defined as having three or more loose stools in a 24-h period or at least one loose stool with blood reported by the mother. A diarrhoeal episode is defined as being separated from another episode by at least two or more diarrhoea-free days. Fever is defined as an axillary temperature > 37.5 °C30. The 24-h food frequency data were collected monthly from ninth month onwards using a 24-h multiple-pass dietary recall approach for assessing the energy intake of the children. The 24-h dietary recall interviews were conducted on non-consecutive days and out of every four recalls, one interview was conducted on a weekend. A locally adapted food composition table was used to convert the dietary intake data to energy31. WAMI index (Water, sanitation, hygiene, Asset, Maternal education, and Income index, ranging from 0 to 1) is a socioeconomic status index that includes access to improved water and sanitation, eight selected assets, maternal education, and household income was used as a representative of socio-economic status of the households32. A higher WAMI index means a better socioeconomic status. The composite environmental enteric dysfunction score or EED score ranging from 0 to 10 was calculated at 15 months from alpha-1-anti-trypsin, myeloperoxidase and neopterin- the three fecal markers of environmental enteric dysfunction following the method published previously33.
Statistical analyses
Secondary analyses of MAL-ED birth cohort study data was conducted. Socio-economic characteristics and demographic factors were described using frequencies with proportions when variables were categorical. Means and standard deviations (SD) were reported when quantitative variables were distributed normally. Medians and interquartile ranges (IQRs) were used when the quantitative variables were not normally distributed. Correlations between the Neu3 abundance and LAZ scores at 18, 21, and 24 months were assessed using Spearman’s correlation test. Association of Neu3 and other predictors to LAZ scores at 18, 21, and 24 months were measured using bivariable and multivariable linear regression models. At first, bivariable analysis was done to explore the unadjusted effect of Neu3, morbidity, socio-economic status, total calorie intake, and birth characteristics on the outcomes. Variables were assessed individually and were included in the multivariable regression model if the p-value was found < 0.20 in bivariable analysis following the recommendation of Greenland et al.34. A p-value < 0.05 was considered statistically significant for multivariable regression analyses. Analyses were performed using Stata version 18 and R version 3.5.335.
Results
Table 1 shows the characteristics of the 189 participants in this study. Half of them were males. The length for age (LAZ) scores of the cohort decreased slightly between 18, 21 and 24 months.
Figure 2 presents the correlation matrix of Neu3 levels at 15 months and LAZ-scores at 18, 21, and 24 months. Data shows that the Neu3 level at 15 months had a weak, yet statistically significant negative correlation with LAZ-scores at subsequent months.
Table 2 shows the associations between Neu3 and other predictors with LAZ scores at different time points. Our findings indicate that LAZ scores at 18, 21, and 24 months were significantly associated with the total Neu3 levels measured at 15 months of age, WAMI index, maternal height, and birth weight of the children. However, the total calorie intake status showed a statistically significant association with LAZ-score only at 18 months of age.
In the multivariable linear regression model, we investigated the adjusted associations of LAZ with biomarkers and socio-demographic characteristics. Table 3 presents the independent variables (p-value ≤ 0.05) included in multivariable analysis. We found that after adjusting for all significant independent variables, total Neu3, maternal height, and birth weight of the children remained significantly associated with the LAZ-score at all three time points. However, the WAMI index was significantly associated at 21 and 24 months.
Discussion
Previous studies indicate that a child’s weight and length at birth, maternal nutritional status, and parents’ height are some of the strongest biological predictors of linear growth 36,37. However, our data shows in addition to the maternal height and birthweight, total neuraminidase-3 at 15 months of age is a predictor of liner growth at later stages, i.e., at 18, 21, and 24 months.
The finding of Neu3 as a predictor of linear growth has biological relevance. Although Neu3 could be endogenous (encoded in chromosome 11 in humans)38, neuraminidases are produced by various pathogens39,40. Thus, a higher level of Neu3 in children suggests possible subclinical infection with such pathogens. Previous studies showed the induction of Neu3 caused severe inflammation in both small and large intestines in animal models23. Other studies supported this finding by showing that Neu3 can activate neutrophils, which are inducers of inflammation41. It is also known that Neu3 impairs the activity of intestinal alkaline phosphatase, which is critical for neutralizing microbial lipopolysaccharide. In the absence of IAP, LPS activates toll-like receptor 4 complex and mediates gut inflammation42, which damages the gut epithelium, causes the infiltration of the gut microbes, activates the local immune cells43, leading to further damage, malabsorption, and malnutrition44,45. All these point to the direction of Neu3, indicating this enzyme is one of the key factors mechanistically involved in malnutrition.
In addition to that, socio-economic factors, including maternal education, sanitation and hygiene practice, economic status of the household, also impact the growth and development of children46. Our data also indicate that socio-economic factors, such as better-quality water, sanitation, maternal education, and higher household income (combined as the WAMI index) can impact children’s linear growth. However, our data suggests that the WAMI factor does not impact the length of a child as early as 18 months of age. It means total Neu3 might be a key predictor at the of linear growth earlier stages of development.
Here, we measured the total neuraminidase-3 in stool in the enrolled children. We could not determine whether the Neu3 is endogenous or exogenous39,47,48. Determining the source of Neu3 would help formulate the appropriate strategies needed to ensure maximum growth and development in children. The samples we collected from patients are from the Bauniabadh area of Mirpur, Dhaka49, a well-known resource-limited urban area in Bangladesh. Even though our sample size is reasonable, having more samples from other parts of this country is required to determine if our findings are generalizable. Previous studies indicate that approximately 40% of stunting is explained by EED, which is one form of chronic inflammatory bowel disorder8. Chronic inflammation in EED suggests lower inhibition of inflammation that could be associated with IAP induced modulation Neu-3 pathways. However, more longitudinal analyses are required to elucidate the role of Neu3 as a predictor or biomarker of EED, liner growth, and stunting. Our current findings demonstrate that use of inhibitors for Neu-3 could be implicated in stunting. This means very likely there are other biological modifiers that impact stunting, still waiting to be discovered.
In conclusion, we found a novel predictor of childhood linear growth, total neuraminidase 3, which can be used as a predictor of linear growth and help clinicians plan a realistic and optimal course of nutritional intervention so that a child can catch up with the linear growth faltering and reach full potential.
Data availability
The dataset generated and analyzed during the current study is available from the corresponding author on reasonable request.
References
De Onis, M. & Branca, F. Childhood stunting: a global perspective. Matern. Child Nutr. 12, 12–26 (2016).
Organization WH. Fund (UNICEF) UNC, Bank W. Levels and trends in child malnutrition: UNICEF. World Health Organization. (2021).
Montenegro, C. R. et al. The pediatric global burden of stunting: Focus on Latin America. Lifestyle Med. 3(3), e67 (2022).
Ahmed, T., Hossain, M., Mahfuz, M., Choudhury, N. & Ahmed, S. Imperatives for reducing child stunting in Bangladesh. Matern. Child Nutr. 12(Suppl 1), 242 (2016).
Black, R. E. et al. Maternal and child undernutrition: Global and regional exposures and health consequences. Lancet 371(9608), 243–260 (2008).
Dewey, K. G. & Begum, K. Long-term consequences of stunting in early life. Matern. Child Nutr. 7, 5–18 (2011).
Das, S. et al. Not water, sanitation and hygiene practice, but timing of stunting is associated with recovery from stunting at 24 months: Results from a multi-country birth cohort study. Public Health Nutr. 24(6), 1428–1437 (2021).
Mahfuz, M. et al. Bangladesh Environmental Enteric Dysfunction (BEED) study: protocol for a community-based intervention study to validate non-invasive biomarkers of environmental enteric dysfunction. BMJ Open 7(8), e017768 (2017).
Kosek, M. N. et al. Causal pathways from enteropathogens to environmental enteropathy: Findings from the MAL-ED birth cohort study. EBioMedicine 18, 109–117 (2017).
Prendergast, A. J. et al. Stunting is characterized by chronic inflammation in Zimbabwean infants. PLoS ONE 9(2), e86928 (2014).
Owino V, Ahmed T, Freemark M, Kelly P, Loy A, Manary M, et al. Environmental enteric dysfunction and growth failure/stunting in global child health. Pediatrics. 138(6). (2016).
Korpe, P. S. & Petri, W. A. Environmental enteropathy: Critical implications of a poorly understood condition. Trends Mol. Med. 18(6), 328–336 (2012).
Prendergast, A. J. & Humphrey, J. H. The stunting syndrome in developing countries. Paediatrics and international child health. 34(4), 250–265 (2014).
Crane, R. J., Jones, K. D. & Berkley, J. A. Environmental enteric dysfunction: An overview. Food Nutr. Bull. 36(1_suppl1), S76–S87 (2015).
Hinnebusch, B. F. et al. Enterocyte differentiation marker intestinal alkaline phosphatase is a target gene of the gut enriched Kruppel-like factor. Am. J. Physiol. Gastrointest. Liver Physiol. 286(1), 23–30 (2004).
Goldberg, R. F. et al. Intestinal alkaline phosphatase is a gut mucosal defense factor maintained by enteral nutrition. Proc. Natl. Acad. Sci. 105(9), 3551–3556 (2008).
Fawley, J. & Gourlay, D. M. Intestinal alkaline phosphatase: A summary of its role in clinical disease. J. Surg. Res. 202(1), 225–234 (2016).
Bates, J. M., Akerlund, J., Mittge, E. & Guillemin, K. Intestinal alkaline phosphatase detoxifies lipopolysaccharide and prevents inflammation in zebrafish in response to the gut microbiota. Cell Host Microbe 2(6), 371–382 (2007).
Koyama, I., Matsunaga, T., Harada, T., Hokari, S. & Komoda, T. Alkaline phosphatases reduce toxicity of lipopolysaccharides in vivo and in vitro through dephosphorylation. Clin. Biochem. 35(6), 455–461 (2002).
Poelstra, K., Bakker, W. W., Klok, P. A., Hardonk, M. J. & Meijer, D. A physiologic function for alkaline phosphatase: endotoxin detoxification. Lab. Investing.: J. Tech. methods Pathol. 76(3), 319–327 (1997).
Bentala, H. et al. Removal of phosphate from lipid A as a strategy to detoxify lipopolysaccharide. Shock 18(6), 561–566 (2002).
Parlato, M. et al. Human ALPI deficiency causes inflammatory bowel disease and highlights a key mechanism of gut homeostasis. EMBO Mol. Med. 10(4), e8483 (2018).
Yang, W. H. et al. Neu3 neuraminidase induction triggers intestinal inflammation and colitis in a model of recurrent human food-poisoning. Proc. Natl. Acad. Sci. 118(29), e2100937118 (2021).
Hoyer, L. L., Hamilton, A. C., Steenbergen, S. M. & Vimr, E. R. Cloning, sequencing and distribution of the Salmonella typhimurlum LT2 siaiidase gene, nanH, provides evidence for interspecies gene transfer. Mol. Microbiol. 6(7), 873–884 (1992).
Monti, E. et al. Identification and expression of NEU3, a novel human sialidase associated to the plasma membrane. Biochem. J. 349(1), 343–351 (2000).
Yang, W. H. et al. Recurrent infection progressively disables host protection against intestinal inflammation. Science 358(6370), eaao5610 (2017).
Murray-Kolb, L. E. et al. The MAL-ED cohort study: Methods and lessons learned when assessing early child development and caregiving mediators in infants and young children in 8 low-and middle-income countries. Clin. Infec. Dis. 59(suppl-4), S261–S272 (2014).
Tg L. Anthropometric standardization reference manual. Human Kinetics Books. 55–68. (1988).
WHO M. Growth, Reference, Study, Group. WHO Child Growth Standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development Geneva: World Health Organization. 312. (2006).
Richard, S. A., Barrett, L. J., Guerrant, R. L., Checkley, W. & Miller, M. A. Disease surveillance methods used in the 8-site MAL-ED cohort study. Clin. Infect. Dis. 59(suppl-4), S220–S224 (2014).
Caulfield, L. E. et al. Infant feeding practices, dietary adequacy, and micronutrient status measures in the MAL-ED study. Clin. Infec. Dis. 59(suppl-4), S248–S254 (2014).
Psaki, S. R. et al. Measuring socioeconomic status in multicountry studies: Results from the eight-country MAL-ED study. Popul. Health Metrics 12(1), 8 (2014).
Kosek, M. et al. Fecal markers of intestinal inflammation and permeability associated with the subsequent acquisition of linear growth deficits in infants. Am. J. Trop. Med. Hyg. 88(2), 390 (2013).
Greenland, S. Modeling and variable selection in epidemiologic analysis. Am. J. Public Health 79(3), 340–349 (1989).
Team RC. R: A language and environment for statistical computing. R Foundation for Statistical Computing. (No Title). (2013).
Aryastami, N. K. et al. Low birth weight was the most dominant predictor associated with stunting among children aged 12–23 months in Indonesia. BMC nutr. 3, 1–6 (2017).
Wu, H., Ma, C., Yang, L. & Xi, B. Association of parental height with offspring stunting in 14 low-and middle-income countries. Front. Nutr. 8, 650976 (2021).
Miyagi, T. & Yamaguchi, K. Mammalian sialidases: Physiological and pathological roles in cellular functions. Glycobiology 22(7), 880–896 (2012).
Soong, G. et al. Bacterial neuraminidase facilitates mucosal infection by participating in biofilm production. J. Clin. Investig. 116(8), 2297–2305 (2006).
Vimr, E. R., Kalivoda, K. A., Deszo, E. L. & Steenbergen, S. M. Diversity of microbial sialic acid metabolism. Microbiol. Mol. Biol. Rev. 68(1), 132–153 (2004).
Kirolos, S. A., Pilling, D. & Gomer, R. H. The extracellular sialidase NEU3 primes neutrophils. J. Leukoc. Biol. 112(6), 1399–1411 (2022).
Soares, J.-B., Pimentel-Nunes, P., Roncon-Albuquerque, R. & Leite-Moreira, A. The role of lipopolysaccharide/toll-like receptor 4 signaling in chronic liver diseases. Hep. Intl. 4, 659–672 (2010).
Lu Q, Yang M-f, Liang Y-j, Xu J, Xu H-m, Nie Y-q, et al. Immunology of inflammatory bowel disease: molecular mechanisms and therapeutics. Journal of inflammation research. 1825–44. (2022).
Valentini, L. et al. Malnutrition and impaired muscle strength in patients with Crohn’s disease and ulcerative colitis in remission. Nutrition 24(7–8), 694–702 (2008).
Massironi S, Viganò C, Palermo A, Pirola L, Mulinacci G, Allocca M, et al. Inflammation and malnutrition in inflammatory bowel disease. The Lancet Gastroenterology & Hepatology. (2023).
Muche, A., Gezie, L. D., Baraki, A. G. & Amsalu, E. T. Predictors of stunting among children age 6–59 months in Ethiopia using Bayesian multi-level analysis. Sci. Rep. 11(1), 3759 (2021).
Feng, C. et al. Endogenous PMN sialidase activity exposes activation epitope on CD11b/CD18 which enhances its binding interaction with ICAM-1. J. Leukoc. Biol. 90(2), 313–321 (2011).
Howlader MA, Demina EP, Samarani S, Guo T, Caillon A, Ahmad A, et al. The Janus‐like role of neuraminidase isoenzymes in inflammation. The FASEB Journal.;36(5). (2022).
Ahmed, T. et al. The MAL-ED cohort study in Mirpur, Bangladesh. Clin. Infec. Di. 59(suppl4), S280–S286 (2014).
Acknowledgements
We express our sincere thanks to the study participants and our colleagues at icddr,b. icddr,b is also grateful to the Governments of Bangladesh, Canada, Sweden and the UK for providing core/unrestricted support.
Funding
The MAL-ED birth cohort study was funded by the Foundation of National Institute of Health, Fogarty International Centre, with overall support from the Bill & Melinda Gates Foundation, grant no. GR-681.
Author information
Authors and Affiliations
Contributions
T.A., and S.D. originated the idea. S.D., and M.A.G. participated in the design of the study and led the protocol. S.D., M.G.R., and M.A.G. were involved in sample and data collection. M.A.G. performed and supervised the sample analysis and laboratory work. S.D., V.P.C., and M.G.R. were involved in data analysis. S.D., V.P.C., and M.A.G. interpreted the results. V.P.C., M.G.R., and R.J.R. were involved in manuscript writing. All the authors read, reviewed, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Das, S., Chowdhury, V.P., Gazi, M.A. et al. Neuraminidase-3 activity in toddlers negatively impacts the linear growth status in Bangladeshi children. Sci Rep 15, 26934 (2025). https://doi.org/10.1038/s41598-025-12763-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-12763-4